Abstract
MRI is used for early detection of inflammation of sacroiliac joints as it shows active lesions of sacroiliitis long before radiographs show damage to the sacroiliac joints. Early diagnosis of arthritis allows early treatment of inflammation and can help delay disease progression and prevent irreversible damage. Also, early identification of axial involvement in juvenile spondyloarthropathy is crucial, as treatment options are different than for peripheral juvenile spondyloarthropathy. In general, standard sequences used in adults are also applied to children. However, interpreting MR images of pediatric sacroiliac joints is more challenging than in adults, because of normal physiological changes during skeletal maturation, which can simulate disease on MR images. Furthermore, classical definitions of sacroiliitis used in adults, for both active inflammatory and structural lesions, can be difficult to extrapolate to children. The development of reliable pediatric-specific definitions for sacroiliitis is still in active study. Understanding both normal and pathological signal changes in children is important to distinguish physiologic findings from disease and to make a correct diagnosis. In this review, the main imaging characteristics of sacroiliitis on MRI in children and its frequent pitfalls will be illustrated, while also citing some discussion points regarding the scan protocol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Juvenile spondyloarthropathy is a generalized term for a group of related inflammatory diseases of the musculoskeletal system, which have an onset in childhood or adolescence [1,2,3]. Most cases of juvenile spondyloarthropathy present with peripheral arthritis and enthesitis of the lower extremities early in the disease course, while sacroiliac joint involvement typically occurs in a later stage [1]. In adult spondyloarthropathy, the axial skeleton which consists of the sacroiliac joints and spine is often involved early in the disease course, especially in the axial spondyloarthropathy group. This is demonstrated by active sacroiliitis being a key criterion for classification [4,5,6,7]. Early diagnosis of arthritis has become more important to treat inflammation as soon as possible and potentially delay progression of the disease [8, 9]. MRI has been increasingly used for early detection of inflammatory changes as it shows active lesions of sacroiliitis long before radiographic changes become evident [4, 10]. Radiography can only detect structural changes, so per definition only the sequelae of inflammation and no early diagnosis (Fig. 1). Imaging of the sacroiliac joints is essential for both diagnosis and surveillance of axial spondyloarthropathy. Imaging findings play a large role in therapeutic management and assessment of treatment response [8, 9].
Added value of MRI compared to radiography of sacroiliac joints. a Anteroposterior pelvic radiographic projection and b semicoronal STIR image of a 16-year-old girl with juvenile spondyloarthropathy; radiography can only detect late, structural changes such as erosion and sclerosis (black arrows), whereas MRI can depict early, active lesions as well such as bone marrow edema (white arrows) and capsulitis (dotted white arrows) (STIR, short tau inversion recovery)
Early identification of axial involvement in juvenile spondyloarthropathy is crucial, as treatment options are different than those for peripheral juvenile spondyloarthropathy [11, 12]. However, diagnosing sacroiliitis with MRI in children and adolescents is more challenging compared to adults due to the normal physiological changes occurring during skeletal maturation, which may be confusing as they can simulate disease if not recognized [13,14,15,16]. Knowledge of the normal imaging features of the immature sacroiliac joint is imperative to avert unnecessary treatment as a result of false-positive diagnosis of sacroiliitis.
In this review, we illustrate frequent pitfalls when diagnosing sacroiliitis in children, summarize the main imaging characteristics of sacroiliitis, and discuss the scan protocol.
Sacroiliac joint MRI scanning protocol
The 2009 ASAS definition for active sacroiliitis on MRI relied on 2 sequences: semicoronal T1 and STIR [4]. Recently, a standardized image acquisition protocol for adult sacroiliac joint MRI has been recommended by an ASAS-SPARTAN collaboration group [17]. Unfortunately, there is no such protocol yet for children. In a European Society of Musculoskeletal Radiology (ESSR)–European Society of Paediatric Radiology (ESPR) paper, an MRI protocol for scanning sacroiliac joints in JIA was presented by Hemke et al., as suggested by the authors in consensus [18]. This protocol consists of a semicoronal T1-weighted sequence, and a semicoronal and axial water-sensitive sequence with fat suppression as recommended sequences, and a semicoronal and axial T1-weighted sequence with fat suppression after contrast administration as optional sequences. However, an internationally approved pediatric sacroiliac joint MRI data acquisition protocol still has to be determined.
The main sequences used are fluid-sensitive sequences with suppressed fat signal and a T1-weighted sequence. Acquisitions are classically made in the semi-coronal plane, parallel to the posterior cortex of the S2 vertebral body, and in the axial oblique plane, perpendicular to the semicoronal images [17, 19, 20], as in adults, although some centers prefer a true axial plane. To date, there is no consensus on which direction should be preferred. It is also recommended to make a (true) axial acquisition with a larger field of view to depict the entire pelvis including the hips, because the hip joint is commonly affected in children with juvenile idiopathic arthritis (Fig. 2) [21,22,23]. This will also give the possibility to assess various entheseal sites of the pelvis, which is useful as well (Fig. 3) [21, 22].
Hip arthritis due to juvenile spondyloarthritis in a 9-year-old girl. Axial STIR image of the pelvis including the hip joints in a sacroiliac joint MRI protocol shows a large effusion of the right hip joint (arrow), illustrating the usefulness of including the hips in sacroiliac joint MRI protocol in juvenile spondyloarthritis (STIR, short tau inversion recovery)
Pelvic enthesitis in a 15-year-old boy with juvenile idiopathic arthritis. Axial STIR image of the pelvis shows enthesitis at the left gluteal insertion at the greater trochanter (arrow), illustrating the usefulness of including axial images through the entire pelvis in sacroiliac joint MRI scanning protocols (STIR, short tau inversion recovery)
Fluid-sensitive sequences for detection of active inflammation
The main sequences used for detection of active disease—primarily bone marrow edema—are short tau inversion recovery (STIR) and fat-suppressed T2-weighted sequences [18, 19, 24, 25]. The choice will depend on the preference of the reader. STIR gives a more homogenous suppression of fat signal, while fat-suppressed T2-weighted images offer better contrast resolution and more optimal signal-to-noise ratio [19].
Sequences for detection of structural lesions
Semicoronal unenhanced T1-weighted images without fat suppression are essential for the assessment of the structural damage to the sacroiliac joints [11, 17, 20]. However, this sequence alone is considered insufficient for proper assessment of the articular surface, and an additional sequence to optimally depict the bone-cartilage interface of the articular surface more sensitive for detection of erosion is recommended, such as T1-weighted images with fat suppression (2D or 3D), which is included in the recently recommended minimal standard acquisition protocol for sacroiliac joints by MRI for adults [17]. In children, it is even more difficult to assess erosion due to normal developmental changes which may simulate erosion [14, 20]. A cartilage-specific MRI sequence might be helpful to distinguish normal growth-related changes from true bony erosions [20].
Imaging characteristics of sacroiliitis in children
The goal of MR imaging is to depict active, inflammatory lesions as well as to diagnose chronic, structural changes. In adults, features of active and structural sacroiliitis have been defined by Assessment of SpondyloArthritis International Society (ASAS) [4,5,6,7]. Also, various MRI scoring methods exist to evaluate sacroiliitis on MRI, of which the Spondyloarthritis Research Consortium of Canada (SPARCC) sacroiliac joint inflammation (SIS) and structural scores (SSS) are most used [26, 27]. These definitions and scoring systems have also been applied to children, in the absence of specific pediatric definitions. However, the classical adult definitions should be used with caution in children [28,29,30]. Developing reliable pediatric-specific definition for sacroiliitis is a difficult task, currently in active research. Recently, the outcome measures in rheumatology clinical trials (OMERACT) MRI in juvenile idiopathic arthritis (JIA) working group has published and updated a scoring system for specific use in the pediatric population, the JAMRIS (juvenile idiopathic arthritis MRI score) scoring system (Table 1) [31,32,33]. Also, an atlas illustrating the different features of this scoring system was published by the OMERACT group for both active and structural lesions [19, 20]. The JAMRIS system is a consensus-driven methodologic and technical survey conducted among a multidisciplinary international expert group, with the objective to develop definitions for the assessment of magnetic resonance imaging pathologies of the sacroiliac joints in JIA [31, 32].
Active inflammatory lesions
Features of active sacroiliitis depicted with MRI are bone marrow edema, osteitis, joint fluid, joint enhancement, inflammation in an erosion cavity, capsulitis, and enthesitis (Fig. 4) [4, 31,32,33]. Active lesions are best evaluated on fat-saturated water sensitive sequences such as STIR or fat-suppressed T2 imaging. Bone marrow edema is the key feature for diagnosing active sacroiliitis. Bone marrow edema in children is more specific for sacroiliitis than in adults (97% vs 88–89%), even though often less extensive. Bone marrow edema also has a lower sensitivity for sacroiliitis in children (26%) than adults (67–79%) [21]. Furthermore, being familiar with the normal developmental variability in children is imperative to accurately distinguish between patterns of normal variation and pathology.
Typical features of both active and structural sacroiliitis in a 16-year-old male with juvenile spondyloarthropathy. Semicoronal (a) STIR and (b) T1-weighted images show typical features of sacroiliitis within the right iliac bone. a Active lesions seen on semicoronal STIR image including bone marrow edema (black arrows), joint inflammation (white arrows) and capsulitis (arrowhead). b Semicoronal T1-weighted image of the same boy 3 months later, showing structural lesions: fat lesion (white arrows), sclerosis (black dotted arrows), and erosion (large white arrow) (STIR, short tau inversion recovery)
Pelvic entheses are also important to assess on sacroiliac joint MRI, as it has been shown that the number of active entheses and joints at onset can predict sacroiliitis at follow-up [22, 23]. Sites of entheses of the pelvis include the symphysis pubis, ramus of pubis, iliac crest, ischial tuberosity, anterior superior and inferior iliac spine, posterior superior iliac spine, longitudinal ligament insertion, vertebral posterior elements, retroarticular part of the sacroiliac joint, and the muscle attachments in the immediate vicinity of the hip [23].
In the JAMRIS definition, all entheseal sites are included, except for the retroarticular part with the interosseous ligaments, which are excluded from scoring—same as in the ASAS definition. The retroarticular or ligamentous part is located in the dorsal and upper 2/3 of the sacroiliac joint, mainly behind the cartilaginous part, where the sacrum and the ilium are connected with restraining ligaments. All other features of the JAMRIS system are scored in the cartilaginous part which is located in the anterior and lower 1/3 of the joint.
Structural lesions
The structural changes in sacroiliitis depicted on MRI are erosion, sclerosis, fat lesion, backfill, and ankylosis (Fig. 4) [4, 31,32,33]. Structural damage is visible mainly in later stages of the disease. The best traditional sequence for the evaluation of structural lesions is a semicoronal T1-weighted sequence; however, the use of gradient echo and other advanced sequences show promise for improved evaluation of erosions. Structural lesions of sacroiliitis are less frequently seen in children. Furthermore, erosions are very difficult to depict on T1 images, due to the normal variation [14]. If seen, they are highly specific for juvenile spondyloarthropathy [21].
Use of intravenous gadolinium-contrast
Most recent papers suggest at most a limited role of gadolinium intravenous contrast in the detection of active sacroiliitis in children [19, 24, 34, 35]. In specific cases—mostly the ambiguous cases—contrast administration may be useful to confirm the presence of disease, or to diagnose sacroiliitis with more confidence [19, 34]. Contrast administration may be helpful to differentiate joint fluid from joint space inflammation. Contrast administration may be preferable when differentiation is required from infectious sacroiliitis or tumor [34]. Compared to inflammatory sacroiliitis, in infective sacroiliitis, the inflammation spreads to involve the peri-articular soft tissues, particularly the iliacus and gluteal muscles and also, peri-articular fluid collection or abscess can develop, which are practically pathognomonic of an infective sacroiliitis.
These possible benefits of gadolinium administration should be weighed against its cost and risks. Obtaining intravenous access in children requires additional materials and personnel in the imaging department, is technically difficult, and can be traumatic and anxiety-provoking for the child and family. There are also concerns regarding gadolinium retention in the brain, bone, skin, liver, spleen, and kidneys, with potential long-term effects that require further study [36, 37].
Frequent pitfalls in the assessment of pediatric sacroiliac joints
Diagnosing sacroiliitis on MRI in the pediatric population is more challenging compared to adults due to normal developmental changes of the immature skeleton [13,14,15,16] including ongoing ossification. The statement “children are not small adults” is very applicable in this context. The sacrum is formed by complex fusion of primary and secondary ossification centers. Primary centers undergo fusion by age ~ 7 years, while multiple small apo- and epiphyses slowly ossify throughout adolescence (Fig. 5) [38, 39]. Ossification of segmental and lateral apophyses of the sacral wings is completed significantly earlier in girls than in boys [40]. Knowledge of the normal MRI appearances of the developing sacroiliac joints is necessary to distinguish normal variants from pathology and avoid false-positive diagnosis of sacroiliitis in healthy subjects [13,14,15,16]. Frequent pitfalls are growth-related signal changes in both subchondral bone and in the joint space itself, cortical irregularities, joint facet defects, and vascular structures.
Normal sacral anatomy and ossification in a 5-month-old boy. a Semicoronal and b axial low-dose CT images illustrating the primary and secondary ossification centers of the sacrum. Each sacral vertebra is developing from 5 primary ossification centers including the centrum (asterisk), two centers for the neural arch (white arrows) and 2 costal processes (black arrows). Later in life multiple small secondary ossification centers will develop, which are small apophyses that fuse to the various surfaces of the primary centers [38]
Normal subchondral high T2 signal vs bone marrow edema
The normal pediatric sacroiliac joints commonly display a subchondral rim of increased signal on fluid-sensitive sequences such as a T2-weighted sequence with fat suppression, or a STIR sequence, also referred to as subchondral flaring (Fig. 6) [13]. This metaphyseal-equivalent high signal presumably results from ossifying epiphyseal cartilage and underlying newly formed subchondral bone [13]. The subchondral “flaring” is typically symmetrical, predominant at the sacral side, gradually fades away with age, and eventually disappears after closure of the segmental apophyses [13, 15]. “Flaring” is most prominently seen in prepubertal and pubertal children.
Example of variability in the normal appearance of the subchondral signal on STIR images of sacroiliac joints in children without low back pain and sent to MRI for other reasons. Semicoronal STIR images in a 10-year-old girl with knee pain shows normal sacroiliac joints with subchondral high signal (“flaring”) along the lateral apophyses of the sacrum (arrows) (STIR, short tau inversion recovery)
If not aware of these normal signal changes, a false-positive diagnosis of sacroiliitis could be made, since they can mimic bone marrow edema (BME). Suspicion for true bone marrow edema and sacroiliitis should arise when the high T2/STIR signal is observed only at the iliac side, is more intense at the iliac than on the sacral side, when there is a definite difference between left and right side, or if there is definite high T2/STIR signal of any pattern in teenagers with closed sacral apophyses [13].
Variations of subchondral bone plate
The subchondral bone plate in adults appears as a regular, sharply defined, thin black line (Fig. 7) [10, 14]. This is often not the case in children. In a 2021 study of 251 normal pediatric sacroiliac joint MRI scans, joint margins were sharply defined and smooth in less than one third of normal children, so at least two-thirds (65%) of normal pediatric sacroiliac joints showed a partial absence of the cortical black line, which is a key component of the adult definition of sacroiliac joint erosions, risking overdiagnosis of sacroiliitis [14]. Partial absence of the cortical black line was present in>85% of normal children at the iliac sacroiliac joint margin, and in nearly 50% at the S1 level.
Examples of normal variation in visibility of the subchondral bone plate on T1-weighted images of normal sacroiliac joints in children without low back pain, sent to MRI for other reasons. Semicoronal T1-weighted images of a normal adult sacroiliac joints in a 21-year-old man with clearly visible and smooth cortical black line (black arrows); b a 14-year old boy with headache showing irregular articular surfaces on both sacral and iliac side of the right sacroiliac joint (white arrows), and blurred iliac articular surface of the left sacroiliac joint (arrowhead); c a 12-year old girl with ankle pain, showing blurred and irregular articular margins on the sacral side bilaterally, and on the iliac side of the left sacroiliac joint (dotted arrows)
The ossifying subchondral bone plate was also frequently irregular, which can mimic erosions—especially when applying adult definitions (Fig. 7) [14]. Irregularity is mostly seen at the S1 border [14].
Also, the underlying bone marrow is still predominantly red and relatively fat-poor, and therefore has a lower T1 signal compared to adults. This results in a lower contrast between cortex and bone marrow with blurring of the articular margins (Fig. 7), making it even more difficult to detect erosions. Blurring of the articular margins is mainly seen at the iliac border [14].
Joint facet defects and ossified nuclei are also known normal variants in children that require some attention not to be confused with structural lesions to avoid false-positive findings by MRI. Articular defects, the so-called joint facet defects, are frequently seen in children and may simulate erosions (Fig. 8). Intra-articular ossified nuclei are frequent after the age of 13 years and can persist up to the age of 18 years in both genders (Fig. 9) [41].
Joint facet defect, a normal variant in the pediatric sacroiliac joint. a Semicoronal T1-weighted b STIR c VIBE and d synthetic-CT images of a 13-year-old boy with juvenile idiopathic arthritis illustrating a focal defect (arrows) at the iliac border of the left sacroiliac joint which is rather difficult to depict on the T1-weighted image, but nicely shown on the VIBE and synthetic CT images. There are no other features of sacroiliitis, no surrounding bone marrow edema or sclerosis, so this is probably a joint facet defect rather than an erosion (STIR, short tau inversion recovery; VIBE, volumetric interpolated breath-hold examination)
Appearance of the joint space and capsule
It is well known that a perceptible quantity of fluid can be present in a normal joint, which should not be mistaken for evidence of a pathological condition and the sacroiliac joint is no exception [16, 42]. The joint space discussed here is the radiographic joint space, which comprises the cartilage and the true anatomical joint space.
Most normal pediatric sacroiliac joints exhibit a mildly increased T2 signal in the joint space (not as bright as cerebrospinal fluid (CSF)), some will even display a very bright T2 signal, equivalent to fluid (Fig. 10) [16, 43]. This should be considered a physiological phenomenon when the high signal line is thin, regular, mostly symmetrical and in absence of other signs of inflammation [16]. When there is doubt about joint space fluid, contrast administration may help to confirm or exclude joint space inflammation [16]. Care must be taken when interpreting post-contrast T1 images, because most healthy pediatric sacroiliac joints will exhibit a thin rim of mild contrast enhancement at the bone/cartilage interface, which also is a growth-related physiological phenomenon (Fig. 11) [16]. This thin enhancing line could be true enhancement of vascular structures, fibrovascular tissue, or the primary spongiosa of newly formed metaphyseal bone; another possible explanation might be the diffusion of contrast into the joint space [16]. True inflammation in the joint space will mostly manifest as focal, intense, and thick enhancement [16, 19].
Normal variability in the sacroiliac joint space signal on STIR. Semicoronal STIR image of a 14-year-old boy with growth hormone deficiency showing increased “fluid-like” signal changes in the joint space (arrows) as illustration of normal variability in the joint space of pediatric sacroiliac joints, not to be confused with joint space inflammation (STIR, short tau inversion recovery)
Normal variability in the sacroiliac joint space signal on T1 after gadolinium. Semicoronal contrast-enhanced fat-saturated T1-sequence in normal sacroiliac joints of an 11-year-old boy with a brain lesion, showing a thin rim of increased signal/enhancement along both iliac and discretely also along the sacral border of both sacroiliac joints (arrows), not to be confused with abnormal joint enhancement in sacroiliitis
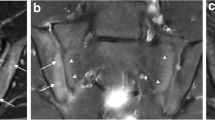
Another possible pitfall to avoid when reading sacroiliac joint MRI is misinterpretation of vascular channels abutting the superior edge of the joint, as capsulitis [19]. These vascular channels are mostly seen as a thin, enhancing line at the cranial side of the joint, crossing from sacral to iliac side, and not usually continuous on one slice (Fig. 12). Scrolling through the slices should demonstrate the continuation of vascular channels beyond the margins of the joint capsule and thus distinguish a vascular channel from capsulitis.
Vascular channels as possible pitfall for diagnosing capsulitis. Semicoronal STIR image in a normal 11-year-old girl illustrating vascular channels abutting the superior edge of the joint (arrows) seen as a thin, enhancing line at the cranial side of the joint, crossing from sacral to iliac side, not to be mistaken for capsulitis. Scrolling through the slices should demonstrate the continuation of vascular channels beyond the margins of the joint capsule and thus distinguish a vascular channel from capsulitis (STIR, short tau inversion recovery)
Future directions
In recent years, considerable progress has been made in new and other sequences that can aid in the assessment of sacroiliitis. Other sequences for evaluation of active inflammation and more specific for better assessment of cartilage and subchondral bone are high-resolution proton density sequences, Dixon sequences, and gradient echo sequences with water-selective excitation specific for cartilage (WATSc), but none of these has been validated in children. Diffusion-weighted imaging (DWI) also seems promising for assessment of inflammation, and could be valuable as a quantitative imaging tool for measuring inflammation, although in everyday practice it is not routinely included in most sacroiliitis scanning protocols [44,45,46,47].
Suitable other sequences that can be helpful for assessment of structural lesions are cartilage-specific MRI sequences, which might be helpful to distinguish normal growth-related changes from true bony erosions. Examples of this are 3D gradient echo sequences such as double echo steady state (DESS) and volumetric interpolated breath-hold examination (VIBE) (Figs. 8 and 13), and gradient echo sequences with water-selective excitation specific for cartilage (WATSc) [19, 20, 48,49,50]. 3D volumetric MRI is playing an increasingly important role in musculoskeletal diagnostic imaging. All these sequences seem very promising, although none of them has been validated in children.
New sequences for evaluation of sacroiliac joints on MRI. Semicoronal a T1-weighted MR image b VIBE image and c reformatted synthetic-CT image in a 14-year-old girl with juvenile spondyloarthropathy and structural lesions of sacroiliitis, all showing multiple erosions (arrows) at the iliac border of the left sacroiliac joint. Note also the sclerosis at the left iliac side (VIBE, volumetric interpolated breath-hold examination)
Other novel sequences and post-processing tools that improve the diagnostic precision for the evaluation of structural damage to the sacroiliac joints in children are susceptibility-weighted or zero echo time (ZTE) images that can depict the cortical bone directly and can be used to create CT-like images by inversion [48, 51, 52]. Another innovative technique is synthetic-CT, which makes use of a deep-learning tool applied to 3D gradient-echo weighted images to generate synthetic radiograph-like and CT-like images, by fully automatic post-processing without user input (Figs. 8 and 13) [48, 53]. Synthetic-CT outperformed routine T1-weighted images in diagnostic accuracy in a prospective study on adult patients with suspected sacroiliitis, while having a reliability comparable to that of true CT images [53]. These CT-like images are almost identical to conventional CT images, but are completely free from ionizing radiation, which of course is very interesting in the pediatric population [53]. However, this technique has not yet been approved for use in children.
Conclusion
MRI depicts multiple features of active inflammation and structural damage in the sacroiliac joints and is the imaging modality of choice for detecting early inflammatory changes. Compared to adults, diagnosing sacroiliitis on MRI in children is challenging mainly due to normal variability in the maturing pediatric sacroiliac joint. Knowledge of these normal variations in pediatric sacroiliac joints is crucial to avoid common pitfalls and thereby prevent a false-positive diagnosis of sacroiliitis. Recently, the JAMRIS scoring system for specific use in the pediatric population has been published by OMERACT, although developing reliable pediatric-specific definitions for sacroiliitis is currently still undergoing active study. Application of adapted, updated scanning protocols can be helpful to improve diagnostic precision. Whereas in adults a standardized image acquisition protocol for sacroiliac joint MRI has been recommended, an internationally approved pediatric sacroiliac joint MRI data acquisition protocol still has to be determined.
References
Burgos-Vargas R (2002) The juvenile-onset spondyloarthritides. Rheum Dis Clin North Am 28:531–560
Petty RE, Southwood TR, Manners P et al (2004) International League of Association for Rheumatology Classification of juvenile idiopathic arthritis: second revision. Edmonton. 2001. J Rheumatol 31:390–392
Weiss PF, Colbert RA (2018) Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatr Clin North Am 65(4):675–690
Sieper J, Rudwaleit M, Baraliakos X, et al. (2009) The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 68(Suppl 2):1–44
Lambert RG, Bakker PA, van der Heijde D (2016) Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis 75:1958–1963
Rudwaleit M, van der Heijde D, Landewé R et al (2011) The Assesment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 70:25–31
Rudwaleit M, van der Heijde D, Landewé R et al (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 68:777–783
Srinivasalu H, Sikora KA, Colbert RA (2021) Recent updates in juvenile spondyloarthritis
Rheum Dis Clin North Am 47:565–583
Tse SM, Laxer RM (2012) New advances in juvenile spondyloarthritis. Nat Rev Rheumatol 8:269–279
Jaremko JL, Liu L, Winn NJ, Ellsworth JE, Lambert RG (2014) Diagnostic utility of magnetic resonance imaging and radiography in juvenile spondyloarthritis: evaluation of the sacroiliac joints in controls and affected subjects. J Rheumatol 41:963–970
Malattia C, Tolend M, Mazzoni M et al. (2020) Current status of MR imaging of juvenile idiopathic arthritis. Best Pract Res Clin Rheumatol 34:101629
Lin C, MacKenzie JD, Courtier JL, Gu JT, Milojevic D (2014) Magnetic resonance imaging findings in juvenile spondyloarthropathy and effects of treatment observed on subsequent imaging. Pediatr Rheumatol Online J 12:1–8.
Herregods N, Jans LB, Chen M et al (2021) Normal subchondral high T2 signal on MRI mimicking sacroiliitis in children: frequency, age distribution, and relationship to skeletal maturity. Eur Radiol 31:3498–3507
Herregods N, Lambert RG, Schiettecatte E et al. (2021) Blurring and irregularity of the subchondral cortex in pediatric sacroiliac joints on T1 images: incidence of normal findings that can mimic erosions. Arthritis Care Res (Hoboken). Jul 7 Online ahead of print. https://doi.org/10.1002/acr.24746
Chauvin NA, Xiao R, Brandon TG et al (2019) MRI of the sacroiliac joint in healthy children. AJR Am J Roentgenol 212:1303–1309
Herregods N, Jans LB, Paschke J et al (2021) Magnetic resonance imaging findings in the normal pediatric sacroiliac joint space that can simulate disease. Pediatr Radiol 51:2530–2538
Lambert R, Baraliakos X, Bernard S et al. on behalf of ASAS SPARTAN MRI Working Group, et al (2022) POS0989 Development of international consensus on a standardized image acquisition protocol for diagnostic evaluation of the sacroiliac joints by MRI – an ASAS-SPARTAN collaboration. Ann Rheum Dis 81:802–803
Hemke R, Herregods N, Jaremko JL et al (2020) Imaging assessment of children presenting with suspected or known juvenile idiopathic arthritis: ESSR-ESPR points to consider. Eur Radiol 30:5237–5249
Herregods N, Maksymowych WP, Jans LB et al (2021) Atlas of MRI findings of sacroiliitis in pediatric sacroiliac joints to accompany the updated preliminary OMERACT pediatric JAMRIS (Juvenile Idiopathic Arthritis MRI Score) scoring system: Part I: Active lesions. Semin Arthritis 51:1089–1098
Herregods N, Maksymowych WP, Jans L et al (2021) Atlas of MRI findings of sacroiliitis in pediatric sacroiliac joints to accompany the updated preliminary OMERACT pediatric JAMRIS (Juvenile Idiopathic Arthritis MRI Score) scoring system: Part II: Structural damage lesions. Semin Arthritis Rheum 51:1099–1107
Herregods N, Dehoorne J, Joos R et al (2015) Diagnostic value of MRI features of sacroiliitis in juvenile spondyloarthritis. Clin Radiol 70:1428–1438
Pagnini I, Savelli S, Matucci-Cerinic M et al (2010) Early predictors of juvenile sacroiliitis in enthesitis-related arthritis. J RHeumatol 37:2395–2401
Herregods N, Dehoorne J, Pattyn E et al (2015) Diagnositic value of pelvic enthesitis on MRI of the sacroiliac joints in enthesitis related arthritis. Pediatr Rheumatol Online J 13(1):46
Weiss PF, Chauvin NA (2020) Imaging in the diagnosis and management of axial spondyloarthritis in children. Best Pract Res Clin Rheumatol 34:101596
Weiss PF, Chauvin NA, Roth J (2016) Imaging in juvenile spondyloarthritis Curr RheumatolRep 18:75
Maksymowych WP, Inman RD, Salonen D et al (2005) Spondyloarthritis Research Consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 53:703–709
Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ (2015) Development and preliminary validation of the Spondyloarthritis Research Consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol 42:79–86
Herregods N, Dehoorne J, Van den Bosch F et al (2017) ASAS definition for sacroiliitis on MRI in SpA: applicable to children? Pediatr Rheumatol Online J 15:24.
Weiss PF, Maksymowych WP, Lambert RG et al (2018) Feasibility and reliability of the Spondyloarthritis Research Consortium of Canada sacroiliac joint inflammation score in children. Arthritis Res Ther 20:56.
Weiss PF, Maksymowych WP, Lambert RG et al (2018) Feasibility and reliability of the Spondyloarthritis Research Consortium of Canada sacroiliac joint inflammation score in children. J Rheumatol 45:1411–1417.
Otobo TM, Conaghan PG, Maksymowych WP et al (2019) Preliminary definitions for sacroiliac joint pathologies in the OMERACT juvenile idiopathic arthritis magnetic resonance imaging score (OMERACT JAMRIS-SIJ). J Rheumatol 46:1192–1197.
Otobo TM, Herregods N, Jaremko J et al (2021) Sacroiliac joint MRI abnormalities in juvenile spondyloarthritis: an update of definitions and scoring of the OMERACT juvenile idiopathic arthritis MRI score. Ann Rheum Dis 80:943–944.
Otobo TM, Herregods N, Jaremko J et al (2021) Reliability of the preliminary OMERACT juvenile idiopathic arthritis MRI score (OMERACT JAMRIS-SIJ). J Clin Med 10:4564.
Weiss PF, Xiao R, Biko DM et al (2015) Detection of inflammatory sacroiliitis in children with magnetic resonance imaging: is gadolinium contrast enhancement necessary? Arthritis Rheumatol 67:2250–2256.
Herregods N, Jaremko JL, Baraliakos X et al (2015) Limited role of gadolinium to detect active sacroiliitis on MRI in juvenile spondyloarthritis. Skeletal Radiol 44:1637–1646.
Uosef A, Villagran M, Kubiak JZ et al. (2020) Side effects of gadolinium MRI contrast agents. Pediatria I Medycyna Rodzinna - Paediatrics and Family Medicine 16:49–52
Elbeshlawi I, AbdelBaki MS (2018) Safety of gadolinium administration in children. Pediatr Neurol 86:27–32.
Broome DR, Hayman LA, Herrick RC et al (1998) Postnatal maturation of the sacrum and coccyx: MR imaging, helical CT, and conventional radiography. AJR Am J Roentgenol 170:1061–1066.
Bowen VA, Cassidy JD (1981) Macroscopic and microscopic anatomy of the sacroiliac joint from embryonic life until the eighth decade. Spine (Phila Pa 1976) 6:620–628
Bollow M, Braun J, Kannenberg J et al (1997) Normal morphology of sacroiliac joints in children: magnetic resonance studies related to age and sex. Skeletal Radiol 26:697–704.
Zejden A, Jurik AG (2017) Anatomy of the sacroiliac joints in children and adolescents by computed tomography. Pediatr Rheumatol Online J 15:1–7.
Weber U, Jurik AG, Zejden A et al (2018) Frequency and anatomic distribution of magnetic resonance imaging features in the sacroiliac joints of young athletes: exploring “background noise” toward a data-driven definition of sacroiliitis in early spondyloarthritis. Arthritis Rheumatol 70:736–745.
Sudol-Szopihska I, Eshed I, Jans L et al. (2018) Classifications and imaging of juvenile spondyloarthritis. J Ultrason 18:224–34
Bray TJP, Vendhan K, Ambrose N et al (2017) Diffusion-weighted imaging is a sensitive biomarker of response to biologic therapy in enthesitis-related arthritis. Rheumatology (Oxford) 56(3):399–407.
Bozgeyik Z, Ozgocmen S, Kocakoc E (2008) Role of diffusion-weighted MRI in the detection of early active sacroiliitis. AJR Am J Roentgenol 191(4):980–986
Orr KE, Andronikou S, Bramham MJ et al (2018) Magnetic resonance imaging of sacroiliitis in children: frequency of findings and interobserver reliability. Pediatr Radiol 48(11):1621–1628.
Vendhan K, Bray TJP, Atkinson D et al (2016) A diffusion-based quantification technique for assessment of sacroiliitis in adolescents with enthesitis-related arthritis. Br J Radiol 89(1059):20150775.
Morbée L, Jans LBO, Herregods N (2022) Novel imaging techniques for sacroiliac joint assessment. Curr Opin Rheumatol 34:187–194.
Diekhoff T, Greese J, Sieper J et al (2018) Improved detection of erosions in the sacroiliac joints on MRI with volumetric interpolated breath-hold examination (VIBE): results from the SIMACT study. Ann Rheum Dis 77:1585–1589.
Magni-Manzoni S, Malattia C, Lanni S, Ravelli A (2012) Advances and challenges in imaging in juvenile idiopathic arthritis. Nat Rev Rheumatol 8:329–336.
Wolharn L, Guggenberger R, Higashigaito K et al. (2022) Detailed bone assessment of the sacroiliac joint in a prospective imaging study: comparison between computed tomography, zero echo time, and black bone magnetic resonance imaging. Skeletal Radiol 1–9
Li Y, Xiong Y, Hou B et al (2022) Comparison of zero echo time MRI with T1-weighted fast spin echo for the recognition of sacroiliac joint structural lesions using CT as the reference standard. Eur Radiol 32:3963–3973.
Jans LB, Chen M, Elewaut D et al (2021) MRI-based synthetic CT in the detection of structural lesions in patients with suspected sacroiliitis: comparison with MRI. Radiology 298:343–349.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of Ghent University Hospital, Belgium. Written informed consent was achieved by all children and parents.
Conflicts of interest
Dr. Jaremko is supported by a Canada CIFAR AI Chair and Medical Imaging Consultants. All other authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herregods, N., Anisau, A., Schiettecatte, E. et al. MRI in pediatric sacroiliitis, what radiologists should know. Pediatr Radiol 53, 1576–1586 (2023). https://doi.org/10.1007/s00247-023-05602-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-023-05602-z