Abstract
Cellulose is the most abundant polymer in nature and constitutes a large pool of carbon for microorganisms, the main agents responsible for soil organic matter decomposition. Cellulolysis occurs as the result of the combined action of fungi and bacteria with different requirements. Earthworms influence decomposition indirectly by affecting microbial population structure and dynamics and also directly because the guts of some species possess cellulolytic activity. Here we assess whether the earthworm Eisenia fetida (Savigny 1826) digests cellulose directly (i.e., with its associated gut microbiota) and also whether the effects of E. fetida on microbial biomass and activity lead to a change in the equilibrium between fungi and bacteria. By enhancing fungal communities, E. fetida would presumably trigger more efficient cellulose decomposition. To evaluate the role of E. fetida in cellulose decomposition, we carried out an experiment in which pig slurry, a microbial-rich substrate, was treated in small-scale vermireactors with and without earthworms. The presence of earthworms in vermireactors significantly increased the rate of cellulose decomposition (0.43 and 0.26% cellulose loss day−1, with and without earthworms, respectively). However, the direct contribution of E. fetida to degradation of cellulose was not significant, although its presence increased microbial biomass (Cmic) and enzyme activity (cellulase and β-glucosidase). Surprisingly, as fungi may be part of the diet of earthworms, the activity of E. fetida triggered fungal growth during vermicomposting. We suggest that this activation is a key step leading to more intense and efficient cellulolysis during vermicomposting of organic wastes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is the largest component of plant residues that enters terrestrial ecosystems [31] and therefore represents a huge source of energy for microorganisms, the main agents responsible for soil organic matter decomposition [22]. In nature, cellulolysis occurs as a result of the combined action of fungi and bacteria with different substrate requirements that shift their biomasses depending on what substrate is being metabolized [17, 32]. The type of microorganisms involved depends on the environmental conditions; under aerobic conditions, they are mainly fungi, actinomycetes, and bacteria, and under anaerobic conditions, they are almost exclusively bacteria [25].
Degradation of cellulose in soils is a slow process that is limited by several factors involving cellulases, such as concentration, location, and mobility of the enzymes [38]. Moreover, production of cellulases is regulated by the speed of accumulation of products [12]. Hemicellulose and lignin content and the degree of crystallinity of cellulose itself also determine the rate at which cellulose is metabolized [25]. Decomposition of lignocellulosic residues is directly mediated by extracellular enzymes [37]; therefore, analysis of the dynamics involved may clarify the mechanisms relating the rate of decomposition with substrate quality and nutrient availability [39].
There is increasing interest in how soil fauna shapes the composition of microbial communities by microbial grazing, disturbance, and dispersal, thereby affecting decomposition and nutrient cycling. Earthworms represent an important portion of soil invertebrate biomass, and, in many ecosystems, earthworms are undoubtedly the key organisms in organic matter decomposition by modifying soil nutrient and microbial dynamics [7]. Reports of cellulolytic activity in the gut of some species of earthworms [20, 21, 42, 49], especially in epigeic earthworms such as Eisenia fetida [48], indicate their ability to digest cellulose, although the effects exerted by earthworms on cellulolysis lie fundamentally in their interactions with microorganisms. These interactions are the subject of a certain amount of controversy, mainly because of the variety of species, substrate, and experimental conditions assayed. It is generally agreed that microorganisms, especially fungi, are part of the diet of earthworms [7]; moreover, earthworms have been shown to graze selectively on fungal species [4, 27]. Although earthworms can digest fungi and bacteria [36], an increase in the number of microorganisms during gut transit has also been reported [9, 19, 36].
Vermicomposting involves the biooxidation and stabilization of organic matter through the joint action of earthworms and microorganisms. The transformations in physicochemical and biochemical properties [6] and the short time in which they occur make them a suitable system for studying microbe–earthworm interactions. The actions of earthworms during vermicomposting include not only digestion and release of easily assimilable substances, such as mucus for microbiota [3], but also the transport and dispersal of microorganisms through casting. Earthworm casts play an important role in decomposition because they have a different nutrient and microbiota composition to the material prior to ingestion [16, 40], which makes possible a better exploitation of resources because of either the appearance of microbial species in fresh substrate or the pool of easily assimilable compounds of cast [7].
In the present study, we question how the earthworm E. fetida affects cellulose decomposition during vermicomposting of pig slurry. We test whether this earthworm species is able to digest cellulose directly (i.e., independently of microorganisms of the substrate but with its associated gut microbiota) and also whether its effects on microbial biomass and activity lead to changes in the relationships between fungi and bacteria.
Materials and Methods
Pig Slurry
Fresh pig slurry was obtained from a pig-breeding farm near the University of Vigo, NW Spain. Pig slurry was homogenized in a slurry pit, then stored in sealed plastic containers and kept at 5°C until use. Some physicochemical characteristics of the pig slurry are summarized in Table 1.
Vermireactor Setup and Functioning
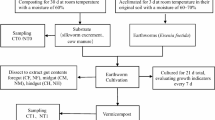
Vermireactors comprised modules that were added sequentially. The modules were made of polyvinyl chloride and resembled sieves. The external diameter of each was 30 cm with a height of 4 cm and the mesh size 5 mm, which allowed mobility of earthworms between modules. Each vermireactor was initially composed of one module containing vermicompost (i.e., a stabilized and nontoxic substrate that serves as bed for earthworms), in which earthworms were placed, and another module containing a layer of fresh pig slurry (3 kg, fresh weight). New modules containing the same amount of fresh pig slurry were added to all the reactors (with and without earthworms) when the earthworms feeding activity required (i.e., changes in the appearance of pig slurry, becoming the coarse fraction, such as seeds and straw, more evident); this procedure allowed us, to date, the addition of each module within the vermireactors.
Experimental Design
We set up a batch of six vermireactors, three without earthworms (control) and three containing an initial population of 500 mature earthworms (E. fetida) each. At the end of the experiment (i.e., after 36 weeks), the vermireactors comprised 12 modules with an increasing gradient of age, resembling a soil profile, from upper to lower layers as follows: 2, 4, 7, 8, 11, 18, 21,25, 27, 29, 33, and 36 weeks.
Sampling Method
At sampling time, the vermireactors were dismantled and the modules isolated to avoid earthworm escape. The earthworms were then manually removed from the substrate; we found earthworms only in layers of 2, 4, 7, 8, 11, and 18 weeks of age. To assess the effect of earthworms on decomposition of pig slurry, we restricted sampling in vermireactors both with and without earthworms (i.e., treatment and control) to the modules corresponding to the above-mentioned times. Five samples of substrate per module were taken randomly and gently mixed for biochemical analyses, i.e., cellulose and hemicellulose content, microbial biomass C (Cmic), ergosterol content, and β-glucosidase and cellulose activities.
Analytical Procedures
Cellulose and Hemicellulose Determination
Cellulose and hemicellulose contents in pig slurry were determined by detergent fiber methods [11]. Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) values were determined using the FibreBag System® (Gerhardt, Königswinter, Germany). This system includes a fine porous mesh bag, into which the dried sample (dw) is weighed (1 g). Sample bags were placed in a carousel, which was then submerged in a 1-L glass beaker containing 360 mL of NDF or ADF solution (it depends on the measurement performed), and the samples were then boiled for 60 min. The carousel containing samples was then rinsed several times with hot water, the bags were dried overnight at 105°C, and finally incinerated at 500°C for 4 h. For determination of ADL, the samples were processed in the same way as above, except that after washing, they were placed in a glass beaker containing 360 mL of 72% H2SO4 for 3 h; the carousel containing samples was then rinsed, dried, and incinerated as described above. The percentage contents of NDF, ADF, and ADL were calculated taking into account the weight of the bags, the samples, and the residues after digestion. Cellulose content was calculated as the difference between ADF and ADL contents and expressed as loss of cellulose with respect to initial cellulose content of pig slurry, 24 ± 5 g 100 g−1 organic matter; hemicellulose content was calculated as the difference between NDF and ADF contents and expressed as g hemicellulose 100 g−1 organic matter.
Microbial Biomass C
Microbial biomass C (Cmic) was determined by the chloroform fumigation–extraction method [43] with field-moist samples (5-g fresh weight). The filtered extracts (0.5 M K2SO4) of both fumigated and unfumigated samples were analyzed for soluble organic C using a Microplate Reader (Bio-Rad Microplate Reader 550, 590 nm). Cmic was estimated as the difference between the organic C extracted from the fumigated and that from the unfumigated sample, multiplied by the K2SO4 extract efficiency factor for microbial C (k c = 2.64) [43].
Ergosterol Content
The ergosterol content of pig slurry was extracted by microwave-assisted extraction (MAE) and determined by high-performance liquid chromatography (HPLC) analysis. All reagents and solvents used for extraction and chromatography were of analytical grade. MAE procedure: Samples (500-mg fresh weight) were placed into 10-mL vials, then 2 mL of methanol and 0.5 mL of 2 M NaOH were added and the vials tightly sealed with Teflon-lined screw caps. Three culture vials were then placed within Teflon PFA® vessels and tightly sealed. These vessels were then placed on the turntable drive stub of a scientific microwave oven (CEM Corporation MDS-2000), processed at 2450 MHz and 630-W maximum output, and irradiated at medium power (60% of maximum output power, manufacturer's setting) for 20 s three times with 1 min of cooling between each time. After cooling for approximately 30 min, the vials were removed from Teflon PSA® vessels. The contents were neutralized with 1 M HCl and then extracted with pentane (3 × ca. 2 mL), all within the 10-mL vials. The combined pentane extracts were evaporated to dryness under a stream of N2 gas, and then redissolved with 1 mL of methanol and filtered through a 0.2-μm syringe filter (MFS) prior to HPLC analysis [47]. HPLC analysis: Ergosterol from pig slurry extracts was separated on a 12.5 × 4 mm Hypersil 5 C18 (366349) reverse-phase column packed with ODS 4 mm and eluted with methanol/water (95:5, v/v) at a flow rate of 2 mL min−1. Ergosterol was detected with a Jasco UV-1570 variable wavelength detector (Jasco) set at 282 nm. Ergosterol content was determined by comparing sample peak areas with those of external standards. Ergosterol was confirmed by comparing retention times with the external standard. Ergosterol standard was purchased from Sigma and redissolved in methanol.
Bacterial/Fungal Ratio
To asses the ratio between the two main fractions of microbiota of pig slurry (bacteria and fungi), values of ergosterol content were converted to fungal C biomass (Cf) using the conversion factor of 5.4 mg ergosterol g−1 Cmic reported by Klamer and Baath [18] for compost samples. We calculated the bacterial C biomass (Cb) as the difference between overall Cmic and Cf, and then calculated the bacterial/fungal ratio (Cb/Cf). Values of this index indicate whether microbial community is predominantly bacterial (Cb/Cf > 1), fungal (0 < Cb/Cf < 1), or if there is an equilibrium between the two main components of microbiota (Cb/Cf = 1).
Enzymatic Activities
β-Glucosidase activity was assessed by determination of the released p-nitrophenol after incubation of samples (1-g fresh weight) with p-nitrophenyl glucoside (0.025 M) for 1 h at 37°C in a Bio-Rad Microplate Reader at 400 nm [8].
Cellulase activity was estimated by determination of released reducing sugars after incubation of samples (5-g fresh weight) with carboxymethylcellulose (CMC) sodium salt (0.7%) for 24 h at 50°C in a Bio-Rad Microplate Reader at 690 nm [35].
Statistical Analysis
Data were analyzed using a split-plot repeated-measures analysis of variance (ANOVAR) where single reactors were subjects, earthworm treatment was fixed as between-subject factor, and week (i.e., each single module) was fixed as within-subject factor. This model assumes correlation between treatment levels within a block, i.e., the modules of each vermireactor [45]. When sphericity assumptions (Mauchly's test) could not be met, we used Huynh–Feldt correction of P whenever values of ɛ were close to 1 [30]. All statistical analyses were performed using SPSS 11.5 software.
Results
On sampling the vermireactors after 36 weeks, we found a mean population of 2800 earthworms per reactor in the presence of earthworms, i.e., more than a fivefold increase in the initial population of 500 mature earthworms. Earthworms were mainly located in the most recent layers, with two different groups being distinguished (Fig. 1). The first group was composed of 2- and 4-week-old layers, with over 1000 earthworms in each layer, and the second group included the 7-, 8-, 11-, and 18-week-old layers, with no more than 200 earthworms per layer. No earthworms were found in the remaining layers (21, 25, 27, 29, 33, and 36 weeks old).
Loss of cellulose content during decomposition of pig slurry significantly increased with age of layers in vermireactors both with and without earthworms (Fig. 2a). In the vermireactors without earthworms, the loss of cellulose ranged between 21 ± 4 and 28 ± 4%, with a maximum loss of 34 ± 2% in the 18-week-old layer. The presence of earthworms in vermireactors highly stimulated the loss of cellulose, especially in 7-, 8-, and 11-week-old layers, in which the cellulose losses were between 40 ± 3 and 47 ± 7%, with a maximum of 54 ± 4% in the 18-week-old layer. Thus, decomposition of cellulose in vermireactors was significantly affected by the age of the layers and was significantly faster in vermireactors with earthworms, as revealed by ANOVAR (Table 2).
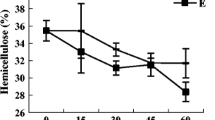
Loss of cellulose (a) and changes in hemicellulose content (b) in layers of vermireactors with (closed squares, n = 3) and without (open squares, n = 3) E. fetida. The vertical distributions of variable values (mean ± SE) are shown on the y-axis, i.e., corresponding to age of pig slurry layers, between 2 and 18 weeks.
The hemicellulose content of pig slurry decreased continuously between 2 and 18 weeks, independently of earthworm presence (Fig. 2b). However, this reduction was slightly more intense in the presence of earthworms, with a minimum of 17 ± 1 g hemicellulose 100 g−1 organic matter registered in the 18-week-old layer, whereas in vermireactors without earthworms, it was 19 ± 1 g hemicellulose 100 g−1 organic matter. Despite this, hemicellulose degradation was not affected by earthworm presence or age of layer, as revealed by ANOVAR (Table 2).
In vermireactors with earthworms, Cmic was significantly higher in almost all layers than in the vermireactors without earthworms except in the 18-week-old layer (Fig. 3a). This was particularly evident in 4-, 7-, 8-, and 11-week-old layers, in which the values of Cmic were either always or almost always higher than Cmic of initial pig slurry (19,000 ± 2000 μg g−1 dw). However, after the maximum of 33,000 ± 4000 μg g−1 dw recorded in the 4-week-old layer, Cmic decreased continuously over time. In vermireactors without earthworms, the values of Cmic decreased from a value of 14,000 ± 3000 μg g−1 dw recorded for the 2-week-old layer to a minimum of 6000 ± 4000 μg g−1 dw (8-week-old layer) with a slight increase in the two oldest layers (11 and 18 weeks old, respectively; Fig. 3a). The results of ANOVAR showed that earthworm presence and age of layers significantly affected values of Cmic. Specific changes in Cmic in different layers in the presence of earthworms were reflected by the high age × earthworm interaction (Table 2).
Changes in microbial C biomass (a), ergosterol content (b), and Cb/Cf index (c) in layers of vermireactors with E. fetida (closed squares, n = 3) and without E. fetida (open squares, n = 3). The vertical distributions of variable values (mean ± SE), i.e., corresponding to the age of layers of pig slurry, between 2 and 18 weeks, are shown on the y-axis.
Ergosterol content was significantly higher in all layers in vermireactors with earthworms than in those layers of vermireactors without earthworms, with a peak of 75 ± 22 μg g−1 dw in the 4-week-old layer (Fig. 3b). In subsequent layers, from 7 to 18 weeks old, the values of ergosterol content decreased with age of the layer, but no significant effects of age of layers were found (Table 2), although they were always higher than in the initial fresh pig slurry (10 ± 1 μg g−1 dw). In vermireactors without earthworms, the ergosterol content was more stable throughout the layers, with values that ranged between 9 ± 1 and 12 ± 2 μg g−1 dw (Fig. 3b). The results of ANOVAR showed that only earthworm presence affected the changes in ergosterol content during decomposition of pig slurry (Table 2).
The bacterial/fungal ratio (Cb/Cf) showed that during decomposition of pig slurry, the bacterial fraction predominated in the microbiota, with values of Cb/Cf > 1 in almost all layers in vermireactors with and without earthworms (Fig. 3c). In 4- and 11-week-old layers (in presence and absence of earthworms, respectively), the Cb/Cf was <1, indicating predominance of fungus (Fig. 3). Neither earthworm presence nor age of layers had significant effects on the Cb/Cf index.
Earthworms significantly increased β-glucosidase activity in comparison with vermireactors without earthworms (Fig. 4a). In vermireactors without earthworms, β-glucosidase activity did not vary as much, with values remaining between 1200 ± 100 and 1400 ± 200 μg PNP g−1 dw in all layers; these values were twice as high than those corresponding to fresh pig slurry (500 ± 100 μg PNP g−1 dw). On the other hand, in vermireactors with earthworms, values of enzymatic activity ranged between 1800 ± 100 and 2100 ± 50 μg PNP g−1 dw in layers between 4 and 18 weeks old, with a maximum of 2120 ± 110 μg PNP g−1 dw recorded in the 8-week-old layer. This variation in time resulted in a significant age × earthworm interaction (Table 2). The results of ANOVAR showed that earthworm presence and age of layers affected β-glucosidase activity during decomposition of pig slurry (Table 2).
Activity of (a) β-glucosidase and (b) cellulase enzymes in layers in vermireactors with E. fetida (closed squares, n = 3) and without E. fetida (open squares, n = 3). The vertical distributions of variable values (mean ± SE), i.e., corresponding to the age of layers of pig slurry, between 2 and 18 weeks, are shown on the y-axis.
Earthworms promoted large increases in cellulase activity in all layers, except in 2- and 7-week-old layers, in which enzymatic activities were higher in vermireactors without earthworms; in general, cellulase activity was almost two times higher in layers from vermireactors with earthworms than in layers from vermireactors without earthworms (Fig. 4b). In both types of vermireactors, the values of enzymatic activity were much higher than in initial fresh pig slurry (4000 ± 1000 μg eq. glucose g−1 dw). Cellulase activity in layers from vermireactors without earthworms remained stable, with values between 50,000 ± 7000 and 60,000 ± 10,000 μg eq. glucose g−1 dw and a maximum of 75,000 ± 9000 μg eq. glucose g−1 dw in the 7-week-old layer (Fig. 4b). Results of ANOVAR showed that only earthworm treatment significantly affected cellulase activity (Table 2).
Discussion
The results of the present experiment indicated that the presence of E. fetida in vermireactors clearly favored cellulose degradation. In vermireactors with earthworms, the rate of cellulolysis was two times higher than in vermireactors without earthworms, resulting in a 1.5-fold increase of cellulose loss after 18 weeks. By contrast, earthworm presence did not affect loss of hemicellulose, a compound that limits the rate of cellulolysis because it is implied in the formation of the crystalline structure [1]. Similar cellulose loss was reported by Vinceslas-Akpa and Loquet [44] during vermicomposting of pruning wastes with E. fetida; Scheu [34] also found that the earthworm Octolasion lacteum increased cellulose mineralization by factors of 1.5 and 1.4, respectively, in 6- and 13-year-old fallow soils. Although microbiota is the main agent responsible for cellulose decomposition, earthworms also play an important role, and their contribution to cellulolysis may be direct through their digestive processes because cellulose is a part of the diet of earthworms [13], and a high cellulase activity in the gut content of E. fetida has been reported [48]. Loss of cellulose would be accelerated by both density and feeding activity of E. fetida, unless availability of labile carbon sources in pig slurry such as dissolved organic carbon contents of pig slurry (11,100 ± 100 μg g−1 dw), a factor limiting growth of earthworms [41], favored their preferential assimilation by earthworms. The present data would therefore suggest that the direct contribution of earthworms to cellulose decomposition may be negligible because the heaviest cellulose loss occurred in layers with the lowest density of earthworms. Moreover, it remains unclear which cellulases are produced by the earthworm and which by the ingested microbiota [24] that could be stimulated in a digestive system involving mutualistic symbioses [22].
Earthworms may also contribute indirectly to cellulolysis through their interactions with microorganisms. Microbial biomass was clearly enhanced in vermireactors with earthworms, although it is generally assumed that microorganisms, especially fungi, are an important part of the earthworm diet [7]. Furthermore, Moody et al. [27] showed that different earthworm species (Lumbricus terrestris, Allolobophora longa, and A. chlorotica) preferentially fed on straw-decomposing fungi and rejected lignin-decomposing fungi. Contrary to expectations, fungal growth was then stimulated in vermireactors with earthworms, and the highest fungal growth was observed in layers containing the highest densities of earthworms. According to this, Pil and Novakova [29] found that in Eisenia andrei vermicomposting facilities, the density of microfungi was higher in earthworm gut and vermicompost than in fresh substrate. In fresh manure, fungi are present as spores [10], and their activation in vermireactors containing earthworms may be attributed to grazing and dispersal of the spores and to physical changes in structure of substrate, favoring fungal growth. In fact, earthworms feeding on pig slurry favored concentration of a coarser fraction of manure such as seeds and fragmented straw that earthworms cannot ingest. Despite the enhancement in fungal growth, bacteria clearly dominated the microbiota of pig slurry in vermireactors, independently of earthworm presence, as shown by the Cb/Cf index. However, the latter type of data must be interpreted carefully because the proportion of algae, rotifers, and particularly protozoa in Cb is not known. Nonetheless, our data suggest that there was microbial succession during cellulose decomposition in vermireactors containing earthworms, with an initial stage (first 7 weeks) during which fungi made an important contribution, and a later stage when bacteria acted as the principal decomposers. Such microbial succession is consistent with the findings of Saito et al. [32], although their experiments were no longer than 5 weeks, and contrary to those of Hu and van Bruggen [17] in which bacteria were involved during a first stage before fungi flourished.
Cellulose can be metabolized by a wide spectrum of microorganisms, including fungi and many genera of bacteria such as those belonging to the aerobic order Actinomycetales and the anaerobic order Clostridiales [25]. Under aerobic conditions, cellulolysis mainly involves fungi, actinomycetes, and nonfilamentous bacteria, whereas in anoxic environments, cellulose is almost exclusively digested by bacteria [23, 25]; moreover, aerobic cellulolysis implies a greater release of extracellular enzymes than anaerobic cellulolysis [25]. Pig slurry is predominantly colonized by anaerobic or facultative anaerobic bacteria [28, 33, 46, 50], and therefore, anaerobic bacteria should be the main agents involved in decomposition of cellulose in vermireactors without earthworms because we did not observe any significant variations in ergosterol content. Conversely, in vermireactors containing earthworms, fungi were the predominant decomposers in the youngest layers with aerobic bacteria becoming more important as age of layers increased. Moreover, access to lignin-protected cellulose as in seeds and straw is restricted to the hyphae of fungi and actinomycetes [25], although many cellulolytic actinomycetes cannot metabolize crystalline cellulose [26]. Nonfilamentous bacteria cannot gain access to protected cellulose; therefore, activation of fungal growth in vermireactors containing earthworms may favor bacterial cellulolysis by releasing more accessible cellulolytic compounds.
We determined the activities of β-glucosidase and cellulase to monitor directly the functional responses of the microbiota community in pig slurry to changes induced by the presence of earthworms; these enzymes are assumed to be produced by fungi in soils [14, 15]. Both β-glucosidase and cellulase activities appeared to show a delay related to fungal appearance because their activities peaked with ergosterol content in the 4-week-old layer. We therefore think that appearance of the fungi was necessary to trigger cellulose decomposition in vermireactors containing earthworms; otherwise, cellulolysis would have been less intense as observed in vermireactors without earthworms. Sinsabaugh and Linkins [39] proposed a model in which the highest activity of cellulolytic enzyme degradation of lignocellulosic substrates would occur when at least half of the potentially mineralizable organic matter has been lost. However, we did not find any relationship between the intense enzymatic activities recorded across all layers and the loss of both cellulose and organic matter content (data not shown). Although these extracellular enzymes can stay active for some time protected in humic complex [2], our experiment was long enough (18 weeks) to indicate that this may explain the prevalence of enzymatic activity across vermireactor layers. In vermireactors containing earthworms, the production of enzymes related to cellulose decomposition appeared to be independent of fungal populations because the activity of two enzymes remained high over time, despite a decrease in ergosterol content. Likewise, the lack of differences in ergosterol content between fresh pig slurry and aged layers cannot explain the stable but high activity compared with the initial enzyme activities in vermireactors without earthworms. Enzyme production by the bacterial fraction of microbiota could account for maintenance of the enzymatic activities, but after an initial increase, microbial biomass decreased with age of layers to close to initial values. Furthermore, in vermireactors without earthworms, some of the highest enzyme activities were recorded in layers with the lowest microbial biomass. One limiting factor in cellulose decomposition is accessibility, which is related to hemicellulose concentration [25]. The more reduced the hemicellulose contents, the higher the availability of cellulose to microorganisms. We recorded a continuous decrease in hemicellulose content, but there were no differences between vermireactors with or without earthworms, and thus, the hypothesis of increased accessibility by earthworms should be rejected. Finally, we consider that enzymatic assays are similar to substrate-induced respiration procedures, where the addition of an assimilable substrate (C, N, or P) stimulates the rate of microbial respiration, and that we may be stimulating and measuring an inactive specific cellulose-metabolizing microbiota, more abundant in vermireactors containing earthworms [5].
References
Atalla, RH, Hackney, JM, Uhlin, I, Thompson, NS (1993) Hemicelluloses as structure regulators in the aggregation of native cellulose. Int J Biol Macromol 15: 109–112
Benitez, E, Sainz, H, Nogales, R (2005) Hydrolytic enzyme activities of extracted humic substances during the vermicomposting of a lignocellulosic olive waste. Bioresour Technol 96: 785–790
Brown, GG, Doube, BM (2004) Functional interactions between earthworms, microorganisms, organic matter, and plants. In: Edwards, CA (Ed.) Earthworm Ecology, 2nd ed. CRC Press, Boca Raton, pp 213–224
Cooke, A (1983) The effects of fungi on food selection by Lumbricus terrestris L. In: Satchell, JE (Ed.) Earthworm Ecology, Chapman & Hall, London, pp 365–373
de Boer, W, Folman, LB, Summerbell, RC, Boddy, L (2004) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29: 795–811
Domínguez, J (2004) State of the art and new perspectives in vermicomposting research. In: Edwards, CA (Ed.) Earthworm Ecology, 2nd ed. CRC Press, Boca Raton, pp 401–425
Edwards, CA (2004) Earthworm Ecology, 2nd ed. CRC Press, Boca Raton
Eivazi, F, Tabatabai, MA (1988) Glucosidases and galactosidases in soils. Soil Biol Biochem 20: 601–606
Fischer, K, Hahn, D, Amann, RI, Daniel, O, Zeyer, J (1995) In situ analysis of the bacterial community in the gut of the earthworm Lumbricus terrestris L., by whole-cell hybridization. Can J Microbiol 41: 666–673
Garrett, SD (1981) Soil Fungi and Soil Fertility. Pergamon Press, Oxford
Goering, HK, Van Soest, PJ (1970) Forage Fiber Analysis, Agr. Handbook No. 379. Agricultural Research Service, USDA, Washington, DC
Goyal, A, Ghosh, B, Eveleigh, D (1991) Characteristics of fungal cellulases. Bioresour Technol 36: 37–50
Hatanaka, K, Ishioka, Y, Furuichi, E (1983) Cultivation of Eisenia fetida using dairy waste sludge cake. In: Satchell, JE (Ed.) Earthworm Ecology from Darwin to Vermiculture, Chapman and Hall, London, pp 323–329
Hayano, K (1986) Cellulase complex in tomato field soil: induction, localization and some properties. Soil Biol Biochem 18: 215–219
Hayano, K, Tubakil, P (1985) Origin and properties of β-glucosidase activity of tomato-field soil. Soil Biol Biochem 17: 553–557
Haynes, RJ, Fraser, PM, Piercy, JE, Tregurtha, RJ (2003) Casts of Aporrectodea caliginosa (Savigny) and Lumbricus rubellus (Hoffmeister) differ in microbial activity, nutrient availability and aggregate stability. Pedobiologia 47: 882–887
Hu, S, van Bruggen, AHC (1997) Microbial dynamics associated with multiphasic decomposition of 14C-labelled cellulose in soil. Microb Ecol 33: 134–143
Klamer, M, Baath, E (2004) Estimation of conversion factors for fungal biomass determination in compost using ergosterol and PFLA 18:2ω6,9. Soil Biol Biochem 36: 57–65
Kristufek, V, Ravasz, K, Pizl, V (1992) Changes in densities of bacteria and microfungi during gut transit in Lumbricus rubellus and Aporrectodea caliginosa (Oligochaeta: Lumbricidae). Soil Biol Biochem 12: 1499–1500
Lattaud, C, Locati, S, Mora, P, Rouland, C (1997a) Origin and activities of glycolytic enzymes in the gut of the tropical geophagous earthworm Millsonia anomala from Lamto (Cote d'Ivoire). Pedobiologia 41: 242–251
Lattaud, C, Zhang, BG, Locati, S, Rouland, C, Lavelle, P (1997b) Activities of the digestive enzymes in the gut and in tissue culture of a tropical geophagous earthworm, Polipheretima elongata (Megascolecidae). Soil Biol Biochem 29: 335–339
Lavelle, P, Spain, AV (2001) Soil Ecology. Kluwer Academic Publishers, London
Leschine, SB (1995) Cellulose degradation in anaerobic environments. Annu Rev Microbiol 49: 399–426
Loquet, M, Vinceslas, M (1987) Cellulolyse et ligninolyse liées au tube digestif d'Eisenia fetida andrei Bouché. Rev Ecol Biol Sol 24: 559–571
Lynd, LR, Weimer, PJ, van Zyl, WH, Pretorius, IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66: 506–577
McCarthy, AJ, Williams, ST (1992) Actinomycetes as agents of biodegradation in the environment—a review. Gene 115: 189–192
Moody, SA, Briones, MJI, Pierce, TG, Dighton, J (1995) Selective consumption of decomposing wheat straw by earthworms. Soil Biol Biochem 27: 1209–1213
Nodar, R, Acea, MJ, Carballas, T (1992) Poultry slurry microbial population: composition and evolution during storage. Biores Technol 40: 29–34
Pižl, V, Novakova, A (2003) Interactions between microfungi and Eisenia andrei (Oligochaeta) during cattle manure vermicomposting. Pedobiologia 47: 895–899
Potvin, C, Lechowicz, MJ, Tardif, S (1990) The statistical analysis of ecological response curves obtained from experiments involving repeated measures. Ecology 71: 1389–1400
Richmond, PA (1991) Occurrence and functions of native cellulose. In: Haigler, CH, Weimer, JP (Eds.) Biosynthesis and Biodegradation of Cellulose. Dekker, New York, pp 5–23
Saito, M, Wada, H, Takay, Y (1990) Development of a microbial community on cellulose buried in waterlogged soil. Biol Fertil Soils 9: 301–305
Salinitro, JP, Blake, IG, Muirhead, PA (1977) Isolation and identification of faecal bacteria from adult swine. Appl Environ Microbiol 33: 79–84
Scheu, S (1993) Cellulose and lignin decomposition in soils from different ecosystems on limestone as affected by earthworm processing. Pedobiologia 37: 167–177
Schinner, F, Von Mersi, W (1990) Xylanase-, CM-cellulase- and invertase activity in soil: an improved method. Soil Biol Biochem 22: 511–515
Schönholzer, F, Hahn, D, Zeyer, J (1999) Origins and fate of fungi and bacteria in the gut of Lumbricus terrestris L. studied by image analysis. FEMS Microbiol Ecol 28: 235–248
Sinsabaugh, RL, Antibus, RK, Linkins, AE, McClaugherty, CA, Rayburn, L, Repert, D, Weiland, T (1992) Wood decomposition over a first order watershed: mass loss as a function of lignocellulase activity. Soil Biol Biochem 24: 743–749
Sinsabaugh, RL, Linkins, AE (1988) Adsorption of cellulase components by leaf litter. Soil Biol Biochem 20: 927–931
Sinsabaugh, RL, Linkins, AE (1993) Statistical modelling of litter decomposition from integrated cellulase activity. Ecology 74: 1594–1597
Tiunov, A, Scheu, S (2000) Microfungal communities in soil, litter and casts of Lumbricus terrestris L. (Lumbricidae): a laboratory experiment. Appl Soil Ecol 14: 17–26
Tiunov, AV, Scheu, S (2004) Carbon availability controls the growth of detritivores (Lumbricidae) and their effect on nitrogen mineralization. Oecologia 138: 83–90
Urbásek, F, Pižl, V (1991) Activity of digestive enzymes in the gut of five earthworm species (Oligochaeta: Lumbricidae). Rev Ecol Biol Sol 28: 461–468
Vance, ED, Brookes, PC, Jenkinson, DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19: 703–707
Vinceslas-Akpa, M, Loquet, M (1997) Organic matter transformations in lignocellulosic waste products composted or vermicomposted (Eisenia fetida andrei): chemical analysis and 13C CPMAS NMR spectroscopy. Soil Biol Biochem 29: 751–758
von Ende, CN (2001) Repeated-measures analysis. In: Scheiner, SM, Gurevitch, J (Eds.) Design and Analysis of Ecological Experiments, Oxford University Press, pp 134–157
Whitehead, TR, Cotta, MA (2001) Characterisation and comparison of microbial populations in swine faeces and manure storage pits by 16S rDNA gene sequence analyses. Anaerobe 7: 181–187
Young, JC (1995) Microwave-assisted extraction of the fungal metabolite ergosterol and total fatty acids. J Agric Food Chem 43: 2904–2910
Zhang, BG, Li, GT, Shen, TS, Wang, JK, Sun, Z (2000) Changes in microbial biomass C, N and P and enzyme activities in soil incubated with the earthworms Metaphire guillelmi or Eisenia fetida. Soil Biol Biochem 32: 2055–2062
Zhang, BG, Rouland, C, Lattaud, C, Lavelle, P (1993) Activity and origin of digestive enzymes in the gut of tropical earthworm Pontoscolex corethurus. Eur J Soil Biol 29: 7–11
Zhu, J (2000) A review of microbiology in swine manure odor control. Agric Ecosyst Environ 78: 93–106
Acknowledgments
This research was supported by CICYT (AGL2003-01570) and Xunta de Galicia (PGIDIT03PXIB30102PR) grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aira, M., Monroy, F. & Domínguez, J. Eisenia fetida (Oligochaeta, Lumbricidae) Activates Fungal Growth, Triggering Cellulose Decomposition During Vermicomposting. Microb Ecol 52, 738–747 (2006). https://doi.org/10.1007/s00248-006-9109-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9109-x