Abstract
Flagellate feeding efficiency appears to depend on morphological characteristics of prey such as cell size and motility, as well as on other characteristics such as digestibility and cell surface characteristics. Bacteria of varying morphological characteristics (cell size) and mineral nutrient characteristics or food quality (as determined by the C:N:P ratio) were obtained by growing Pseudomonas fluorescens in chemostats at four dilution rates (0.03, 0.06, 0.10, and 0.13 h−1) and three temperatures (14°C, 20°C, and 28°C). Cells of a given food quality were heat-killed and used to grow the flagellate Ochromonas danica. Ingestion and digestion rates were determined by using fluorescently labeled bacteria of the same food quality as the bacteria supporting growth. Ingestion rates were affected by both food quality and cell size. Cells of high food quality (low carbon:element ratio) were ingested at higher rates than cells of low food quality. Multiple regression analysis indicated that cell size also influenced ingestion rate but to a much lesser extent than did food quality. Digestion rates were not correlated with either food quality or cell size. Results suggest that flagellates may adjust feeding efficiency based on the quality of food items available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considerable literature exists that characterize the role of flagellate nanozooplankton in planktonic systems. Predation by flagellates is a major mortality factor for aquatic bacteria [23, 48, 55] and it is clear that, in some systems, flagellates are capable of cropping as much as 100% of bacterial production [10, 45]. Given the abundance of bacteria in both marine and freshwaters, their potential for rapid growth, their ability to use dilute organic and inorganic nutrients, and their high assimilation efficiencies, flagellates must play a crucial role in transferring nutrients sequestered in bacterial biomass to consumers in higher trophic levels [2, 50].
Flagellate grazing can also influence the characteristics of bacterial communities and lead to changes in their structural and taxonomic composition [29, 32, 67]. Some of the characteristics of bacterial communities attributed to flagellate grazing include small average cell size [1, 15, 23, 49], high frequency of motile cells [23], and cell types of complex inedible morphologies [47, 59].
Flagellate feeding efficiency and/or prey selection appears to depend on prey morphology characteristics such as cell size and motility [23, 34, 42], as well as other features such as digestibility and cell surface characteristics [22, 30, 38, 43]. Nisbet [44] speculated that feeding selectivity may, in part, be receptor-mediated and regulated by “signal substances” on prey cell surfaces. Thus, nutritional and biochemical food quality may influence prey selection and ingestion [8].
Food quality has been shown to greatly influence the rate at which metazoan zooplankton exploit prey [12, 18, 62]. However, analogous studies on predator–prey food quality interactions in nanozooplankton communities are lacking. In this work, the mixotroph nanoflagellate, Ochromonas danica was fed bacteria (Pseudomonas fluorescens) of known, but varying food quality, where food quality was measured as the C:N:P ratio, an index of macronutrient composition that has proven useful in studies on metazoan zooplankton. Ingestion and digestion rates were determined for the protozoan during short-term feeding experiments. The relationships among food quality, ingestion, and digestion are discussed for this predator–prey couple.
Materials and Methods
Organisms and Analytical
P. fluorescens (ATCC 3214) stock cultures were maintained on Standard Mineral Base (SMB) [65] supplemented with 10 mM glucose (SMBg). Bacteria-free O. danica (UTEX 1298) was maintained in Ochromonas Medium [61]. Attempts to grow this strain as an exclusive phototroph have not been successful.
P. fluorescens was grown in SMBg in continually stirred and aerated 800 mL chemostats (Applikon) at four dilution rates (d=0.03, 0.06, 0.10, and 0.13 h−1) and three temperatures (14°C, 20°C, and 28°C). Reactors were assumed to be in steady state after three complete turnovers at a given temperature and dilution rate. Outflow was aseptically captured into presterilized 1-L bottles (Nalgene). Cells harvested from chemostats (720 mL) were distributed into conical polypropylene centrifuge tubes (Nalgene) and pelleted (Sorvall RT6000B refrigerated centrifuge, Sorvall Instruments; 5000 rpm, 15°C, 25 min). Pellets were combined into 180 mL phosphate-buffered saline (PBS) resulting in a 4× concentration of original sample. The homogenized 4× sample was subsequently divided into two portions: 150 mL to prepare heat-killed (HK) bacteria [53] and 30 mL to prepare fluorescently labeled bacteria (FLB) [57], according to the modifications of Chrzanowski and Šimek [15].
Bacterial abundance was determined by direct epifluorescent microscopic enumeration (1250×) of formaldehyde-preserved (2% final concentration) samples using DAPI as the fluorochrome [51]. Cell volume (V) was determined from length and width of at least 100 cells according to the formula: \(V = {\left[ {\pi \left( {{\left( {0.5W^{2} } \right)}{\left( {L - W} \right)}} \right.} \right]} + {\left[ {\left. {{\left( {4 \mathord{\left/ {\vphantom {4 3}} \right. \kern-\nulldelimiterspace} 3} \right)}\pi } \right){\left( {0.5W^{3} } \right)}} \right]}\), where W is the maximum cell width and L is the maximum cell length (μm). Length and width of individual cells was determined from digital images (Olympus DP70) and Simple PCI imaging software (Compix, Inc., Brandywine, PA, USA). Bacteria contained in 2 mL were collected on precombusted glass-fiber filters (Whatman GF/F) for element analysis. Two sets of triplicate filters were prepared for each culture condition; one set for carbon (C) and nitrogen (N) analysis and one set for particulate phosphorus (P) analysis. The C and N content of cells was determined using a CHN analyzer (PerkinElmer series 2200 CHN Analyzer). The P content of cells was determined from persulfate digests and subsequent analysis of soluble reactive P [63]. Ratios of elements are reported as mol:mol.
Grazing Experiments
Culture conditions have been found to influence flagellate grazing [8, 52, 66]. Bacteria used in grazing experiments were of varying element composition or food quality (Table 1). Consequently, O. danica was preconditioned to grow (50 mL cultures) on HK P. fluorescens of a given food quality (range 1.2×108 to 4.6×108 bacteria mL−1) immediately prior to assessing ingestion and digestion rates of that food item. Three feeding trials were conducted for 10 of the 12 food qualities at prey densities (range 1.0×108 to 4.2×108 bacteria mL−1) sufficient to ensure that O. danica ingestion and growth rates were saturated [68].
Ingestion and digestion of a bacteria of a given food quality was assessed during the time O. danica was in exponential growth (2–4 days after the start of preconditioning). FLB were used as tracers to monitor ingestion and digestion rates. FLB (1–1.5 mL) were added to 12 mL of preconditioning culture to yield a final concentration of approximately 10% of the total HK bacteria. Following FLB addition, 1-mL aliquots were removed from each replicate at 5, 20, 40 and 60 min and preserved in ice-cold glutaraldehyde (2% final concentration) to prevent egestion of FLB [58]. After 60 min (post-FLB addition), 2 mL was removed from each replicate and diluted with a mixture of medium and bacteria (10 mL SMB and 8 mL HK bacteria) of the same food quality and concentration used in preconditioning thereby reducing the total concentration of FLB tenfold. Samples were taken at various time intervals up to 100 min after dilution and preserved in ice-cold glutaraldehyde.
As soon as possible after preservation, a 10-μL aliquot of each preserved sample was placed directly on a microscope slide and covered with a cover slip. The slides were placed in a refrigerator and processed within 2 h. FLB contained in food vacuoles in each of 50 O. danica cells were counted.
The rate at which bacteria were ingested by Ochromonas was determined from the slope of a regression line fitting FLB protozoan−1 to time and subsequently normalizing FLB uptake to their proportion of the population of HK bacteria supporting growth. Visual inspection of plots of FLB loss over time suggested an exponential decline similar to that reported by Dolan and Šimek [19] (Fig. 1). Therefore, digestion rate constants (DRC) were determined from the slope of a regression line fitting the natural log of FLB protozoan−1 to time after dilution. The half-life of bacteria contained in food vacuoles (Td1/2) was determined from DRC according to the formula: Td1/2=ln(0.5)(DRC−1).
Results and Discussion
There are few data characterizing the element stoichiometry of nanoflagellates largely due to the difficulties in separating elements associated with prey from elements associated with the flagellates. In protozoa, C and N appear to be maintained in a constant ratio [30], whereas considerably less is known about the C:P ratio. We have used element ratios as an index of food quality, assuming that nanozooplankton will have stoichiometric constraints similar to those of macrozooplankton (but see [24]).
The element stoichiometry of bacteria can vary depending on growth rate, temperature, and nutrient sources [54, 64]. We grew P. fluorescens of varying element composition in chemostats by adjusting the dilution rate and temperature (Table 1) but without altering medium composition. Heat killing cells resulted in a significant loss of C, N, and P and, as a consequence, C and N were enriched relative to P (Table 1). Nevertheless, resulting HK cells were of distinct element composition. If the metric of food quality is considered as the ratio of C:P, then prey food quality spanned almost a twofold range; the best food quality cells had C:P ratios of 59:1 and the worst food quality cells had C:P ratios of 139:1. A similar twofold range in food quality is found when food quality is considered as N:P. N:P ratio of prey cells ranged between 13:1 and 26:1. When compared to both C:P and N:P, C:N ratios varied little and ranged between 4.3 and 5.4.
Average volumes of HK bacteria ranged between 0.0753 and 0.1652 μm3 (Table 2). Although there was considerable scatter in the data, C:P and N:P ratios were weakly correlated to average cell volume (C:P: R 2=0.13, N=30, P=0.051; N:P: R 2=0.13, N=30, P=0.055). The C:N ratio was not correlated to cell volume.
Grazing Experiments
Grazing studies followed a pulse-chase design using FLB as tracers of HK incorporation. Abundance of HK bacteria used as prey in the various experiments ranged between 1.0×108 and 4.2×108 cells mL−1, whereas FLB concentrations ranged between 1.3×107 and 2.5×107 cells mL−1. The final concentration of FLB in grazing studies ranged from 4% to 19%, well within the range reported in previous studies [4, 6, 33, 57].
Two examples of typical feeding experiments are shown in Fig. 1. Table 2 shows ingestion rates and the DRC obtained for each feeding trial. Ingestion rates ranged from approximately 2 bacteria protozoan−1 min−1 when Ochromonas was fed high-quality prey (C:N:P=59:13:1) to approximately 0.5 bacteria protozoan−1 min−1 when fed poor food quality prey (C:N:P=139:26:1). Overall, ingestion rates were within the range of rates reported previously [5, 9, 19, 22, 28, 57].
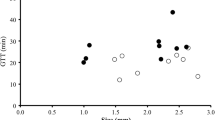
It is difficult to display the relationship between food quality and ingestion rate without separating the overall measure of quality (C:N:P) into components (C:P, C:N, or N:P). The effects of prey food quality on ingestion and digestion were analyzed by simple regressions of ingestion rate or DRC upon a food quality determinant (C:P, C:N, or N:P). In each regression, 30 separate measurements were used (three independent trials from each of 10 separate feeding conditions). There was a significant correlation between ingestion and each metric of food quality: C:P, R 2=0.49, P<0.0001; C:N, R 2=0.44, P<0.0001; N:P, R 2=0.43, P<0.0001 (Fig. 2). It is somewhat arbitrary to consider separate indexes of food quality in this manner when Ochromonas feeds upon bacterial cells represented by a complete C:N:P ratio; however, the data clearly indicate that bacteria of high food quality were ingested at more rapid rates than cells of poor food quality. The experimental design did not permit an identification of a particular element that promoted grazing; ingestion rates were high if P was abundant relative to C (low C:P), if N was abundant relative to C (low C:N), or if P was abundant relative to N (low N:P). Thus, the similarity among the coefficients of determination probably reflects the composite measure of food quality (C:N:P) rather than variability due to an individual element.
Ingestion rate (closed circles) and digestion rate constants (open circles) for Ochromonas danica as a function of the element ratio of prey (Pseudomonas fluorescens). Each point is the rate for a single trial. Error estimates are not shown, but given in Table 2.
Bacterial cell size is a function of growth rate: rapidly growing cells are larger than slowly growing cells. Bacteria used as prey were generated in chemostats where dilution (growth) rate was manipulated to generate cells of differing food quality; consequently, the bacteria also varied in size (Table 2). Prey selection by protozoa has been linked to size distribution of the prey items [1, 15, 26, 49]. The combination of variation in cell size and variation in the element composition of the bacteria could potentially confound interpretations of grazing experiments. To examine the relative effect of size and food quality on ingestion rate, stepwise multiple regression analysis was used to assess the importance of each factor. In the multiple regression models (Table 3), both cell size and food quality were important determinants of ingestion rate, but food quality accounted for a much greater proportion, approximately 45%, of the overall variation in ingestion rate. Variation in size of prey items accounted for 9–23% of the overall variation in ingestion rate.
The DRC (see Table 2) varied little and averaged 0.00916±0.0002 min−1. In contrast to the strong correlations observed between ingestion rates and element ratios, no correlation was found between the DRC and the element ratio of the prey. The half-life of bacteria in a food vacuole averaged 77 min and was similar to the half-life of vacuole contents reported for Bodo sp. [19]. Digestion rates appear to be independent of food concentration [8, 9, 19, 23], and are also more likely a factor of the physiology of the protozoan than of the prey. Digestion rate is likely affected by accessibility of digestive enzymes [8], accumulation efficiencies [69], metabolic rate [7], or growth state [23, 56].
Many aspects of the impact of flagellate predators on bacterial communities have been examined previously. Much of this research focuses on the outcomes of the predator–prey interaction, for example, the size of cells remaining in the community that are more or less resistant to grazing, the development of a prey community dominated by grazer resistant morphologies [38], or the taxonomic classes of cells more or less subject to grazing [29]. Often alluded to, but less frequently investigated, are those features of the prey items that make them susceptible to predation [39]. Those features that have been considered—capsules, surface hydrophobicity and charge—have been, to a large extent, found to be unimportant regulators of predation [23, 36, 38, 39].
Clearly, some flagellates can discriminate between high-quality and poor-quality prey items. John and Davidson [30] found that Paraphysomonas vestita ingested low C:N algal cells (N-replete) at higher rates than high C:N cells (N-deplete). When ample food is available, flagellates will discriminate between low-quality (as microbeads) and high-quality food (as bacteria), preferring to feed upon the bacteria [31]. In situations where microbeads were found to be ingested at rates similar to those at which bacteria were ingested, the beads were also rapidly egested [8]. All bacteria are not of equivalent quality as prey. Bacteria grown under P limitation were captured by flagellates with high frequency, yet most were immediately egested [39]. These studies, as well as the work reported herein, suggest that other features of bacterial cells, apart from the well-studied aspect of cell size, may supply important cues to predators.
Several lines of evidence are beginning to converge, reinforcing the notion that flagellates may be selecting the rapidly growing members of the bacterial community [3, 17, 29, 46, 56, 60]. Part of the selection process, as revealed in this work, may be associated with the element composition of prey items. Bacterial cell size and macromolecular composition are functions of growth rate: rapidly growing cells are larger than slowly growing cells; and RNA, protein, and to a lesser extent DNA, all increase as growth rate increases [11, 27]. Basic microbial physiology and evolving theory in ecological stoichiometry predicts that rapidly growing cells are characterized by low C:P and N:P ratios [16]. The low ratios are brought about by an increase in P-rich macromolecules, largely RNA, required to meet the demands of protein synthesis. Therefore, selection based on size may simply be a consequence of a different selection cue, the element composition of cells (see discussion in [67]). Multiple regression analysis (this article) lends support to this position; however, it is important to realize that in the experimental design of this work, Ochromonas was not given a choice among prey types. Clearly, high-quality prey items were ingested at higher efficiencies than low-quality prey items, but it remains to be determined if Ochromonas would select high-quality items when presented a mixture of high- and low-quality prey. In addition, a potential source of error in this experimental design is the use of HK bacteria. Flagellates have been shown to discriminate between live and HK bacteria of the same strain [35]. Thus it also remains to be determined if prey selection would be similar if live bacteria of varying quality were used as prey.
Features of the bacterial cells acting as cues to feeding or ingestion also remain unknown. Clearly, cell size is important in the overall process and although element composition of cells has suggested some potential links to metabolic processes, it seems unlikely that a given mineral element itself accounts for ingestion efficiency (see above). Moreover, as heat-killed cells were used in this work, capsules and surface characteristics of cells were likely removed or altered compared to that of live cells. Soluble cytoplasmic signals could also be disregarded because heating and washing of cells probably greatly diminished their potential influence. However, recent findings relating to digestion processes seem to suggest that some aspect of the protein content of prey may be important in regulating flagellate predation. Microbeads are generally a poor food surrogate in grazing studies; however, microbeads coated with protein (bovine serum albumin) seem to be ingested at higher rates than uncoated microbeads [37]. Bacterial cells with low C:N content, which may be indicative of high protein content, also appear to be preferentially ingested ([30], this study). Zubkov et al. [69] have shown that bacterial proteins appear to be digested by flagellates more easily than other bacterial macromolecules and this finding, when coupled with data indicating high N regeneration by flagellates [70], suggests a flagellate metabolism largely based on protein degradation.
Food quality has long been associated with predator prey selection through optimal foraging theory [40]. In terrestrial and aquatic food webs, predators have been found to exploit resources (prey) having the greatest food quality whether the metric of food quality is mineral content ([41], but see [14]), ratios of essential elements [20, 25, 62], or growth rate [12, 13, 18, 21]. Only recently have similar concepts been reported for flagellate nanozooplankton of microbial food webs ([30], this study). Although we cannot assert that differential ingestion rates based on food quality is a concept that fully extends to all flagellate nanozooplankton, it certainly appears to be a feature that warrants further study.
References
Andersson, A, Larsson, U, Haström, A (1986) Size-selective grazing by a microflagellate on pelagic bacteria. Mar Ecol Prog Ser 33: 51
Azam, F, Fenchel, T, Field, JG, Gray, JS, Meyer-Reil, LA, Thingstad, F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10: 257–263
Beardsley, C, Pernthaler, J, Wosniok, W, Amann, R (2003) Are readily culturable bacteria in coastal North Sea water suppressed by selective grazing mortality? Appl Environ Microbiol 69: 2624–2630
Bloem, J, Ellenbroek, F, Bar-Gilissen, M, Cappenberg, TE (1989) Protozoan grazing and bacterial production in stratified Lake Vechten estimated with fluorescently labeled bacteria and by thymidine incorporation. Appl Environ Microbiol 55: 1787–1795
Boenigk, J, Arndt, H (2000) Particle handling during interception feeding by four species of heterotrophic nanoflagellates. J Eukaryot Microbiol 47: 350–358
Boenigk, J, Arndt, H, Cleven, EJ (2001) The problematic nature of fluorescently labeled bacteria (FLB) in Spumella feeding experiments—an explanation by using video microscopy. Arch Hydrobiol 152: 329–338
Boenigk, J, Matz, C, Jürgens, K, Arndt, H (2001) Confusing selective feeding with differential digestion in bacterivorous nanoflagellates. J Eukaryot Microbiol 48: 425–432
Boenigk, J, Matz, C, Jürgens, K, Arndt, H (2001) The influence of pre-culture conditions and food quality on the ingestion and digestion process of three species of heterotrophic nanoflagellates. Microb Ecol 42: 168–176
Boenigk, J, Matz, C, Jürgens, K, Arndt, H (2002) Food concentration-dependent regulation of food selectivity of interception—feeding bacterivorous nanoflagellates. Aquat Microb Ecol 27: 195–202
Borsheim, KY (1984) Clearance rates of bacteria-sized particles by freshwater ciliates measured with monodispersed fluorescent latex beads. Oecologia 63: 286–288
Bremer, H, Dennis, PP (1987) Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt, FC (Ed.) Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Am Soc Microbiol, Washington, DC, pp 1527–1542
Butler, NM, Suttle, CA, Neill, WE (1989) Discrimination by freshwater zooplankton between single algal cells differing in nutritional status. Oecologia 78: 368–372
Cebrián, J, Duarte, CM (1994) The dependence of herbivory on growth rate in natural plant communities. Funct Ecol 8: 518–525
Cebrián, J, Williams, M, McClelland, J, Valiela, I (1998) The dependence of heterotrophic consumption and C accumulation on autotrophic nutrient content in ecosystems. Ecol Lett 1: 165–170
Chrzanowski, TH, Šimek, K (1990) Prey-size selection by freshwater flagellated protozoa. Limnol Oceanogr 35: 1429–1436
Chrzanowski, TH, Kyle, M, Elser, JJ, Sterner, RW (1996) Element ratios and growth dynamics of bacteria in an oligotrophic Canadian shield lake. Aquat Microb Ecol 11: 119–125
Cottrell, MT, Kirchman, DL (2004) Single-cell analysis of bacterial growth cell size and community structure in the Delaware estuary. Aquat Microb Ecol 34: 139–149
Cowles, TJ, Olson, RJ, Chisholm, SW (1988) Food selection by copepods: discrimination on the basis of food quality. Mar Biol 100: 41–49
Dolan, JR, Šimek, K (1998) Ingestion and digestion of an autotrophic picoplankter Synechococcus by a heterotrophic nanoflagellate Bodo saltans. Limnol Oceanogr 43: 1740–1746
Elser, JJ, Sterner, RW, Gorokhova, E, Fagan, WF, Markow, TA, Cotner, JB, Harrison, JF, Hobbie, SE, Odell, GM, Weider, LJ (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3: 540–550
Elser, JJ, Acharya, K, Kyle, M, Cotner, J, Makino, W, Markow, TA, Watts, T, Hobbie, S, Fagan, WF, Schade, J, Hood, J, Sterner, RW (2003) Growth rate—stoichiometry couplings in diverse biota. Ecol Lett 6: 936–943
Gonźalez, JM, Iriberri, J, Egea, L, Barcina, I (1990) Differential rates of digestion of bacteria by freshwater and marine phagotrophic protozoa. Appl Environ Microbiol 56: 1851–1857
Gonźalez, JM, Sherr, E, Sherr, B (1993) Differential feeding by marine flagellates on growing versus starving and on motile versus non-motile bacterial prey. Mar Ecol Prog Ser 102: 257–267
Grover, JP (2003) The impact of variable stoichiometry on predator–prey interactions: a multinutrient approach. Am Nat 162: 29–43
Güsewell, S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164: 243–266
Hahn, MW, Höfle, MG (1999) Flagellate predation on a bacterial model community: interplay of size-selective grazing specific bacterial cell size and bacterial community composition. Appl Environ Microbiol 65: 4863–4872
Herbert, D (1976) Stoichiometric aspects of microbial growth. In: Dean, ACR, Ellwood, DD, Evans, CGT, Melling, J (Eds.) Continuous Culture 6: Applications and New Fields. Ellis Horwood, Chichester, pp 1–30
Holen, DA, Boraas, ME (1991) The feeding behavior of Spumella sp. as a function of particle size: implications for bacterial size in pelagic systems. Oecologia 220: 73–88
Jezbera, J, Horňák, K, Šimek, K (2005) Food selection by bacterivorous protests: insight from the analysis of the food vacuole content by means of fluorescence in situ hybridization. FEMS Microbiol Ecol 52: 351–363
John, EH, Davidson, K (2001) Prey selectivity and the influence of prey carbon:nitrogen ratio on microflagellate grazing. J Exp Mar Biol Ecol 260: 93–111
Jürgens, K, DeMott, WR (1995) Behavioral flexibility in prey selection by bacterivorous nanoflagellates. Limnol Oceanogr 40: 1503–1507
Jürgens, K, Matz, C (2002) Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Anton van Leeuwenhoek 81: 413–422
Keller, MD, Shapiro, L, Haugen, E, Cucci, TL, Sherr, E, Sherr, B (1994) Phagotrophy of fluorescently labeled bacteria by an oceanic phytoplankter. Microb Ecol 28: 39–52
Kiorboe, T, Titelman, J (1998) Feeding prey selection and prey encounter mechanism in the heterotrophic dinoflagellate Noctiluca scintillans. J Plankton Res 20: 1615–1636
Landry, MR, Lehner-Fournier, JM, Sundstrom, JA, Fagerness, VL, Selph, KE (1991) Discrimination between living and heat-killed prey by a marine zooflagellate Paraphysomonas vestita (Stokes). J Exp Mar Biol Ecol 146: 139–151
Matz, C, Jürgens, K (2001) Effects of hydrophobic and electrostatic cell surface properties of bacteria on feeding rates of heterotrophic nanoflagellates. Appl Environ Microbiol 67: 814–820
Matz, C, Boenigk, J, Arndt, H, Jürgens, K (2002) Role of bacterial phenotypic traits in selective feeding of the heterotrophic nanoflagellates Spumella sp. Aquat Microb Ecol 27: 137–148
Matz, C, Deines, P, Jürgens, K (2002) Phenotypic variation in Pseudomonas sp. CM10 determines microcolony formation and survival under protozoan grazing. FEMS Microbiol Ecol 39: 57–65
Matz, C, Jürgens, K (2003) Interaction of nutrient limitation and protozoan grazing determines the phenotypic structure of a bacterial community. Microb Ecol 45: 384–398
MacArthur, RH, Pianka, ER (1966) On optimal use of a patchy environment. Am Nat 100: 603–609
McNaughton, SJ (1990) Mineral nutrition and seasonal movements of African migratory ungulates. Nature 345: 613–615
Monger, BC, Landry, MR (1992) Size-selective grazing by heterotrophic nanoflagellates: an analysis using live-stained bacteria and dual-beam flow cytometry. Arch Hydrobiol Beih 37: 173–185
Monger, BC, Landry, MR, Brown, SL (1999) Feeding selection of heterotrophic marine nanoflagellates based on the surface hydrophobicity of their picoplankton prey. Limnol Oceanogr 44: 1917–1927
Nisbet, B (1987) Nutrition and Feeding Strategies in Protozoa. Croom Helm, London
Nygaard, K, Hessen, DO (1990) Use of 14C-protein-labeled bacteria for estimating clearance rates by heterotrophic and mixotrophic flagellates. Mar Ecol Prog Ser 68: 7–14
Pernthaler, J, Sattler, B, Šimek, K, Schwarzenbacher, A, Psenner, R (1996) Top–down effects on the size-biomass distribution of a freshwater bacterioplankton community. Aquat Microb Ecol 10: 255–263
Pernthaler, J, Posch, T, Šimek, K, Vrba, J, Amann, R, Psenner, R (1997) Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl Environ Microbiol 63: 596–601
Peters, F, Marrase, C, Havskum, H, Rassoulzadegan, F, Dolan, J, Alcaraz, M, Gasol, JM (2002) Turbulence and the microbial food web: effects on bacterial losses to predation and on community structure. J Plankton Res 24: 321–331
Pfandl, K, Posch, T, Boenigk, J (2004) Unexpected effects of prey dimensions and morphologies on the size selective feeding by two bacterivorous flagellates (Ochromonas sp. and Spumella sp.). J Eukaryot Microbiol 51: 626–633
Pomeroy, LR (1974) The ocean’s food web: A changing paradigm. BioScience 24: 499–504
Porter, KG, Feig, YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25: 943–948
Sanders, RW, Caron, D, Davidson, J, Dennett, M, Moran, D (2001) Nutrient acquisition and population growth of a mixotrophic alga in axenic and bacterized cultures. Microb Ecol 42: 513–523
Sanders, RW, Porter, KG, Caron, DA (1990) Relationship between phototrophy and phagotrophy in the mixotrophic chrysophyte Poterioochromonas malhamensis. Microb Ecol 19: 97–109
Schaechter, E, Maaloe, O, Kjeldgaard, N (1958) Dependence on medium and temperature of cell size and chemical composition during balance growth of Salmonella typhimurium. J Gen Microbiol 19: 592–606
Sherr, EB, Sherr, BF (2002) Significance of predation by protists in aquatic microbial food webs. Anton van Leeuwenhoek 81: 293–308
Sherr, BF, Sherr, EB, McDaniel, J (1992) Effect of protistan grazing on the frequency of dividing cells in bacterioplankton assemblages. Appl Environ Microbiol 58: 2381–2385
Sherr, BF, Sherr, EB, Fallon, RD (1987) Use of monodispersed fluorescently labeled bacteria to estimate in situ protozoan Bacterivory. Appl Environ Microbiol 53: 958–965
Sieracki, ME, Haas, LW, Caron, DA, Lessard, EJ (1987) Effect of fixation on particle retention by microflagellates: underestimation of grazing rates. Mar Ecol Prog Ser 38: 251–258
Šimek, K, Vrba, J, Pernthaler, J, Posch, T, Hartman, P, Nedoma, J, Psenner, R (1997) Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl Environ Microbiol 63: 587–5955
Šimek, K, Macek, M, Seda, J, Vyhnalek, V (1990) Possible food chain relationships between bacterioplankton, protozoan, and cladocerans in a reservoir. Int Rev Ges Hydrobiol 75: 583–596
Starr, RC (1978) The culture collection of algae at the University of Texas at Austin. J Phycol 14: 47–100
Sterner, RW, Smith, RF (1993) Daphnia growth on varying quality of Scenedesmus: mineral limitation of zooplankton. Ecol 74: 2351–2360
Strickland, JDH, Parsons, TR (1972) A practical handbook of seawater analysis, 2nd ed. Bull Fish Res Board Can 167: 1–310
Tempest, D, Hunter, J (1965) The influence of temperature and pH value on the macromolecular composition of magnesium-limited and glycerol-limited Aerobacter aerogenes growing in a chemostat. J Gen Microbiol 41: 267–273
White, D, Hegeman, GD (1998) Microbial Physiology and Biochemistry Laboratory: A Quantitative Approach. Oxford Univ. Press, NY
Wilhelm, RO, Heller, M, Bohland, C, Tomaschewski, I, Klein, P, Klauth, W, Tappe, J, Groeneweg, C, Soeder, J, Janse, P, Meyer, W (1998) Biometric analysis of physiologically structured pure bacterial cultures recovering from starvation. Can J Microbiol 44: 399–404
Wu, Q, Boenigk, J, Hahn, M (2004) Successful predation of filamentous bacteria by a nanoflagellate challenges current models of flagellate bacterivory. Appl Environ Microbiol 70: 332–339
Zubkov, MV, Sleigh, MA (1995) Bacterivory by starved marine heterotrophic nanoflagellates of two species which feed differently, estimated by uptake of dual radioactive-labeled bacteria. FEMS Microbiol Ecol 17: 57–66
Zubkov, MV, Zöllner, E, Jürgens, K (2001) Digestion of bacterial macromolecules by a mixotrophic flagellate Ochromonas sp. compared with that by two heterotrophic flagellates Spumella pudica and Bodo saltans. Eur J Protistol 37: 155–166
Zwart, KB, Darbyshire, JF (1992) Growth and nitrogenous excretion of a common soil flagellate Spumella sp.—a laboratory experiment. J Soil Sci 43: 145–157
Acknowledgments
This work was supported by the Texas Advanced Research Program grant 003656-0153-2001 and by National Science Foundation grant DEB-0444844. Special thanks to Marnie Rout, Natalie Hanna and Guimel Molina for assistance with analytical work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shannon, S.P., Chrzanowski, T.H. & Grover, J.P. Prey Food Quality Affects Flagellate Ingestion Rates. Microb Ecol 53, 66–73 (2007). https://doi.org/10.1007/s00248-006-9140-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9140-y