Abstract
Total 427 yeast strains from seawater, sediments, mud of salterns, guts of the marine fish, and marine algae were obtained. After inulinase activity of the yeast cultures was estimated, we found that four strains (OUC1, G7a, OUC2, and G7a1) of the marine yeasts grown in the medium with inulin could secrete a large amount of inulinase into the medium. The results of routine identification and molecular methods show that they belong to Pichia guilliermondii OUC1, Cryptococcus aureus G7a, Yarrowia lipolytica OUC2, and Debaryomyces hansenii G7a1, respectively. The optimal pHs of inulinase activity produced by them were 6.0, 5.0, 5.0, and 5.0, respectively, while the optimal temperatures of inulinase activity produced by them were 60°, 50°, 60°, and 50°C, respectively. A large amount of monosaccharides and a trace amount of oligosaccharides were detected after the hydrolysis by the crude inulinase produced by P. guilliermondii OUC1, indicating that the crude inulinase had a high exoinulinase activity while a large amount of monosaccharides and oligosaccharides were detected after inulin hydrolysis by the crude inulinase produced both by C. aureus G7a and D. hansenii G7a1. However, no monosaccharides and disaccharides were detected after inulin hydrolysis by the crude inulinase produced by Y. lipolytica OUC2, suggesting that the crude inulinase had no exoinulinase activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yeast has been used in food and other industries for ages. They have earned acceptability for long and are considered natural. Yeasts are also considered to be easier to handle and grow, in comparison to bacteria. Many hydrolytic enzymes such as amylase, lipase, protease, and phytase have been commercially produced by yeasts. Among the enzymes, inulinase has received much attention as it can be widely applied to production of fuel ethanol and high fructose syrup from inulin. Inulin is a linear β-(2, 1)-linked fructose polymer that occurs as a reserve carbohydrate in Jerusalem artichoke, dahlia tubers, or chicory root [13, 15]. Fructose is widely used in many foods and beverages instead of sucrose. Inulin can be converted into fructose by chemical approach. However, the chemical approach is currently associated with some drawbacks [6, 13]. Fructose can also be produced from starch by enzymatic methods involving α-amylase, amyloglucosidase, and glucose isomerase [13]. The best procedure involves the use of microbial inulinase, which after one step enzymatic hydrolysis of inulin, yields 95% pure fructose. Inulinase is produced by many microorganisms, such as Kluyveromyces, Aspergillus, Staphylococcus, Xanthomonas, and Pseudomonas. Yeasts such as Kluyveromyces fragilis, Kluyveromyces marxianus, Candida kefyr, Debaryomyces cantarelli and fungi, Penicillium and Aspergillus species are common inulinase producers [13]. Among the yeasts which can produce inulinases, strains of Candida sp., Sporotrichum sp., Pichia sp., and Kluyveromyces sp., two species of K. fragilis and K. marxianus, have high potential for producing commercially acceptable yields of the enzyme [13]. However, there has been no report about inulinase and inulinase production from marine yeasts [3].
After we screened over 400 marine yeast strains from different marine environments, we found that some marine yeast strains could secrete a large amount of inulinase into medium prepared with seawater or artificial seawater. The main purpose of the present study was to analyze diversity of inulinase-producing marine yeasts. We also identified the hydrolysis products of inulin by the crude inulinases produced by the inulinase-producing marine yeasts. To our knowledge, this is the first report that the yeasts derived from the marine environment can produce extracellular inulinase.
Methods
Sampling
Different samples of seawater and sediments in China South Sea (100 m depth, 20°C, pH 8.1 and 2.89% salinity, summer of 2004), Indian Ocean (50 m depth, 25°C, pH 8.1 and 2.89% salinity, summer of 2005), and the Pacific Ocean (200 m depth, 20°C, pH 8.1 and 2.89% salinity, winter of 2004) were collected during the Antarctic exploration in 2004 and hypersaline sea water (1 m depth, 26°C, pH 8.1 and 15% salinity, Spring of 2004), sediments of the salterns (1 m depth, 26°C, pH 8.1 and 15% salinity, Autumn of 2004), different species of marine animals and algae along the coast of Qingdao, China (10 m depth, 15°C, pH 8.1 and 2.89% salinity, Autumn of 2005) were also collected.
Isolation of Marine Yeasts
Two milliliters of the seawater or 2.0 g of the sediments or 2.0 ml of homogenized guts of marine animals or homogenized marine algae were suspended in 20.0 ml of YPD medium containing 2.0% glucose, 2.0% polypeptone, and 1.0% yeast extract prepared with seawater and supplemented with 0.05% chloramphenicol immediately after sampling and cultivated at natural temperature on the ship for 5 days. After suitable dilution of the cell cultures, the dilute was plated on YPD plates with 0.05% chloramphenicol and the plates were incubated at 20–25°C for 5 days. Different colonies from the plates were transferred to the YPD slants, respectively.
Inulinase Production
One loop of the cells of each yeast strain from the slants was transferred to 50 ml of YPD medium in 250 ml flask and aerobically cultivated for 24 h. The cell culture (5 ml, OD600 nm = 20.0) was transferred to 45 ml of the production medium which contained inulin 4.0%, K2HP04 0.3%, yeast extract 0.5%, pH 5.0 prepared with seawater and grown by shaking at 170 rpm and 28.0°C for 3 days.
Determination of Inulinase Activity
The fermented broth was centrifuged at 2823×g and 4°C for 5 min and the supernatant obtained was taken as a crude enzyme. The reaction mixture containing 0.1 ml of the crude enzyme and 0.9 ml of acetate buffer (0.1 M, pH 5.0 or pH 6.0) with 2.0% inulin was incubated at 50° or 60°C for 10 min. The reaction was inactivated immediately by keeping the reaction mixture at 100°C for 10 min. The same mixture to which the same amount of the inactivated crude enzyme (heated at 100°C for 10 min) was added before the reaction was used as the control. The amount of reducing sugar in the reaction mixture was assayed by the method of Nelson–Somogyi [18]. One inulinase unit (U) was defined as the amount of enzyme that produces one micromole of reducing sugar per minute under the assay conditions used in this study.
DNA Extraction and PCR
The total genomic DNA of the yeast strains was isolated and purified by using the methods as described [16]. Amplification and sequencing of 18S rDNA and ITS from the yeasts were performed according to the methods described [4].
Phylogenetic Analysis and Identification of the Yeasts
The sequences obtained above were aligned by using BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST). For comparison with currently available sequences, several sequences were retrieved with over 98% similarity belonging to different genera from NCBI (http://www.ncbi.nlm.nih.gov) and multiple alignment was performed by using Bioedit 7.0. The routine identification of the yeasts was performed by using the methods as described [9]. The fermentation tests and assimilation tests were carried out at 25°C and with the media at natural pH and with 2.81% salinity.
Effects of pH and Temperature on Inulinase Activity
The effects of pH on the enzyme activity were determined by incubating the culture supernatant at different pH between 4.0 and 7.0 using the standard assay conditions described above. The buffers used were 0.1 M citric acid–sodium citrate buffer (pH 3.0), 0.1 M acetate buffer (pH 4.0–5.0), and 0.1 M phosphate buffer (pH 6.0–8.0). The optimal temperature for activity of the enzyme was determined at 40°, 45°, 50°, 55°, 60°, and 65°C in the same buffer as described above.
Inulin Hydrolysis
Inulin hydrolysis was carried out by incubating the reaction mixture containing 900 μl of 2.0% inulin in 0.1 M acetate buffer (pH 5.0 or pH 6.0) and 900 μl of 120 U ml−1 of the concentrated crude enzyme at 50° or 60°C for 16 h. One microliter of the end products of inulin hydrolysis after the incubation at 50° or 60°C were withdrawn and identified to ascertain the extent of hydrolysis by ascending thin layer chromatography (Silica gel 60, MERCK, Germany) with the solvent system of n-butanol–pyridine–water (6:4:3) and a detection reagent comprising 2.0% diphenylamine in acetone–2.0% aniline in acetone–85% phosphoric acid (5:5:1 by volume).
Results
Screening of the Inulinase-producing Yeasts
Many terrestrial yeasts have been confirmed to have capacity to produce a large amount of inulinase [6, 13]. Therefore, we want to know if such yeasts exist in marine environments. After over 400 yeast strains from seawater, sediments, guts of the marine fish, and marine algae were screened, we found that four strains (OUC1, OUC2, G7a, and G7a1) of the marine yeasts grown in the medium with inulin could secrete a large amount of inulinase into the medium (data not shown). As shown in Table 1, the four marine yeast strains were isolated from marine algae at Changdao island in China and sediment of China South Sea, respectively. The colony of the yeast strain G7a was pink and butyrous, and its vegetative cells were reproduced by budding (Figs. 1 and 2). There were not elaborate pseudohyphae and ascospores (data not shown). The colony of the yeast strain OUC2 was white to cream and butyrous to membranous and its vegetative cells reproduced by budding (Figs. 1 and 2). There were elaborate pseudohyphae and/or septate hyphae and persistent asci containing up to four rough oval, round and hat saturn- or walnut-shaped ascospores (data not shown). The colony of the yeast strain OUC1 was white to cream and butyrous. The vegetative cells of this strain were reproduced by budding (Figs. 1 and 2). There was a pseudohyphae. The ascospores formed were hat-shaped (data not shown). The colony of the yeast strain G7a1 was white to cream and butyrous and its vegetative cells reproduced by budding (Figs. 1 and 2). There were filaments, none, or simple pseudohyphae. The persistent asci containing 1 or 2 rough, round ascospores was formed (data not shown).
Physiological and Biochemical Characterization
Tables 2 and 3 show fermentation spectra and carbon source assimilation spectra of the marine yeasts obtained in this study. Based on the fermentation spectra and carbon source assimilation spectra of the marine yeasts and those of the type strains listed in “The yeast: a taxonomic study” [9] and the type strain identified [19], we found that strains OUC2, OUC1, G7a, and G7a1 looked similar to Yarrowia lipolytica, Pichia guilliermondii, Cryptococcus aureus, Debaryomyces hansenii, respectively.
Phylogenetic Analysis of Partial Sequences of the 18S rRNA Genes and ITS
According to [9], traditional and routine identification methods which depend on phenotype, are usually leading to uncertain and inaccurate interpretations of species interaction. Sequence analysis of phylogeny for microbial taxonomy is a more accurate method for determining inter- and intraspecific relationships. Therefore, 18S rDNA partial sequences and ITS of the yeast strains were determined and aligned by using BLAST analysis (http://www.ncbi.nlm.nih.gov/BLAST). Phylogenetic trees were constructed by using PHILIP software package version 3.75c [5]. Distance matrices were generated by the DNADIST program based on Kimura’s two-parameter model [8]. Neighbor-joining analysis of the data sets was carried out with the program Neighbor of the PHYLIP package. Cafeteria roenbergensis (heterotrophic flagellates) and Gianda intestinalis were used as out-groups during the construction of consensus trees of the isolates based on 18S rRNA gene sequences and ITS, respectively. The search for the similarity between 18S rDNA sequences and ITS of the isolates and those in the NCBI database shows that many phylogenetically related yeast species were similar to the marine yeast strains obtained in this study. Phylogenetic relationships of 18S rDNA sequences and ITS of the marine yeast strains were shown in Figs. 3 and 4 and their Genbank accession numbers are shown in Table 4. The topology of the phylogram in Fig. 3 confirms that a 98% bootstrap value, a 100% bootstrap value, a 97% bootstrap value, and a 84% bootstrap value were detected between 18S rRNA gene sequence of strain G7a and that of C. aureus, between 18S rRNA gene sequence of strain OUC1 and that of Y. lipolytica, between 18S rRNA gene sequence of strain G7a1 and that of D. hansenii, between 18S rRNA gene sequence of strain OUC1, and that of P. guilliermondii, respectively, while ITS sequence of strain G7a had 100% match with that of C. aureus, ITS sequence of strain OUC2 had 93% match with that of Y. lipolytica, ITS sequence of strain G7a1 had 83% match with that of D. hansenii, and ITS sequence of strain OUC1 had 51% match with that of P. guilliermondii. Therefore, strain OUC2 was closely related to Y. lipolytica, whereas strain OUC1 could be in close relationship to P. guilliermondii. The strain G7a was assigned to C. aureus. 18S rDNA sequences and ITS of yeast strain G7a1 was identical to those of D. hansenii, respectively.
Effects of Different Temperature and pH on Activity of the Crude Inulinases
The results in Fig. 5 show that the optimal pH for the crude inulinase produced by the marine yeast strain OUC1 were 6.0 while the optimal pHs for the crude inulinase produced by the marine yeast strain OUC2, G7a, and G7a1 were 5.0. It can be observed from the results in Fig. 6 that the optimal temperatures of the crude inulinases produced by the marine yeast strains OUC2 and OUC1 were 60°C, while the optimal temperatures of the crude inulinases produced by the marine yeast strains G7a and G7a1 were 50°C.
The optimal pHs of the crude inulinases produced by the four marine yeasts. Data are given as means ± SD, n = 3. Strain OUC2 (filled diamond); strain OUC1 (filled square); strain G7a (filled triangle); strain G7a1 (x mark). The buffers with 1.8% (w/v) inulin; temperature: 50°C; incubation time: 10 min.
The optimal temperatures of the crude inulinases produced by the four marine yeasts. Data are given as means ± SD, n = 3. Strain OUC2 (filled diamond); strain OUC1 (filled square); strain G7a (filled triangle); strain G7a1 (x mark). Acetate buffer or phosphate buffer (0.1 M, pH 5.0 or 6.0) with 1.8% (w/v) inulin; incubation time: 10 min.
Inulin Hydrolysis
The products of hydrolysis of inulin were studied using a combination of the concentrated crude inulinases and inulin. A total of 900 μl of the concentrated supernatant was added to 900 μl of 2.0% inulin in acetate buffer (0.1 M, pH 5.0 or pH 6.0). The mixtures were water-bathed at 50° or 60°C for 16 h. The hydrolysis products were analyzed by thin-layer chromatography (TLC). The results in Fig. 7 show that only oligosaccharides, but no monosaccharides and disaccharides, were detected after inulin hydrolysis by the crude inulinase produced by Y. lipolytica OUC2, suggesting that the crude inulinase of the strain had no exoinulinase activity. However, it can be seen from the data in Figs. 7 and 8 that a large amount of both monosaccharides and oligosaccharides were detected after inulin hydrolysis by the crude inulinase produced both by D. hansenii G7a1 and C. aureus G7a. Meanwhile, Fig. 9 shows that a large amount of monosaccharides and a trace amount of oligosaccharides were detected after the hydrolysis by the crude inulinase produced by P. guilliermondii OUC1, indicating that the crude inulinase of these strains had a high exoinulinase activity.
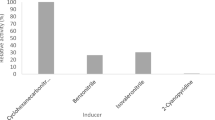
Thin layer chromatography of hydrolysis products of inulin of OUC2 and G7a1. Lane 1 Control (inulin + inactivated inulinase produced by yeast strain OUC2); 2 the hydrolysis products of inulin at the catalysis of inulinase produced by yeast strain OUC2; 3 fructose; 4 sucrose; 5 kestose(trisaccharides); 6 tetrasaccharide;7 control (inulin + inactivated inulinase produced by yeast strain G7a1); 8 the hydrolysis products of at the catalysis of inulinase produced by yeast strain G7a1. The inactivated inulinase was obtained by heating at 100°C for 10 min. The end products of inulin hydrolysis were analyzed by using TLC plate (Silica gel 60, MERCK, Germany) with the solvent system n-butanol–pyridine–water (6:4:3) and a detection reagent comprising 20 g/l diphenylamine in acetone–20 g/l aniline in acetone–850 g/l phosphoric acid (5:5:1 by volume).
Thin layer chromatography of hydrolysis products of inulin with concentrated inulinase produced by yeast strainG7a. Lane 1 control (inulin + inactivated inulinase); 2 hydrolysis products; 3 fructose; 4 sucrose; 5 kestose (trisaccharides); 6 tetrasaccharides. The inactivated inulinase was obtained by heating at 100°C for 10 min. The end products of inulin hydrolysis were analyzed by using TLC plate (Silica gel 60, MERCK, Germany) with the solvent system n-butanol–pyridine–water (6:4:3) and a detection reagent comprising 20 g/l diphenylamine in acetone–20 g/l aniline in acetone–850 g/l phosphoric acid (5:5:1 by volume).
Thin layer chromatography of hydrolysis products of inulin with concentrated inulinase produced by yeast strain OUC1. Lane 1 control (inulin + inactivated inulinase); 2 hydrolysis products; 3 fructose; 4 sucrose; 5 kestose (trisaccharides). The inactivated inulinase was obtained by heating at 100°C for 10 min. The end products of inulin hydrolysis were analyzed by using TLC plate (Silica gel 60, MERCK, Germany) with the solvent system n-butanol–pyridine–water (6:4:3) and a detection reagent comprising 20 g/l diphenylamine in acetone–20 g/l aniline in acetone–850 g/l phosphoric acid (5:5:1 by volume).
Discussion
In recent years, we have found that diversity of marine yeasts is very rich. However, to our knowledge, marine yeast is still an untouched bioresource for extracellular enzyme production [3]. It is very interesting to observe from the results obtained in this study that many species of inulinase-producing marine yeasts occurred in the marine environments (Table 1).
The results of routine identification and molecular methods show that the marine yeast strains OUC1, G7a, OUC2, and G7a1 obtained in this study belong to P. guilliermondii, C. aureus, Y. lipolytica, and D. hansenii, respectively (Figs. 1, 2, 3, and 4; Tables 2 and 3). So far, the terrestrial yeasts such as K. fragilis, K. marxianus, C. kefyr, D. cantarelli, and fungi, Penicillium and Aspergillus species have been found to be the common inulinase producers [13]. Among the terrestrial yeasts which can produce inulinases, strains of Candida sp., Sporotrichum sp., Pichia sp., and Kluyveromyces sp., two species of K. fragilis and K. marxianus, have high potential for producing commercially acceptable yields of the enzyme [13]. Therefore, it is the first time for us to report that P. guilliermondii, C. aureus, Y. lipolytica, and D. hansenii from the marine environments can secret over 40 U/ml of inulinase into the medium. Some strains of terrestrial P. guilliermondii have been widely applied to biocontrol of molds on grains, fruit and vegetable [14]. C. aureus has been described only in recent years [19]. Many strains of terrestrial Y. lipolytica have been found to have wide uses in dairy and fermentation industries [12] and some strains of marine Y. lipolytica were found to have high ability to degrade oil pollutants in marine environments [11]. D. hansenii is a common member in the salty environments [1]. So, we think that the four marine yeasts obtained in this study could be safely applied in food and fermentation industries.
The results in Fig. 5 show that the optimal pH for the crude inulinase produced by the marine yeast strain OUC1 was 6.0 and the optimal pHs for the crude inulinase produced by the marine yeast strain OUC2, G7a, and G7a1 were 5.0 while the results in Fig. 6 demonstrate that the optimal temperatures of the crude inulinases produced by the marine yeast strains OUC2 and OUC1 were 60°C and the optimal temperatures of the crude inulinases produced by the marine yeast strains G7a and G7a1 were 50°C. The optimum pH for inulinase secreted by K. marxianus was 4.4 and optimal temperatures for inulinase from K. fragilis and K. marxianus were 55°C [10, 13]. The maximum inulinase activities for K. marxianus var. bulgaricus were observed at 50° and 60°C [2]. This means that the optimal pH and temperature for the crude inulinases produced by the marine yeast strains were in agreement with those for inulinases produced by terrestrial yeasts.
The results in Table 5 indicate that inulinase activities by the four marine yeasts reached over 40.0 U ml−1. Using inulin as substrate, the highest production of inulinase was 2.8 U ml−1 using Candida pseudotropicalis. When the yeasts grew in Jerusalem artichoke tubercles extract, around 90% of the enzymatic activity was accumulated in the medium and much higher levels of inulinase activity were obtained: 14.6 U ml−1 for C. kefyr, 18.7 U ml−1 for C. pseudotropicalis, 18.4 U ml−1 for K. marxianus var. bulgaricus, and 14.3 U ml−1 for K. fragilis. In dry Jerusalem artichoke extract, higher inulinase production was obtained after 7-day fermentation, using K. marxianus var. bulgaricus [2]. Inulinase yield of 40.5 U ml−1 of K. marxianus var. bulgaricus in an optimized medium containing inulin (3.5%), beef extract (0.5%), MnSO4 (0.5 mM), CoCl2 (0.05 mM), SDS (0.4 mM), and pH 6.5 at 30°C under agitation (150 rpm) for 60 h has been obtained at shake flask level [17]. Inulinase gene inuA1 from Aspergillus niger AF10 was overexpressed in Pichia pastoris and inulinase activity reached 50.6 U ml−1 in the fermentation liquid after 72 h [20]. The INU1 gene encoding an exoinlinase from K. marxianus KW02 was expressed in P. pastoris. The enzyme activity of recombinant P. pastoris in the fermentation liquid was 52.0 U ml−1 [21]. This demonstrates that inulinase activity produced by the marine yeasts obtained in this study reached very high level and the four marine yeast strains may also have highly potential applications in inulin hydrolysis.
The results in Fig. 9 indicate that the crude inulinase produced by P. guilliermondii OUC1 had a high exoinulinase activity. Such inulinase could be used to hydrolyze inulin to produce ultra-high fructose syrups and high concentration ethanol as a large amount of monosaccharides, such as fructose will be released from inulin at the catalysis of the enzyme [6, 13, 15]. The data in Fig. 7 reveal that the crude inulinase produced by Y. lipolytica OUC2 had no exoinulinase activity. Such inulinase could be used to hydrolyze inulin to yield inulooligosaccharides which have wide applications in various types of foods like confectionery, fruit preparations, milk desserts, yogurt and fresh cheese, baked goods, chocolate, ice cream, and sauces [7].
To develop some new techniques for production of ultra-high fructose syrups, high concentration ethanol and inulooligosaccharides from inulin by using the unique inulinases produced by the marine yeasts, the further research work on the inulinases and the marine yeasts is being undertaken in this laboratory.
References
Alba-Lois, L, Segal, C, Rodarte, B, Valdes-Lopez, V, DeLuna, A, Cardenas, R (2004) NADP-glutamate dehydrogenase activity is increased under hyperosmotic conditions in the halotolerant yeast Debaryomyces hansenii. Curr Microbiol 48: 68–72
Cazetta, ML, Martins, PMM, Monti, R, Contiero, J (2005) Yacon (Polymnia sanchifolia) extract as a substrate to produce inulinase by Kluyveromyces marxianus var. bulgaricus. J Food Eng 66: 301–305
Chi, Z, Liu, Z, Gao, L, Gong, F, Ma, C, Wang, X, Li, H (2006) Marine yeasts and their applications in mariculture. J Ocean Univ China 5: 251–256
Chi, Z, Ma, C, Wang, P, Li, H (2007) Optimization of medium and cultivation conditions for alkaline protease production by the marine yeast Aureobasidium pullulans. Bioresour Technol 98: 534–538
Felsenstein, J (1995) PHYLIP (Phylogenetic Inference Package), Version 3.75. Distributed by author, Department of Genetics, University of Washington, Seattle, WA
Gill, PK, Manhas, RK, Singh, P (2006) Hydrolysis of inulin by immobilized thermostable extracellular exoinulinase from Aspergillus fumigatus. J Food Eng 76: 369–375
Kaur, N, Gupta, AK (2002) Applications of inulin and oligofructose in health and nutrition. J Biosci 27: 703–714
Kimura, M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies on nucleotide sequences. J Mol Evol 2: 87–90
Kurtzman, CP, Fell, JW (2000) In: The yeasts: a taxonomic study, 4th revised and enlarged edition, Elsevier, Amsterdam
Kushi, RT, Monti, R, Contiero, J (2000) Production, purification and characterization of an extracellular inulinase came from Kluyveromyces marxianus var. bulgaricus. J Ind Microbiol Biotech 25: 63–69
Jain, MR, Zinjarde, SS, Deobagkar, DD, Deobagkar, DN (2004) 2,4,6-Trinitrotoluene transformation by a tropical marine yeast, Yarrowia lipolytica NCIM 3589. Mar Pollut Bull 49: 783–788
Madzak, C, Gaillardin, C, Beckerich, JM (2004) Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica: a review. J Biotechnol 109: 63–81
Pandey, A, Soccol, CR, Selvakumar, P, Soccol, VT, Krieger, N, Jose, D, Fontana, JD (1999) Recent developments in microbial inulinases, its production, properties and industrial applications. Appl Biochem Biotechnol 81: 35–52
Reyes, MEQ, Rohrbach, KG, Paull, RE (2004) Microbial antagonists control postharvest black rot of pineapple fruit. Postharvest Biol Technol 33: 193–203
Rocha, JR, Catana, R, Ferreira, BS, Cabral, JMS, Fernandes, P (2006) Design and characterization of an enzyme system for inulin hydrolysis. Food Chem 95: 77–82
Sambrook, J, Fritsch, EF, Maniatis T (1989) In: Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Beijing, (Chinese translating ed.), pp 367–370
Singh, RS, Dhaliwal, R, Puri, M (2006) Production of inulinase from Kluyveromyces marxianus YS-1 using root extract of Asparagus racemosus. Proc Biochem 41: 1703–1707
Spiro, RG (1966) Analysis of sugars found in glycoproteins. Methods Enzymol 8: 3–26
Takashima, M, Sugita, T, Shinoda, T, Nakase, T (2003) Three new combinations from the Cryptococcus laurentii complex: Cryptococcus aureus, Cryptococcus carnescens and Cryptococcus peneaus. Int J Syst Evol Microbiol 53: 1187–1194
Zhang, L, Wang, J, Ohta, Y, Wang, Y (2003) Expression of the inulinase gene from Aspergillus niger in Pichia pastoris. Proc Biochem 38: 1209–1212
Zhang, L, Zhao, C, Wang, J, Ohta, Y, Wang, Y (2005) Inhibition of glucose on an exoinulinase from Kluyveromyces marxianus expressed in Pichia pastoris. Proc Biochem 40: 1541–1545
Acknowledgments
This research was supported by grants 30670058 from National Natural Science Foundation of China and LvKaRenCaiGongCheng program from Ocean University of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, L., Chi, Z., Sheng, J. et al. Inulinase-producing Marine Yeasts: Evaluation of their Diversity and Inulin Hydrolysis by Their Crude Enzymes. Microb Ecol 54, 722–729 (2007). https://doi.org/10.1007/s00248-007-9231-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9231-4