Abstract
Fetal central nervous system MRI is a well-established method to complement a high-quality fetal ultrasound and to clarify sonographically detected abnormalities in complex pregnancies. However, there is still worldwide heterogeneity and confusion regarding the indications of fetal central nervous system MRI, which has roots in differences among countries regarding the performance of ultrasound examinations and legislation on pregnancy termination. The purpose of this article is to clarify the indications for fetal central nervous system MRI by focusing on the ultrasound findings that guide further investigation with MRI and highlight the strengths and the weaknesses of each modality on imaging the fetal central nervous system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ultrasonography is the primary technique for assessing the fetal brain, an area of particular concern due to the relatively high frequency of developmental abnormalities (approximately 3/1,000 pregnancies), many of which affect the child’s outcome. Despite the high performance of ultrasound in highly trained operators with ultra-advanced machines, some abnormalities may be overlooked. This happens due to poor local conditions, such as pelvic engagement of the fetal head, maternal obesity or twin pregnancies, to technical difficulties encountered during the third trimester when the cranium is more ossified, or even to the nature of some anomalies, which makes them hardly detectable on ultrasound but better appreciated on fetal MRI [1]. Following the impressive technical advent of MRI technology during the last three decades, fetal MRI has been established as a specialized tool, complementary to an optimal ultrasound in complex pregnancies, and evaluation of the fetal central nervous system has been historically the main indication [2]. In recent years, 3.0-tesla (T) MRI has provided higher resolution and better signal-to-noise ratio with superior tissue contrast compared to 1.5-T MRI units [3]. Interestingly, prenatal neurocounseling remains challenging, despite all these diagnostic weapons, as the accuracy of each modality and the natural course of certain pathologies remain uncertain; these factors result in possible disagreement between pre- and postnatal imaging [4]. Additionally, there is still confusion regarding the indications and the timing of fetal MRI performance. Setting the indications for fetal MRI is quite demanding because some of them depend entirely on the quality of the ultrasound, which is very heterogeneous worldwide [5]. On the other hand, the timing of fetal MRI performance is strongly influenced by local legislation regarding the legal limit for pregnancy termination, which is different among countries. This eventually affects the diagnostic accuracy of fetal MRI because certain central nervous system pathologies become evident late in pregnancy [2, 5]. The purpose of this article is to clarify the indications for routine fetal central nervous system MRI focusing mainly on the sonographic findings that trigger further imaging investigation. On purpose, some potential applications of MRI, which are currently studied in a research context, are not discussed.
Fetal central nervous system imaging
Fetal ultrasound is the fundamental imaging modality of the fetal central nervous system while fetal MRI reserves a complementary role. Ultrasound presents absolute technical advantages over MRI: it is not limited by fetal motion, it shows superiority in real-time fetal study and vessel flow evaluation with color Doppler study, it is more pleasant, it is easily accessed and repeated, it is inexpensive, it presents better spatial resolution than MRI and it depicts calcifications more confidently.There are, however, local conditions that limit performance of even a high-quality ultrasound, some of which are identical despite the anatomical area examined, i.e. oligohydramnios, maternal obesity, multiple gestations and fetal positioning. Certain technical sonographic limitations are specific to the fetal central nervous system, such as poor beam penetration through the ossified calvarium during the third trimester, resulting in reverberation artifact that obscures detail from the hemisphere proximal to the transducer; poor analysis of the posterior fossa structures, mainly the brainstem; and poor visualization of the skull base structures. However, some abnormalities are genuinely less conspicuous on ultrasound, including white matter myelination or neuron migrational disorders [1]. However, the sonographer’s skills play a pivotal role in the quality of fetal ultrasound, even in the absence of other technical difficulties. They not only affect the accuracy of the ultrasound but also the added diagnostic value of fetal MRI, making it difficult to draw statistically significant results by comparing results from different institutions or countries.
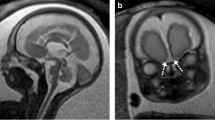
On the other hand, MRI is not hampered by maternal body habitus, oligohydramnios or fetal presentation, although it is frequently associated with maternal anxiety in advanced gestational age. It is not operator dependent, in contrast to ultrasound, which is affected by the sonographer’s technical skills. MRI has higher contrast resolution than ultrasound and provides supra- and infratentorial brain measurements [2]. Additionally, it shows superiority in identifying hemorrhage, ischemia or small cavitary lesions, while it maps the involvement of adjacent structures [6]. It easily produces images on three planes, which are better appreciated by clinicians and parents, showing superiority in revealing anomalies or structures that require sagittal views [6]. Certain skull base structures, such as the olfactory bulbs, the pituitary stalk and gland, and the semicircular canals (Fig. 1), are appreciable on MRI, which alters the characterization of specific anomalies [2].
Normal fetal brain structures seen clearly on MRI but poorly — or not at all — on US. a, b Coronal T2-weighted MR slices at 30 weeks of gestation show normal olfactory bulbs (a, arrows) and optic chiasm (b, arrow). c A midsagittal T1-weighted MR slice at 33 weeks of gestation marks the pituitary gland as an area of diffusely increased signal intensity corresponding to neuro- and adenohypophysis (arrow). d An axial T2-weighted MR slice at the level of the petrous bone at 32 weeks of gestation illustrates normal configuration of semicircular canals (arrows)
The relative advantages of MRI over ultrasound include the evaluation of diffuse white matter abnormalities, the good visibility of the cortical ribbon, especially during the end of the third trimester when the pericerebral fluid spaces are thin, and the advantageous depiction of the posterior fossa structures, mainly the brainstem [2]. Overall, its performance, in contrast to ultrasound, is improved with the advent of gestation, as the fetal size increases and motion is minimized while at the same time there is impressive evolution of the fetal brain [6].
Fetal central nervous system magnetic resonance imaging
Fetal MRI is well accepted by clinicians as a useful imaging adjunct for the fetal brain [7]. Despite reports highlighting increased maternal stress because of the request for a fetal MRI scan, the prospective MERIDIAN (MRI to enhance the diagnosis of fetal developmental brain abnormalities in utero) study has shown that patient acceptability for fetal MRI is high (95–97%) as the relative population is usually “information hungry” in order to decide about the course of their pregnancy [7].
The timing of fetal MRI performance is strongly affected by local legislation, depending on the legal limit for pregnancy termination in different countries. Overall, early examination of the brain, usually guided by increased parental anxiety and pressure, is not recommended because the information collected from the immature brain is limited [1]. The ideal period for performing fetal brain MRI is between 28 weeks and 32 weeks of gestation to allow time for migration and gyration development [1]. However, because some central nervous system abnormalities develop late in pregnancy while others present more pathognomonic features at that time, the optimal gestational age for MRI scanning, in the absence of legal limitation, should be tailored to each individual case accordingly. Thus, structural abnormalities, such as corpus callosum agenesis or posterior fossa anomalies, may be scanned early, whereas evaluation of gyration and parenchyma requires waiting until well into the third trimester [1].
Technical considerations of fetal central nervous system MRI include a light meal before the scan to minimize fetal movement, a friendly environment and comfortable maternal placement in the unit, including toes first, a left lateral decubitus position and the use of phased-array surface coils. Certain institutions apply maternal sedation by oral administration of 1 mg flunitrazepam before the scan [1], although this is not implemented globally. Scan protocol is based on ultra-fast sequences; single-shot fast spin-echo T2-weighted sequences are fundamental and should be acquired in the three planes of the fetal head and spine with 2- to 3-mm slice thickness. The fetal head should be further investigated with T1-weighted axial images, performed breath-hold in breech presentation, and gradient-echo T2* images to better investigate focal lesions, such as hemorrhage and calcifications, with the understanding that these sequences lack anatomical detail [1]. In standard fetal brain MRI, other sequences to consider are diffusion-weighted imaging (DWI) on the axial plane with apparent diffusion coefficient (ADC) mapping to assess fiber development, myelination process and age of ischemic lesions, and less often fluid-attenuated inversion recovery (FLAIR) sequence [1]. Intravenous injection of contrast media is not recommended because gadolinium chelates cross the placenta with unknown effects to the fetus [1].
During the last few years, 3.0-T MRI scanners have enhanced MRI in the diagnostic evaluation of the fetal central nervous system. Given the increased signal-to-noise ratio of these scanners, with appropriate sequence adaptations that reduce susceptibility artifacts, they result in higher imaging resolution with improved image quality and better visualization of small brain structures, keeping radiofrequency energy deposition low [3].
Main indications
Indications of fetal central nervous system MRI, based on literature research and personal experience, can be divided into cases with negative findings and cases with positive findings on prenatal ultrasound, as follows [1, 2, 6, 8]:
-
1.
A familial history of severe brain abnormality in a previous pregnancy, in the absence of possible early genetic testing, to look for subtle similar signs, if this remains questionable or if this is considered easily overlooked from detailed ultrasound (i.e. posterior fossa malformations, gyration anomalies, tuberous sclerosis).
-
2.
In high risk for development of brain abnormalities cases, such as fetal infections with positive amniotic fluid polymerase chain reaction — particularly cytomegalovirus, ischemic events in a stressful maternal environment (twin-to-twin transfusion syndrome, in utero death of a monochorionic twin, maternal use of toxic agents), or other anomalies, such as open dysraphism.
-
3.
In abnormal skull biometry, expressed as increased or decreased circumference and other abnormal biometric sonographic measurements of the brain structures, i.e. length of corpus callosum, transverse cerebellar diameter.
-
4.
One or several brain abnormalities identified by ultrasound, even in the case of a technically suboptimal scan, to clarify whether they are isolated.
A checklist of points for systemic evaluation when reporting a fetal brain MRI is provided in Table 1. A detailed description of anticipated or pathological findings on each abnormality is beyond the scope of this paper.
Maternal cytomegalovirus infections
Congenital cytomegalovirus infections represent a worrying condition for the normal development of the fetal brain, as they may be associated with neurodevelopmental sequelae and hearing loss, especially if seroconversion/fetal infection occurs early in pregnancy. They remain underdiagnosed because systematic screening during pregnancy is lacking in some countries [8, 9]. The current consensus is that serology tests should be offered to pregnant women with flu-like symptoms or when imaging suggests fetal cytomegalovirus infection [9]. In case of positive blood tests, intrauterine infection can be confirmed by polymerase chain reaction detection of cytomegalovirus deoxyribonucleic acid (DNA) in amniotic fluid after the 21st week of gestation and at least 6 weeks after infection. In such cases, a normal ultrasound examination performed by expert sonographers is a good predictor of normal neurodevelopmental outcome [9]. Otherwise, abnormalities detected on ultrasound (ventriculomegaly, microcephaly, subependymal pseudocyst, lenticulostriate vasculopathy, polymicrogyria, intracranial calcifications) raise suspicion of congenital cytomegalovirus infection and should be further investigated with MRI, unless the diagnosis is straightforward on ultrasound with many of the abovementioned lesions present. In the absence of these lesions with a positive amniotic fluid polymerase chain reaction, a fetal brain MRI should be performed to rule out subtle gyration anomalies and/or white matter parenchymal abnormalities [9].
Sonographic findings that require magnetic resonance imaging
When deciding to perform fetal MRI following the identification of abnormalities on ultrasound, we come across crucial questions that needs to be answered: What are we anticipating by exploring the ultrasound finding further with MRI? What is the benefit of a fetal MRI?
Abnormal skull biometry: microcephaly/macrocephaly
The small size of the fetal skull is a factor considerably related to neurodevelopmental outcome. It is measured and compared with normative charts both on ultrasound and fetal MRI [6]. The suggested crucial level of microcephaly, in order to justify the cases that will benefit from the additional information from fetal MRI, is head circumference on ultrasound below the 3rd centile. Fetal MRI is important in order to scrutinize the etiology of isolated microcephaly [2]. Moreover, it presents a substantial advantage over ultrasound in correctly identifying a disproportionately small brain size, termed micrencephaly from the ancient Greek word encephalos/εγκέφαλος meaning brain, because it allows accurate measurements of the brain itself that are not feasible by ultrasound, which can measure the skull only. Micrencephaly may represent a more fundamental issue, especially when encountered in a normal-size skull, where the pericerebral fluid spaces are prominent [7, 8]. Macrocephaly (defined as a head circumference >97th centile) may be related to enlarged pericerebral spaces, and fetal MRI has the potential to differentiate it from true megalencephaly and to examine associated gyration or white-matter anomalies, sometimes seen in overgrowth syndromes [2].
Midline supratentorial anomalies
Ultrasound may suggest midline pathology by depicting an abnormality of a midline structure (corpus callosum, cavum septum pellucidum, interhemispheric fissure) or an abnormal midline lesion (cyst, meningocele).
Corpus callosum agenesis/dysgenesis
Because spatial resolution is increased on ultrasound, the morphology of the corpus callosum is better appreciated in good sonographic conditions than with MRI. The anterior part of the corpus callosum in particular is usually poorly visualized using MRI due to the thinness of the interhemispheric fissure and MRI is usually not helpful for diagnosing corpus callosum partial agenesis or for characterizing callosal dysgenesis [2]. Tubulonodular lipomas are easily depicted by ultrasound and do not require MRI [2]. On the contrary, curvilinear lipomas are much more challenging to diagnose because they may be very subtle on ultrasound, whereas fat T1 intensity is usually absent on fetal MRI. Therefore, particularly in a short corpus callosum, MRI is required to look for abnormal T2 intensity of the lipoma (Fig. 2) [10].
A fetus at 30 weeks of gestation with a curvilinear pericallosal lipoma revealed on ultrasound by a short corpus callosum. a A sagittal T2-weighted MRI slice shows marked hypointensity of the corpus callosum, which is too short with an absence of development of the rostrum and the splenium. Even though no abnormal signal intensity was observed on the T1-weighted sagittal slice (not shown), the marked T2-hypointensity suggested pericallosal lipoma. b A T1-weighted sagittal slice at 3 months of age shows linear hyperintensity lining the corpus callosum, in keeping with a curvilinear lipoma
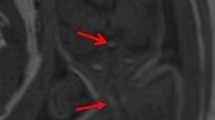
Whenever a callosal anomaly is identified or suspected on ultrasound, MRI is indicated in order to search for possible associated anomalies that could be overlooked, particularly sulcation anomalies, but also abnormalities of the olfactory bulbs, the optic pathways and the pituitary gland because these structures cannot be analyzed by ultrasound (Fig. 1) [1, 2, 4]. MRI is also indicated in midline cysts, which may be associated with callosal or other midline anomalies. It may help depict focal cortical polymicrogyria adjacent to the cyst wall (Fig. 3). It should be underlined, however, that ventriculomegaly, sometimes severe, represents part of corpus callosum agenesis and should not be counted as an associated abnormality to differentiate isolated from non-isolated cases [4].
A fetus at 31 weeks of gestation with partial agenesis of the corpus callosum, huge interhemispheric cyst and dysplastic parenchyma surrounding the cyst. a A coronal T2-weighted MRI shows blurring and thickening of the cortex (arrow) adjacent to the right aspect of the interhemispheric cyst. b A coronal T2-weighted MRI at 6 weeks of age confirms right parietal dysplasia (arrow)
Abnormal septum pellucidum
The cavum septum pellucidum is evaluated during routine screening with fetal second-trimester ultrasound and represents a vital landmark of normal brain development [11]. The absence of cavum septum pellucidum may be observed in septal agenesis or necrotic disruption. It serves as an important marker for several associated brain abnormalities, which can be better demonstrated with MRI. Thus, MRI is warranted in an ultrasound finding of absent cavum septum pellucidum to look for holoprosencephaly spectrum, septo-optic dysplasia and associated schizencephaly [4, 11]. However, the prenatal diagnosis of septo-optic dysplasia is extremely difficult because hypoplasia of the optic chiasm and the optic nerves may either not be appreciable in any modality or may even develop after birth [1]. The cavum septum pellucidum may also be deviated at ultrasound, which should lead to thorough evaluation of the entire brain because such a feature may be observed in tubulinopathies.
Underdevelopment or distortion of the interhemispheric fissure
Interhemispheric fissure can be thoroughly visualized with ultrasound and underdevelopment or distortion can be identified. The interhemispheric fissure is typically underdeveloped in holoprosencephaly as a result of abnormal ventral induction with the failure of midline cleavage of the fetal cerebral hemispheres. Holoprosencephaly presents a heterogeneous range of anatomical abnormalities. Unusual cases of subtle interhemispheric abnormalities, i.e. types of holoprosencephaly with hypothalamic fusion, are better demonstrated with fetal MRI. In any case of interhemispheric abnormality, fetal MRI can supplement the sonographic evaluation by confirming the diagnosis, classifying the severity and recognizing associated brain abnormalities [11]. Distortion of the anterior part of the interhemispheric fissure has been reported in tubulinopathies, which encompass other imaging features: asymmetrical brainstem, cerebellar anomalies, callosal dysgenesis and gyration abnormalities [12]. Some of these findings are better depicted by MRI (Fig. 4).
A fetus at 33 weeks of gestation with tubulinopathy (TUBbb mutation). a A coronal T2-weighted MRI through the frontal lobes shows dysmorphic left frontal horn and asymmetrical insulae, the right one (star) being widely open. b An axial T2-weighted MRI confirms asymmetrical insulae with widening on the right (star) together with asymmetrical ventricles, mild distortion of the cavum septum pellucidum (arrow) and dysmorphic, mildly enlarged left ventricle, c A midsagittal T2-weighted MRI reveals suboptimal bulge of the pons, whose anteroposterior diameter correlates to the 3rd centile. d An axial T2-weighted MRI through the posterior fossa confirms typical asymmetry of the pons (arrow)
Midline encephalocele or meningocele
Encephalocele is a rare malformation characterized by the herniation of brain tissue with or without the meninges through a defect in the cranium. It is classified by location and usually presents associated midline anomalies. Whereas posterior encephalocele can be identified with ultrasound, especially if the fetal head is positioned away from the uterine wall, an anterior meningocele through the ethmoid plate is difficult to identify because it protrudes into the nasal cavity. Coronal thin-slice MR images may set the suspicion of this entity because the associated abnormal configuration of the inferior aspect of the frontal lobes is better appreciated in three-plane imaging.
Ventricular abnormalities
Ventricular abnormalities identified by ultrasound refer to abnormal biometry (ventriculomegaly) or shape (porencephalic communicating cavities, cortical dysplasia), abnormalities of the subependymal area (heterotopia, hemorrhage) and intraventricular anomalies (hemorrhage).
Ventriculomegaly is the most frequent finding of prenatal ultrasound and the most common indication for referral for fetal MRI, yet it is still incompletely understood, with a lot of related controversy, despite having been the focus of much research in the literature [2, 7]. It may be associated with developmental or acquired abnormalities detected with higher sensitivity by fetal MRI rather than ultrasound. Consequently, the definition of isolated to complex ventriculomegaly changes and the prognosis worsens in about 19% of the fetuses [7]. A recent large-scale MRI-based study resulted in the significant correlation of additional MRI-proven central nervous system anomalies with the increase of ventricular width and in bilateral involvement [8]. These findings justify fetal MRI in moderate-to-severe ventriculomegaly (≥12-mm width) on ultrasound.
In subependymal hemorrhage, sonographic findings vary from hyperechoic nodular ependyma, increased periventricular white matter echogenicity to porencephaly. When a porencephalic cyst is formed, there is additional white matter loss with ventricular dilation and abnormal outline in communication between the cyst and the ventricles. MRI is superior in clarifying the location of the cysts (intra-axial in porencephalic cysts versus extra-axial in arachnoid cysts) and their anatomical relationship with the adjacent structures.
MRI is superior to ultrasound in detecting an abnormal ventricular outline in subependymal heterotopias. It plays a key role in demonstrating subependymal nodules and abnormally differentiated cells (tubers) in tuberous sclerosis as T2-hypointense and T1-hyperintense lesions, thus suggesting the possibility of tuberous sclerosis (Fig. 5) [1, 2]. On the other hand, subependymal pseudocysts, located in the wall of the lateral ventricles, typically below the frontal horns, in the caudothalamic notch or lateral to the external angle of the frontal horns, can be easily visualized and accurately measured with ultrasound. However, they should be further investigated with fetal MRI if they are adjacent to the occipital horns or posterior to the caudothalamic notch, and if they present atypical morphology or large size, to rule out additional brain abnormalities in variable associated pathological conditions (hemorrhage, hypoxic–ischemic injury, infections, metabolic diseases, chromosomal abnormalities and, less commonly, toxic intake) [13].
Hyperechogenicity of the choroid plexuses as a result of hemorrhage may be underestimated by inexperienced sonographers or during a third-trimester scan. It will, however, be accurately depicted on fetal MRI performed due to associated ventriculomegaly in T1-weighted and T2* gradient-echo sequences [14, 15].
Parenchymal lesions
Parenchymal lesions are suspected in a stressful fetal environment, such as during twin pregnancies or maternal infections. Because ultrasound shows limitations in identifying small focal or diffuse parenchymal lesions, MRI should be performed to detect small intraparenchymal hemorrhages of different ages, especially with the use of T1-weighted and T2* gradient echo sequences, ischemic cavitary (T2-weighted sequences) and non-cavitary lesions and, very rarely, periventricular calcified leukomalacia (T1 hyperintensities), which may all be missed on ultrasound [1, 2, 4, 14, 15]. MRI has no “hidden” areas underneath the cranium and shows superiority to ultrasound for the identification and the detailed localization of the lesion, which affects the prognosis [4]. Diffuse parenchymal lesions are, however, more difficult to identify. Subsequently, ADC mapping from a DWI sequence has shown value in these cases and should be routinely included in the protocol of fetal brain MRI because it identifies ischemic lesions earlier than conventional T2-weighted images. Therefore, fetal MRI is indicated as soon as there is concern for focal or diffuse parenchymal lesions on ultrasound or whenever the pregnancy is considered at risk for hemorrhagic and/or ischemic lesions [14].
Fetal intracranial tumors are rare. Ventriculomegaly and macrocephaly, together with a mass, are the most common findings observed at ultrasound. Their mixed consistency, with hemorrhage and calcification, is well depicted by ultrasonography, while MRI can better demonstrate their location and extension to adjacent structures.
Cortical abnormalities
Ultrasound presents inherent difficulty in demonstrating cortex and sulcation, which is a good marker of fetal brain maturation [6]. In contrast, fetal MRI is more accurate in assessing sulcal development. Proper timing for fetal MRI is crucial. The suggested period is between 28 weeks and 34 weeks of gestation (optimally, 30–32 weeks of gestation); if fetal MRI is performed too early during the second trimester, some anomalies could be missed because the primary sulcation has not completed yet [1, 8]. If it is performed too late, sulcation can be difficult to evaluate because of the reduction in pericerebral fluid spaces and development of secondary sulcation. On the other hand, diagnosing polymicrogyria with fetal MRI is challenging during the late third trimester of pregnancy, because overfolding of the cortical ribbon may be difficult to differentiate from normal secondary sulci [8]. Therefore, earlier (around 28–30 weeks of gestation) MRI examination is favored when polymicrogyria is suspected.
In schizencephaly, the cleft is lined by polymicrogyria. Such a lesion must be thoroughly searched for in case of absent septum pellucidum or in association with ischemic lesions with or without ventriculomegaly. Diagnosis by ultrasound is challenging, particularly in closed-lip schizencephaly (Fig. 6). Therefore, in such a context, MRI is indicated because it is much more sensitive than ultrasound for delineating the cleft [1].
Posterior fossa
The most common, yet nonspecific, sonographic finding that alerts further detailed investigation of the posterior fossa with MRI is the enlargement of the pericerebellar fluid. It is encountered in several different conditions (mega cisterna magna, Blake pouch, retrocerebellar arachnoid cyst and Dandy-Walker malformation) on the standard axial plane of the second-trimester US [16]. Other ultrasound findings may raise suspicion of posterior fossa malformation: small transverse cerebellar diameter, unilateral small cerebellar hemisphere or abnormal morphology of the 4th ventricle. The precise diagnosis of posterior fossa malformations is based on the analysis of the vermis, the cerebellar hemispheres, the brainstem, the 4th ventricle and the tentorium cerebelli. Ultrasound can evaluate these anatomical structures, but for many reasons (the fetal position, the maternal wall, the possibility of using the posterior fontanelle as an acoustic window, etc.), MRI is often more accurate and should be performed as soon as there is concern for a posterior fossa anomaly. Because MRI may well identify posterior fossa structures by the second trimester, early (from around 22–24 weeks of gestation) application of this modality in questionable cases is advised and three-plane T2-weighted sequences should be performed. However, associated sulcation abnormalities, if present, are only depicted by MRI in the third trimester.
Small unilateral cerebellar hemisphere is usually observed in the setting of destructive lesions that cause prominence of fluid spaces on ultrasound with a rather typical loss of the anatomical structure. However, the prognosis depends on the vermis being involved or not, which requires both ultrasound and MRI analysis [16]. T2-weighted slices must be acquired in three planes, keeping in mind that the vermis may not be visible on the midsagittal view but on an oblique view, due to displacement of its axis in marked reduction of the size of one cerebellar hemisphere (Fig. 7). Gradient echo T2* sequences can show deposits of hemosiderin [17].
While it is difficult to visualize the brainstem on ultrasound, it is well demonstrated and measured on fetal MRI. Thus, when there is concern for pontocerebellar hypoplasia (decreased transverse cerebellar diameter at ultrasound and/or positive family history), MRI should be performed. However, it must be kept in mind that flattening of the pons may appear quite late during the pregnancy and even only postnatally (Fig. 8) [1]. MRI is also useful after ultrasound depicts supratentorial findings raising suspicion for tubulinopathies. In this setting, the typical asymmetrical appearance of the brainstem, which may be overlooked on ultrasound, is clearly demonstrated on axial T2-weighted images.
A fetus at 30 weeks of gestation with poor cerebellar growth and ventriculomegaly due to pontocerebellar hypoplasia. a A midsagittal T2-weighted MRI shows a flattened brainstem without any bulge of the pons. Vermian height corresponds to the 3rd centile. b A coronal T2-weighted MRI shows bilateral ventriculomegaly and small cerebellar hemispheres
Pericerebral spaces
According to their location, some pericerebral lesions, such as subdural hematomas and arachnoid cysts, can be better visualized with MRI because ultrasound inherently has limited visualization of the subcranial areas. However, the border of arachnoid cysts is better depicted by ultrasound than by MRI.
Spinal cord
The spinal cord is more accurately demonstrated by ultrasound than by MRI due to the better spatial resolution of ultrasound and the use of high-frequency probes when the back of the fetus is more superficially placed [2]. Therefore, there is usually no indication for MRI except in poor sonographic conditions. The quality of MR images is hampered by the small size of the spinal cord and the canal, making it impossible to identify details. Thus, when fetal spinal MRI is suggested, advanced gestational age is preferred for imaging if this is allowed by legislation for pregnancy termination. Spinal cord MRI protocol includes three planes of single-shot T2-weighted gradient echo sequences, usually of 2-mm thickness. Imaging is enhanced with axial T1-weighted images. Finally, cine sequences, usually on the sagittal spinal plane, provide appreciation of fetal lower limbs motion, which adds further information regarding fetal motility in large spinal defects and myelomeningocele. The course of the spinal cord, the localization of the conus medullaris and large lipomas can be visualized.
Diastematomyelia is usually easily depicted by ultrasound and can also be visualized by MRI. The bony spur is usually better appreciated by ultrasound, but it can hamper good visibility of the spinal cord. In such cases, MRI may be useful [18].
Because there is strong association of “open” spinal dysraphism and brain malformations (callosal dysgenesis, subependymal heterotopias), reciprocal investigation with fetal MRI is crucial in order to conduct appropriate counseling and prenatal management [8, 18]. Hindbrain herniation through the foramen magnum (Chiari II malformation) is easily depicted by ultrasound and does not require MRI.
Conclusion
We tried to clarify the indications of fetal central nervous system MRI with the knowledge of worldwide variations in the application of prenatal ultrasound and the legislations of pregnancy termination, focusing not only on cases with technical limitations for ultrasound performance (obese mothers, oligohydramnios) and familial history of brain abnormality, but also on sonographic findings that necessitate detailed investigation and further characterization. Prenatal neurocounseling remains challenging and is mainly based on the detailed description of the cerebral anatomy and precise localization of the abnormality, which makes pivotal the role of high-quality prenatal imaging, both ultrasound and MRI. Fetal MRI could help future parents adjust and prepare for the probable outcome. The future challenge of fetal MRI is to add metabolic and functional studies, address the challenge of objectively detecting diffuse white matter lesions and mainly early damage with the help of DWI and ADC mapping, and visualize abnormal white matter tracts with prenatal tractography. Functional MRI can be a useful tool for evaluating blood oxygenation in human fetuses to monitor high-risk fetuses and sensorial fetal activity.
References
Salomon LJ, Garel C (2007) Magnetic resonance imaging examination of the fetal brain. Ultrasound Obstet Gynecol 30:1019–1032
Garel C (2008) Imaging the fetus: when does MRI really help? Pediatr Radiol 38:S467–S470
Krishnamurthy U, Neelavalli J, Mody S et al (2015) MR imaging of the fetal brain at 1.5T and 3.0T field strengths: comparing specific absorption rate (SAR) and image quality. J Perinat Med 43:209–220
Garel C, Moutard M-L (2014) Main congenital cerebral anomalies: how prenatal imaging aids counceling. Fetal Diagn Ther 35:229–239
Cassart M, Garel C (2020) European overview of current practice of fetal imaging by pediatric radiologists: a new task force is launched. Pediatr Radiol 50:1794–1798
Rossi AC, Prefumo F (2014) Additional value of fetal magnetic resonance imaging in the prenatal diagnosis of central nervous system anomalies: a systematic review of the literature. Ultrasound Obstet Gynecol 44:388–393
Griffiths PD, Bradburn M, Campbell MJ et al (2019) MRI in the diagnosis of fetal developmental brain abnormalities: the MERIDIAN diagnostic accuracy study. Health Technol Assess 23:1–144
Griffiths PD, Mooney C, Bradburn M, Jarvis D (2018) Should we perform in utero MRI on a fetus at increased risk of a brain abnormality if ultrasonography is normal or shows non-specific findings? Clin Radiol 73:123–134
Diogo MC, Glatter S, Binder J et al (2020) The MRI spectrum of congenital cytomegalovirus infection. Prenat Diagn 40:110–124
Atallah A, Lacalm A, Massoud M et al (2018) Prenatal diagnosis of pericallosal curvilinear lipoma: specific imaging pattern and diagnostic pitfalls. Ultrasound Obstet Gynecol 51:269–273
Griffiths PD, Jarvis D (2016) In utero MR imaging of fetal holoprosencephaly: a structures approach to diagnosis and classification. AJNR Am J Neuroradiol 37:535–543
Cabet S, Karl K, Garel C et al (2020) Two different prenatal imaging cerebral patterns of tubulinopathies. Ultrasound Obstet Gynecol 57:493–497
Esteban H, Blondiaux E, Audureau E et al (2015) Prenatal features of isolated subependymal pseudocysts associated with adverse pregnancy outcome. Ultrasound Obstet Gynecol 46:678–687
Putbrese B, Kennedy A (2017) Findings and differential diagnosis of fetal intracranial haemorrhage and fetal ischaemic brain injury: what is the role of fetal MRI? Br J Radiol 90:20160253
Garel C, Delezoide A-L, Elmalech-Berges M et al (2004) Contribution of fetal MR imaging in the evaluation of cerebral ischemic lesions. AJNR Am J Neuroradiol 25:1563–1568
Garel C (2010) Posterior fossa malformations: main features and limits in prenatal diagnosis. Pediatr Radiol 40:1038–1045
Massoud M, Cagneaux M, Garel C et al (2014) Prenatal unilateral cerebellar hypoplasia in a series of 26 cases: significance and implication for prenatal diagnosis. Ultrasound Obstet Gynecol 44:447–454
Bulas D (2010) Fetal evaluation of spine dysraphism. Pediatr Radiol 40:1029–1037
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Papaioannou, G., Klein, W., Cassart, M. et al. Indications for magnetic resonance imaging of the fetal central nervous system: recommendations from the European Society of Paediatric Radiology Fetal Task Force. Pediatr Radiol 51, 2105–2114 (2021). https://doi.org/10.1007/s00247-021-05104-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05104-w