Abstract
Rapid microbial monitoring technologies are invaluable in assessing contamination of spacecraft and associated environments. Universal and widespread elements of microbial structure and chemistry are logical targets for assessing microbial burden. Several biomarkers such as ATP, LPS, and DNA (ribosomal or spore-specific), were targeted to quantify either total bioburden or specific types of microbial contamination. The findings of these assays were compared with conventional, culture-dependent methods. This review evaluates the applicability and efficacy of some of these methods in monitoring the microbial burden of spacecraft and associated environments. Samples were collected from the surfaces of spacecraft, from surfaces of assembly facilities, and from drinking water reservoirs aboard the International Space Station (ISS). Culture-dependent techniques found species of Bacillus to be dominant on these surfaces. In contrast, rapid, culture-independent techniques revealed the presence of many Gram-positive and Gram-negative microorganisms, as well as actinomycetes and fungi. These included both cultivable and noncultivable microbes, findings further confirmed by DNA-based microbial detection techniques. Although the ISS drinking water was devoid of cultivable microbes, molecular-based techniques retrieved DNA sequences of numerous opportunistic pathogens. Each of the methods tested in this study has its advantages, and by coupling two or more of these techniques even more reliable information as to microbial burden is rapidly obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The microbial colonization of spacecraft and cleanroom assembly facility surfaces is of major concern to those commissioning modern-day space-related experimentation. The search for life elsewhere in the solar system will rely heavily on validated cleaning and sterility methods. It would be devastating for the integrity of a mission, or far worse for a pristine environment, such as the possible Europan subsurface ocean to be compromised as a result of terrestrial microbial contamination. To this end, new molecular-based methods are being used to ensure the cleanliness and sterility of mission-critical spacecraft component surfaces.
It is important to emphasize both cleanliness and sterility, and to validate the effectiveness and sensitivity of selected methods. Microorganisms have been found to inhabit nearly every niche imaginable on Earth, including the manmade environments of spacecraft surfaces and cleanrooms. Our previous studies suggest that spacecraft surfaces, during assembly in cleanrooms, contain 0 to 4 spore-forming cells cm−2 [13, 30]. So, not only is it crucial to work to minimize and, if possible, eradicate such microbial contaminants, but also it is important to characterize and identify the recurring, prevalent microbes associated with such cleanroom and spacecraft assembly environments. Studies have repeatedly shown species of the extremely hardy, spore-forming members of the Bacillus genus to be the most highly represented in samples collected from spacecraft and facility surfaces [13, 24]. The extremely oligotrophic, low-humidity, temperature-controlled conditions of a spacecraft assembly facility exert a selective pressure: only microbes able to withstand such unfavorable conditions persist. It is here that we see the overwhelming advantage that spore-forming bacteria possess. They enter a dormant state in the form of spores until favorable conditions permit them to germinate. Indeed, time and time again it has been shown that spore-forming bacteria, particularly species of Bacillus, are the most commonly isolated when assaying such environments [13, 22, 24, 30, 32].
The bias in Bacillus incidence might be due to the use of current culture-dependent microbial detection technologies. In our studies, we proved that when newly developed molecular microbial detection techniques were implemented, incidence and prevalence of non-spore-forming microbial species were also observed in both spacecraft and associated environments. Culture-dependent microbial detection methods underestimate the viable microbial population [8, 13, 16, 19, 30] because of the noncultivability of certain microbes and/or the presence of viable, but noncultivable, microbes [7, 25].
Identification and characterization of microbial bioburden must be restricted to a certain number of detection methodologies because practical limitations exist in detecting every microbe in a given sample. Targeting various “biomolecules” and “biomarkers” through use of the newer molecule-based methods allows for the rapid and thorough monitoring of microbial presence within such spacecraft and cleanroom surface samples [5, 18, 31]. Microbial detection based on enzymatic activity and several gene marker-based techniques were evaluated and are presented here. Recently, these techniques have found application in the assessment of drinking water quality of the International Space Station (ISS).
Methods
Microbial Detection Technologies
Samples
Although many opportunities exist to enhance the collection of samples, a discussion of sampling techniques is beyond the scope of this communication. Samples listed in Table 1 were collected using either spacecraft qualified swabs or wipes. Drinking water samples processed for the STS-113 mission and aboard the ISS were also collected and analyzed. Whenever necessary, suitable negative and positive controls were included. Based on the method, positive controls were either live microbial cultures or pure chemicals.
Culture-Dependent Methods
NASA Standard Assay
This assay is intended for the enumeration of spores and heterotrophic microbial populations. A swab sample (25 cm2 area) was placed into 10 mL of sterile phosphate buffered rinse solution [1] and sonicated for 2 min. The rinse solution was aseptically pipetted out and split into 2 parts. One part of the rinse solution was subjected to heat-shock (80°C for 15 min) and the other part was not. Appropriate aliquots of samples were placed in petri dishes and total aerobic counts were enumerated by the pour plate technique using TSA as growth medium (32°C for 3 to 7 days). All microorganisms isolated, including fungi, yeasts, and bacteria, were stored at −80°C in glycerol until they were further characterized.
Plate Count Assay
This assay is routinely used in environmental microbiology for the cultivation of mesophilic and heterotrophic microbial populations [2]. Samples were prepared as described above, spread plated onto prepoured R2A agar plates, and incubated at 25°C for 7 days. Samples were not heat-shocked. Although this minimal medium facilitates the growth of a diverse range of microbes, there was not a significant difference in microbial counts between TSA and R2A when “clean” spacecraft surface samples were analyzed.
Culture-Independent Methods
ATP-Based Microbial Detection
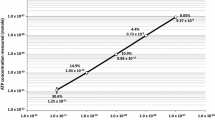
ATP-based microbial detection was made popular in the early 1960s to evaluate industrial hygiene quality. However, recent innovations have promoted this technique to the very forefront of state-of-the-art microbial monitoring [6]. Today, it is possible to measure strictly intracellular ATP (viable microbes) within a given sample, as well as total ATP (total microbial burden), inclusive of extracellular and intracellular ATP [29]. To determine total ATP, 0.1 mL of sample was combined with 0.1 mL of a detergent for lysing cells (benzalkonium chloride) and then incubated at room temperature for 1 min prior to the addition of 0.1 mL of luciferin-luciferase reagent, in which the luciferase is an enzyme selected for resistance to inactivation by the detergent. The sample was mixed and the amount of bioluminescence measured with a luminometer. To determine intracellular ATP, 0.1 mL of an ATP-eliminating reagent (apyrase, adenosine deaminase) was added to a 1-mL portion of the sample, mixed, and allowed to incubate for 30 min to remove any extracellular ATP, after which the assay for ATP was carried out as described above. This rapid and simple assay (60-min) is commercially available (Kikkoman Corp., San Francisco, CA).
LPS-Based Microbial Detection
When microorganisms invade an animal, the immune system responds by initiating a highly specific enzyme cascade in its blood cells (amebocytes). It is known that this cascade is initiated by the presence of lipopolysaccharide found in the outer membrane of Gram-negative bacteria and beta glucan in yeasts, and results in the formation of a gelclot that destroys the invading microbes. The LPS-based microbial detection assay exploits this principle as it occurs in amoebocytes of horseshoe crab (Limulus polyphemus) coupled with a chromogenic substrate. Hereafter, we refer to this as the limulus amebocyte assay (LAL). This method is most commonly used to quantify endotoxin and is the basis for the American Society for Testing and Materials method E2144-01 [26]. Commercially available kits (Charles River Laboratories, Wilmington, MA) were used to perform this rapid and simple assay (2-h) where appropriate aliquots of samples were mixed with reaction reagents and clotting was measured colorimetrically.
DNA-Based Microbial Detection
DNA was extracted directly from samples and was used to analyze molecular microbial diversity without cultivation. Suitable aliquots of each sample were subjected to DNA extraction using either a manual [9] procedure or an automated IG Autolyser DNA extractor (Instant Genetics, Menlo Park, CA) per the manufacturers’ protocol (www.instantgenetics.com ). Bacterial small-subunit rRNA genes (rrn) were PCR-amplified with eubacterially biased primers B27F and B1512R, and eukaryotic 18S rRNA genes were amplified using NS1F and NS8R primers. PCR conditions and cloning procedures were followed as described elsewhere [13]. Ribosomal DNA inserts were PCR-amplified from plasmid clones and amplification products were digested with restriction endonuclease for 3 h at 37°C. Banding patterns were grouped according to similarity, and representative members of each distinct pattern group were fully, bidirectionally sequenced. The phylogenetic relationships of organisms covered in this study were determined by comparison of individual 16S or 18S rDNA sequences to sequences in the public database (http://www.ncbi.nlm.nih.gov/ ). Evolutionary trees were constructed using PAUP software [25].
Specific Detection of Problematic Microbial Species
Research has identified spore-forming microorganisms as problematic because of their resistance to varying chemicals and conditions in spacecraft and associated environment. In order to quantify prevalent microbial species, a strategy to detect spore-forming microorganisms was developed. The gene splB is unique to spore-forming bacteria and encodes spore-photoproduct-lyase, an enzyme involved in direct reversal of UV-induced pyrimidine dimerization. Twenty-eight Bacillus species were procured from various sources, and their DNA was extracted. The rrn and splB genes were then PCR amplified, and species showing positive splB gene amplification were sequenced. Alignment of the splB sequences enabled us to identify highly conserved domains for the design of semidegenerate “universal” Bacillus splB primers for PCR amplification of unknown samples. Spacecraft-qualified titanium plates artificially inoculated with B. subtilis ATCC 6051 were swabbed and PCR assayed for splB presence. Number of B. subtilis cells using splB and the sensitivity of the TaqMan quantitative-PCR (Q-PCR) method were determined.
Results
Microbial Burden
The aerobic heterotrophic microbial population (total heterotrophs) was enumerated using both R2A and TSA media, whereas TSA was chosen to count endospore-forming microbes since spore-formers tend to grow faster in this medium. The microbial populations aboard naturally falling air particulates both trapped on witness plates and swabbed from floors, tables, and cabinet tops of class 100K (number of particles >0.5 µm in size per cubic foot) cleanroom spacecraft assembly facility surfaces are depicted in Table 1. This includes culture-dependent microbial counts enumerated for both spore-formers and heterotrophs. In addition, microbial density values derived from assaying two biomolecules, ATP and LPS, are given in Table 1. Based on Escherichia coli standard culture counts, microbial densities were calculated on the assumption that one RLU (a measure of ATP) and 1 pg endotoxin (a measure of LPS) is equivalent to one CFU. Number of samples tested in each category is also tabulated.
Fallout Air Particulates
The total heterotrophs of the cleanroom air ranged between 5 and 2.4 × 102 CFU, and the spore-forming microbes were between 0 and 2.8 × 101 CFU per cm2 on the surface area of witness plates. Paint-coated witness plates captured more microbes than did stainless steel plates. Witness plates coated with NS43G, an off-white conductive paint, trapped more particulates [28], and the number of microorganisms isolated was also higher with NS43G when compared to other witness plates. In most cases, the number of heterotrophs present ranged from less than the detectable level to 5.0 × 101 CFU per cm2.
Spacecraft and Assembly Facilities
Twenty-five surface samples of the Mars Odyssey spacecraft were pooled and showed, on average, total heterotrophs and spore-formers at 1 and 0.1 CFU per cm2, respectively (Table 1). The pooled swab samples collected from flight-ready circuit boards (http://aarchive-lib.jpl.nasa.gov ), prepared for a possible future mission to Europa, contained microbial densities somewhat similar to those observed for the Mars Odyssey [13]. The microbial burden determined by the ATP assay was within an order of magnitude of that dictated by the plate count assay for the Mars Odyssey surfaces.
Floor, table, and cabinet top surface samples from two class 100K cleanroom assembly facilities were examined. In general, the number of heterotrophs present in these locations was in the range of 0 to 102 CFU, and abundance of spore-formers was less than 1 CFU per cm2. Many of the tested samples had no cultivable microbial counts. The microbial burden associated with the JPL-SAF cleanroom floors, tables, and cabinet tops, as determined by biomolecule enumeration, showed similar microbial densities.
However, the microbial burden of the KSC SAEF-II floors, as measured by the ATP assay, exhibited at least 1 to 2 logs higher viable microbial population than cultivable counts.
ISS Drinking Water
Untreated municipal water samples (City of Cocoa, FL) processed for the ISS contained 1.4 × 104 CFU/100 mL, whereas cultivable counts were not observed in the biocide-treated samples. The water used to rehydrate foods on board the ISS revealed 5.1 × 103 CFU/100 mL cultivable counts, whereas the drinking water did not show any CFU. The ATP assay exhibited 1.3 × 105 microorganisms per 100 mL present in the untreated municipal water samples, an order of magnitude higher. Based on culture-independent analysis (ATP and LPS measurements), the absence of any viable microbial populations in these water samples suggests that biocide treatment is effective in removing all cultivable microbes from the water system. Intracellular ATP determinations were unable to detect any measurable microbial burden in the ISS drinking water samples, unlike LAL analysis that measured 1.1 × 101 pg endotoxin. Whether this contaminating endotoxin arose from living or dead cells could not be determined, as this assay detects the presence of all endotoxin within a sample, regardless of its source. Results of the ATP assay in combination with LAL analysis stress the need to develop an approach to analyze the presence of noncultivable microbes [15].
Cultivable Microbial Diversity
Excluding the ISS drinking water samples, the most common microbes isolated from the environments sampled in this study were Gram-positive bacteria, particularly species of Bacillus (Table 2). Among the members of the Bacillus, about 12 known and 10 novel species were encountered. One of these novel species was characterized and named B. nealsonii [30], and another round-spore-forming bacillus was described as “B. odysseyi” [14]. Without exception, B. pumilus was repeatedly isolated from all of the various surface locations analyzed. Although B. pumilus was not isolated from the ISS drinking water, this bacterial species was isolated from ISS hardware surfaces (data not shown). With the exception of B. odysseyi, all of the Bacillus species isolated in this study fall into the Bacillus rRNA group-1, phylogenetically.
In addition to Bacillus, species of other spore-forming genera such as Sporosarcina and Paenibacillus, actinomycetes, and fungi (Aureobasidium) were isolated. A. pullulans was isolated from the Mars Odyssey spacecraft but was not cultivated from any of the other surrounding surfaces. Unlike the surrounding facility surfaces, several genera were cultivated from the surfaces of the Mars Odyssey spacecraft, whose components were manufactured across various geographical locations. Asporogenous rods, such as Corynebacterium, Curtobacterium, Filibacter, Microbacterium, and cocci, Kytococcus, Micrococcus, Planococcus, Staphylococcus, and Terracoccus, were cultivated from the various surfaces assayed in this study.
Comparatively, fewer Gram-negative microbes were isolated from the many surface samples analyzed. Species of the β- and γ-proteobacterial lineages were cultivated from the spacecraft surfaces only. Of the water collected from the ISS, only the water used to rehydrate foods yielded any cultivable microbes, those being Acinetobacter radioresistens and Acidovorax temperans. Interestingly, A. radioresistens was isolated from both the surface of the Mars Odyssey spacecraft and the ISS drinking water.
Molecular Microbial Diversity
Because this method is not dependent on culturing of microbes, a much larger diversity was observed (Table 2). Phylogenetic analyses of the 16S rDNA sequences showed equal representation of Gram-positive and Gram-negative bacteria in the spacecraft and facility surface samples. Among the sequences retrieved directly from the samples analyzed in this study were representatives of α-, β-, γ-proteobacteria and Gram-positive bacteria. Sequences of Bacillus and Taxeobacter species are repeatedly retrieved from JPL-SAF surfaces, whereas Aquaspirillum and Variovorax sequences are the most commonly retrieved from KSC facility surfaces. For the most part, sequences retrieved from JPL-SAF surfaces derive from low-humidity, oligotrophic tolerant microbes, similar to those found in desert-like environments. Conversely, most of the sequences retrieved from KSC facility surfaces arise from microbes more tolerant of humid, brackish environments.
The ISS drinking water samples were quite diverse, as sequences arising from aerobic heterotrophs, halogen-reducing bacteria, and commensals were observed. Of greatest importance was the retrieval of several 16S rDNA sequences of opportunistic pathogens. Sequences of Afipia, Ochrobactrum, Stenotrophomonas, and Propionibacterium were all retrieved from the drinking and/or regenerative waters of the ISS. The untreated municipal water harbored sequences of Pseudomonas only, while after treatment with iodine sequences of α- and β-proteobacteria were retrieved. This is suggestive of a posttreatment contamination event, either linked with the storage practices or transport, or otherwise.
Specific Detection of Problematic Microbial Species
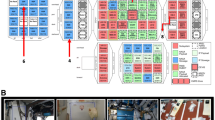
About 800 base pairs of the splB sequences from 17 Bacillus species were sequenced. The splB gene nucleotide sequence is highly heterogeneous, and ~70% nucleotide sequence similarity was observed among various species of Bacillus, as well as between inter-genus spore-forming bacteria (Fig. 1). Such heterogeneity of gene sequences has been exploited to design effective probe-primer sets specific for a given problematic species. For example, a specific TaqMan splB probe-primer set was synthesized that allowed us to perform quantitative real-time PCR to detect B. subtilis from environmental surface samples. Surfaces contaminated with as few as 103 CFU per mL were effectively detected and this TaqMan system was specific to the detection of the microbial species targeted (Fig. 1).
Phylogenetic tree of the splB sequences (~800 bp each) of 17 Bacillus sp. The numbers above the lines are the percent bootstrap values of that branch of the tree after 500 replications. Inset gel image shows splB amplification (~150 bp) of varying DNA extractions from artificial inoculations of either B. subtilis on Ti plates. Lane 1, DNA ladder; lane 2; automated DNA extraction; lane 3; manual DNA extraction; lane 4; 103 B. subtilis per Ti plate; lane 5, 102 B. subtilis per Ti plate; lane 6, B. pumilus ATCC 7061 DNA; lane 7, negative control, no DNA.
Discussion
For decades microbiologists and ecologists alike have battled with “the great plate count anomaly,” that is, how to go about culturing microbes from the environment while minimizing the inherent bias associated with using any one particular medium. It is well known that only ~1% of the microorganisms reported are cultivable, so if humanity is to gain a better understanding of the role that microbes play in any given environment, superior methods of investigation will need to be developed and put into practice. In this study we explored several state-of-the-art techniques, ranging from enzyme-based biomolecule enumeration schemes to universal gene-based cloning strategies. Whereas cultivation-dependent methods are reliant upon prior knowledge of the microbes in question, modern day cultivation-independent techniques require no such foreknowledge, and elicit no such bias for or against previously reported microorganisms.
From the Viking mission (1976) to the present day, each and every attempt at microbial enumeration and isolation from spacecraft and associated environments has been carried out using traditional culture-based techniques [3, 4, 12, 16, 19, 20, 21, 23, 24, 30]. In this communication we compared the results generated using both traditional and advanced molecular approaches while examining the abundance of microorganisms from spacecraft and associated environments. As expected, populations were far less diverse when examined by culture-dependent methods, most likely because of the limitations in the methods employed.
One of the culture-independent methods, microscopy, is a powerful tool and is invaluable for qualitatively assessing microbial populations. However, because of the drastically low number of microbes present in spacecraft and associated environments (0 to 102 CFU per cm2), and the limitations associated with this technique (autofluorescence, false-positives, nonspecific binding of stain, insufficient penetration resulting in false negatives [21]) it is impractical. Despite an appreciable number of visible cells (2 log higher than spacecraft surfaces) in the assembly floor samples, the nonspecific binding of stains to particulates was problematic. We did not perform in situ nucleic acid hybridizations since previous attempts have shown that such techniques necessitate >107 cells per reaction (data not shown).
The molecular microbial detection methods revealed the presence of noncultivable microbes in the ISS drinking water, confirming the fact that the measures taken to cultivate microbes from the drinking water samples were estimating only 10% of the microbial contamination. Although the drinking water collected from the ISS did not yield any measurable microbes, DNA-based procedures retrieved ribosomal sequences of opportunistic pathogens. Likewise, molecular cloning-based methods showed more diverse populations from both the spacecraft and facility samples than culture-dependent methods employed. This is reasonable since recovery of DNA fragments yields information from the total microbial population present, dead or alive, unlike culturing microorganisms that require specific conditions for growth. In this communication, we have shown that Q-PCR approaches can be used to detect and quantify problematic microbial species (splB for spore-forming microbes) once the problem is made known by use of molecular microbial diversity approaches.
The ATP assay has long been recognized as being “by far the most convenient and reliable method for estimating total microbial biomass in most environmental samples” [10]. In many samples, notably cleanroom floors, the levels of free extracellular ATP were extremely high, sometimes 2 or 3 orders of magnitude greater than the intracellular values (data not shown). This ATP is probably attributable to lysed, dead cells. The present study demonstrates the value of knowing culturable as well as total microbial burden. Although it can be presumed that the level of ATP is as valid as are viable cell counts for estimating the cleanliness of a facility, it is evidently not possible for the amount of ATP measured to be used to estimate a cell number or a quantitative bioburden, because of large differences in volumes and metabolic states of different cells. However, the use of culturable cell counts to determine total bioburden is also subject to scrutiny, since with any given medium only a small and indeterminable fraction of the organisms present will grow [11, 26]. Similarly, the rapid inferences derived from the LAL assay allowed quick decisions to be made in regard to recleaning necessary surfaces.
Finally, these molecular microbial detection assays report clumped-cell numbers accurately, whereas plate counts do not. Moreover, the molecule(s)-based assays show no preference to cultivable species; they tell of the presence of any and all contaminating microbes, regardless of growth requirement, nutrient availability, syntrophy, or cellular health. These assays are particularly applicable for monitoring samples from environments with extremely low microbial burden. Single-handedly, each of the above-described technologies has its limitations and can yield only so much relevant information. The real power of such rapid techniques comes in their use in concert, and when combined, they yield additional insight into the microbial population.
References
Anonymous (1980) NASA standard procedures for the microbiological examination of space hardware, NHB 5340.1B, 1980, Jet Propulsion Laboratory communication, National Aeronautics and Space Administration
AD Eaton LS Clesceri AE Greenberg (Eds) (1995) Standard Methods for the Examination of Water and Wastewater, 19th ed. APHA Washington, DC
MS Favero (1971) ArticleTitleMicrobiological assay of space hardware. Environ Biol Med 1 27–36 Occurrence Handle1:STN:280:CSmD383it1w%3D Occurrence Handle4951037
TL Foster L Winans Jr (1975) ArticleTitlePsychrophilic microorganisms from areas associated with the Viking spacecraft. Appl Microbiol 4 546–550
SJ Giovannoni TB Britschgi CL Moyer KG Field (1990) ArticleTitleGenetic diversity in Sargasso Sea bacterioplankton. Nature 345 60–63 Occurrence Handle1:CAS:528:DyaK3cXktFymu7s%3D Occurrence Handle2330053
MW Griffiths (1996) ArticleTitleThe role of ATP bioluminescence in the food industry: new light on old problems. Food Technol 50 62–72 Occurrence Handle1:CAS:528:DyaK28XksFegtbk%3D
DJ Grimes AL Mills KH Nealson (2000) The importance of viable but nonculturable bacteria in biogeochemistry. RR Colwell DJ Grimes (Eds) Nonculturable Microorganisms in the Environment. ASM Press Washington, DC 209–227
V Guarnieri E Gaia L Battocchio M Pitzurra A Savino C Pasquarella T Vago V Cotronei (1997) ArticleTitleNew methods for microbial contamination monitoring: an experiment on board the MIR orbital station. Acta Astronaut 40 195–201 Occurrence Handle10.1016/S0094-5765(97)00102-1 Occurrence Handle1:STN:280:DC%2BD3MnlvFeguw%3D%3D Occurrence Handle11540769
JL Johnson (1981) Genetic characterization. P Gerhardt RGE Murray RN Costilaw EW Nester WA Wood NR Krieg GB Phillips (Eds) Manual of Methods for general bacteriology. ASM Press Washington, DC 450–472
D Karl (1980) ArticleTitleCellular nucleotide measurements and applications in microbial ecology. Microbiol Rev 44 739–796 Occurrence Handle1:CAS:528:DyaL3MXmtFWkug%3D%3D Occurrence Handle7010116
TL Kieft (2000) Size matters: dwarf cells in soil and subsurface terrestrial environments. RR Colwell DJ Grimes (Eds) Nonculturable Microorganisms in the Environment. ASM Press Washington, DC 19–46
DW Koenig DL Pierson (1997) ArticleTitleMicrobiology of the Space Shuttle water system. Wat Sci Technol 35 59–64 Occurrence Handle10.1016/S0273-1223(97)00235-7 Occurrence Handle1:CAS:528:DyaK2sXmt1KhtLo%3D
MT La Duc WL Nicholson R Kern K Venkateswaran (2003) ArticleTitleMicrobial characterization of the Mars Odyssey spacecraft and its encapsulation facility. Environ Microbiol (accepted) . .
MT La Duc M Satomi K Venkateswaran (2003) ArticleTitle Bacillus odysseyi sp. nov. isolated from the Mars Odyssey spacecraft. Intern J Syst Evol Microbiol (accepted) . .
La Duc, MT, Sumner, R, Pierson, D, Venkateswaran, K (2003) Characterization and monitoring of microbes in the International Space Station drinking water. Proceeding of the International Conference on Environmental Systems. 03ICES-188. July 6 to 11th 2003, Vancouver, Canada
K Makimura R Hanazawa K Takatori Y Tamura R Fujisaki Y Nishiyama S Abe K Uchida Y Kawamura T Ezaki H Yamaguchi (2001) ArticleTitleFungal flora on board the Mir-Space Station, identification by morphological features and ribosomal DNA sequences. Microbiol Immunol 45 357–363 Occurrence Handle1:CAS:528:DC%2BD3MXltVSrurk%3D Occurrence Handle11471823
A Moter UB Gobel (2000) ArticleTitleFluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods 41 85–112 Occurrence Handle10.1016/S0167-7012(00)00152-4 Occurrence Handle1:STN:280:DC%2BD3cvis1Cnuw%3D%3D Occurrence Handle10991623
NR Pace DA Stahl DJ Lane GJ Olsen (1985) ArticleTitleThe analysis of natural microbial communities by ribosomal RNA sequences. Microb Ecol 9 1–56
DL Pierson (2001) ArticleTitleMicrobial contamination of spacecraft. Gravit Space Biol Bull 14 1–6 Occurrence Handle1:STN:280:DC%2BD387jtFartA%3D%3D Occurrence Handle11865864
JR Puleo ND Fields B Moore RC Graves (1970) ArticleTitleMicrobial contamination associated with the Apollo 6 spacecraft during final assembly and testing. Space Life Sci 1 48–56
JR Puleo GS Oxborrow ND Fields HE Hall (1970) ArticleTitleQuantitative and qualitative microbiological profiles of the Apollo 10 and 11 spacecraft. Appl Microbiol 3 384–389
JR Puleo GS Oxborrow ND Fields CM Herring LS Smith (1973) ArticleTitleMicrobiological profiles of four Apollo spacecraft. Appl Microbiol 26 838–845 Occurrence Handle1:STN:280:CSuD28jivFY%3D Occurrence Handle4148913
JR Puleo MS Favero GS Oxborrow CM Herring (1975) ArticleTitleMethod for collecting naturally occurring airborne bacterial spores for determining their thermal resistance. Appl Microbiol 30 786–790 Occurrence Handle1:STN:280:CSmD1cnmtlI%3D Occurrence Handle1106321
JR Puleo ND Fields SL Bergstrom GS Oxborrow PD Stabekis R Koukol (1977) ArticleTitleMicrobiological profiles of the Viking spacecraft. Appl Environ Microbiol 33 379–384 Occurrence Handle1:STN:280:CSiC2MngvFU%3D Occurrence Handle848957
DB Roszak RR Colwell (1987) ArticleTitleSurvival strategies of the bacteria in the natural environment. Microbiol Rev 51 365–379 Occurrence Handle3312987
E Stackebrandt TM Embley (2000) Diversity of uncultured microorganisms in the environment. RR Colwell DJ Grimes (Eds) Nonculturable Microorganisms in the Environment. ASM Press Washington DC 19–46
Swofford D (1990) PAUP: phylogenetic analysis using parsimony, version 3.0. Computer program distributed by the Illinois Natural History Survey, Champaign, IL
PS Thorne KH Bartlett J Phipps K Kulhankova (2003) ArticleTitleEvaluation of five extraction protocols for quantification of endotoxin in metalworking fluid aerosol. Ann Occup Hyg 47 31–36 Occurrence Handle10.1093/annhyg/meg009 Occurrence Handle1:CAS:528:DC%2BD3sXhs1Wns74%3D Occurrence Handle12505904
VB Vasin VI Trofimov (1995) ArticleTitleThe experimental study of microbial contamination of the space hardware. Adv Space Res 3 273–276 Occurrence Handle10.1016/S0273-1177(99)80096-9
K Venkateswaran M Satomi S Chung R Kern R Koukol C Basic DC White (2001) ArticleTitleMolecular microbial diversity of a spacecraft assembly facility. Syst Appl Microbiol 24 311–320 Occurrence Handle1:CAS:528:DC%2BD3MXmsl2itL0%3D Occurrence Handle11518337
K Venkateswaran N Hattori MT La Duc R Kern (2003) ArticleTitleATP as a biomarker of viable microorganisms in clean-room facilities. J Microbiol Methods 52 367–377 Occurrence Handle10.1016/S0167-7012(02)00192-6 Occurrence Handle1:CAS:528:DC%2BD3sXjtFyquw%3D%3D Occurrence Handle12531506
K Venkateswaran M Kempf F Chen M Satomi W Nicholson R Kern (2003) ArticleTitle Bacillus nealsonii sp. nov., isolated from a spacecraft assembly facility, whose spores are gamma-radiation resistant. Int J Syst Evol Microbiol . .
Acknowledgements
The research described in this paper was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration. Funding was provided by the Mars Program Office, and Advanced Environmental Monitoring and Control. Technical assistance by D. Pierson, R. Sumner, W. Nicholson, S. Chung, G. Kazarians, F. Chen, M. Kempf, G. Kuhlman, and T. Ma is appreciated. We are thankful to D. Jan, K. Buxbaum and T. Luchik for support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
La Duc, M., Kern, R. & Venkateswaran, K. Microbial Monitoring of Spacecraft and Associated Environments . Microb Ecol 47, 150–158 (2004). https://doi.org/10.1007/s00248-003-1012-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-003-1012-0