Abstract
Ultrasound contrast agent (UCA) use in radiology is expanding beyond traditional applications such as evaluation of liver lesions, vesicoureteral reflux and echocardiography. Among emerging techniques, 3-D and 4-D contrast-enhanced ultrasound (CEUS) imaging have demonstrated potential in enhancing the accuracy of voiding urosonography and are ready for wider clinical adoption. US contrast-based lymphatic imaging has been implemented for guiding needle placement in MR lymphangiography in children. In adults, intraoperative CEUS imaging has improved diagnosis and assisted surgical management in tumor resection, and its translation to pediatric brain tumor surgery is imminent. Because of growing interest in precision medicine, targeted US molecular imaging is a topic of active preclinical research and early stage clinical translation. Finally, an exciting new development in the application of UCA is in the field of localized drug delivery and release, with a particular emphasis on treating aggressive brain tumors. Under the appropriate acoustic settings, UCA can reversibly open the blood–brain barrier, allowing drug delivery into the brain. The aim of this article is to review the emerging CEUS applications and provide evidence regarding the feasibility of these applications for clinical implementation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contrast-enhanced ultrasound (CEUS) has been increasingly utilized in children for a wide variety of indications, including tumor characterization, trauma evaluation and hypoxic–ischemic brain injury [1,2,3,4,5,6,7]. Offering an excellent safety profile, a lack of ionizing radiation, and bedside access, CEUS is an appealing imaging modality, particularly in our sickest and most vulnerable pediatric patients. The clinical use of CEUS in children is gaining momentum and expanding as emerging and evolving techniques and therapeutic applications provide exciting improvements to diagnosis and treatment. Here we introduce some of these emerging applications that are driving the field of CEUS in innovative directions. Specifically, we explore applications such as three- and four-dimensional (3-D/4-D) CEUS imaging, visualization of the lymphatics using CEUS, and intraoperative applications of CEUS. In addition, we briefly present the evolution of CEUS as a therapeutic modality via molecular imaging and drug delivery.
These new applications are opening the window to advanced capabilities that were previously not possible using CEUS. For example, 3-D/4-D CEUS provides the advantage of visualizing perfusion within an entire volume of tissue, organ or lesion rather than the perfusion within just a single two-dimensional (2-D) slice, thereby providing the full picture. The added capability of 3-D CEUS means that the vascular information within these volumes can be better understood and the underlying complexities clearly depicted [8,9,10]. Four-dimensional CEUS adds the dimension of real time to the 3-D acquisition of images. This ability to visualize in real time the 3-D movement of UCA microbubbles within a region of interest is a significant advancement in US imaging.
Lymphatic imaging with CEUS is a newer technique that is being explored as an alternative approach to dynamic contrast magnetic resonance (MR) lymphangiography [11,12,13]. In this application, CEUS provides the advantage of being able to image in real time at the patient bedside, a capability especially beneficial in the pediatric population where it is sometimes challenging for younger patients to undergo dynamic contrast MR lymphangiography.
Intraoperative CEUS involves the use of UCA during an operative procedure to guide, confirm, or obtain real-time diagnostic information during the course of the operation [14,15,16]. Again, the major advantage of intraoperative CEUS is its portability, which allows both easy performance of the study at the child’s bedside and flexible maneuvering of the US transducer to the procedural access points, providing real-time images during the procedure.
Therapeutic applications of CEUS are rapidly evolving and include the ability to perform targeted/molecular imaging and targeted drug delivery [17,18,19,20]. These applications have expanded the capacity of CEUS from a purely diagnostic modality to one with both diagnostic and therapeutic applications. This article reviews these emerging applications and provides supporting evidence regarding the feasibility and effectiveness of these applications for clinical implementation.
Emerging/evolving techniques
Three-dimensional (3-D)/four-dimensional (4-D) ultrasound applications
Three-dimensional US is a longstanding, valuable technology that enables the volumetric visualization of a region of interest instead of the single 2-D US image slice. Four-dimensional US adds the temporal component to 3-D US by capturing a series of 3-D volumes in real time. While 3-D and 4-D US have been used to monitor fetal development for decades, the addition of 3-D/4-D technology to CEUS has made it possible to evaluate the movement of UCA microbubbles within a tissue in multiple dimensions simultaneously. This is a significant factor for studying complex vascular features that cannot be fully appreciated using a single 2-D image slice. Furthermore, 4-D CEUS demonstrates 3-D perfusion behavior in real time. This added functionality provides a more comprehensive picture of the mechanisms under investigation and could aid in making a more informed diagnostic decision.
The current primary application of 3-D/4-D CEUS in children is an effort to augment the diagnostic accuracy of 2-D contrast-enhanced voiding urosonography (VUS). Recent studies showed that the sensitivity of 3-D/4-D contrast-enhanced VUS in vesicoureteral reflux (VUR) detection is comparable to standard 2-D contrast-enhanced VUS [21]. Some studies suggest that 2-D and 3-D/4-D contrast-enhanced VUS detect more reflux than does fluoroscopic voiding cystourethrography (VCUG), but 3-D/4-D techniques might provide additional information and improve the grading accuracy of VUR compared to 2-D contrast-enhanced VUS alone, which can have important clinical implications [21,22,23]. Specifically, 3-D/4-D contrast-enhanced VUS with advanced post-processing options (e.g., rendering, sharpening, contrasting, zooming, rotating the image) as well as artifact removal, can improve depiction of the pelvicalyceal system and ureter by showing visible borders distinct from the surrounding structures, thus offering more detailed morphological visualization of the reflux (Fig. 1) [21, 22]. Such a precise visualization of the collecting system might be of value in children with abnormal renal rotation or position. Another considerable advantage of the 3-D/4-D technique is the capability to document reflux in a manner analogous to fluoroscopic VCUG, e.g., in the anatomical directions of the kidneys. This approach seems to be more comprehensive when interacting with the referring physicians [21,22,23]. Recently, the 3-D/4-D technique has also been applied during the micturition part of the contrast-enhanced VUS examination for improving visualization of urethral abnormalities in both female and male patients [22].
Three- and four-dimensional contrast-enhanced voiding urosonography (VUS). a Sagittal contrast-enhanced VUS in a 7-month-old boy with dual display of two-dimensional (2-D) images of the right kidney in gray-scale (left) and contrast (right) modes demonstrates US contrast agent filling the right renal collecting system (arrow). b, c Three-dimensional (3-D)-rendered contrast-enhanced VUS images of the right kidney in anterior projection (b) and posterior projection (c) demonstrate contrast opacification of the right renal collecting system including the pelvis (arrows), calyces (asterisks) and ureter (arrowheads) to better advantage than 2-D contrast-enhanced VUS static images; the latter images also demonstrate multiple angles, with improved visualization of the calyceal dilation, possibly allowing for more accurate grading of the vesicoureteral reflux
Among the possible limitations of 3-D/4-D contrast-enhanced VUS to be considered are the need to use specific US transducers, limited access to US scanners offering high-quality 3-D/4-D contrast imaging software, additional time necessary for post-processing, and the relatively steep learning curve for performance of the examination and subsequent post-processing techniques. Furthermore, because 2-D and 3-D/4-D contrast-enhanced VUS examinations cannot be performed simultaneously, 3-D/4-D contrast-enhanced VUS can only complement 2-D contrast-enhanced VUS and not replace it for diagnostic purposes; therefore additional examination time is needed to perform both modalities. Finally, it is possible that some of the observed differences between 2-D and the subsequently performed 3-D/4-D contrast-enhanced VUS might in part be explained by the inherently dynamic nature of the reflux phenomenon, whose degree can change over the course of the examination [21]. Three-dimensional contrast-enhanced VUS has specific limitations: static character and higher susceptibility to artifacts. The 4-D contrast-enhanced VUS as a real-time dynamic modality visualizes the pelvicalyceal system better and for a longer time compared to 3-D contrast-enhanced VUS, and thus can be used as the sole volumetric technique, though preceded by 2-D contrast-enhanced VUS (Supplementary Online Material 1) [21]. Furthermore, 4-D contrast-enhanced VUS acquires images faster than 3-D, making it particularly advantageous in pediatrics, wherein a lack of cooperation by children often creates difficulties in good-quality acquisitions. However, the main limitation of 4-D contrast-enhanced VUS is the intrinsic loss of resolution/voxel size, although this loss does not seem to influence the reliability of VUR diagnosis and grading [21,22,23].
Three-dimensional/four-dimensional CEUS could improve the evaluation of focal liver lesions such as focal nodular hyperplasia, hemangiomas, hepatocellular carcinomas and metastases. In adults, tumor vascularity, feeding vessels and enhancement patterns can be visualized with better accuracy with 3-D/4-D CEUS compared to 2-D imaging [8, 9]. Three-dimensional/four-dimensional CEUS has been utilized to guide interventional procedures for tumor local treatment and assessment of treatment response [10, 24, 25]. However, studies describing similar applications for pediatric patients have not been performed.
Contrast-enhanced ultrasound of the lymphatics
Percutaneous interventions for the treatment of lymphatic flow disorders, such as plastic bronchitis and protein-losing enteropathy, have prompted a need for imaging modalities to ensure accurate diagnosis and appropriate follow-up [26, 27]. Dynamic contrast-enhanced MR lymphangiography has become the most common imaging modality for diagnosing these disorders, but CEUS offers an alternative approach for real-time imaging that might aid in interventions and can be performed at the bedside [28, 29].
Lymphatic imaging with CEUS in children is limited, but it has been studied in adults. Most commonly, UCAs have been administered intradermally to identify sentinel lymph nodes. This technique was originally described in swine but has been successfully used for the identification of sentinel lymph nodes in women with breast cancer [13, 30,31,32,33]. The efferent lymphatics in adults have also been directly imaged by UCA injection of inguinal lymph nodes into the groin to confirm needle placement prior to dynamic contrast-enhanced MR lymphangiography [12].
To our knowledge, similar studies using CEUS to perform lymphatic imaging in children have not been published, but similar to the adult application, we have been successfully using CEUS to confirm needle placement prior to dynamic contrast-enhanced MR lymphangiography. When imaging the central lymphatic system with MR lymphangiography, it is necessary to inject a gadolinium-based contrast agent into the inguinal nodes via a needle while dynamically performing MR imaging. Usually verification of needle position and opacification of the efferent lymphatics is performed under fluoroscopy during the injection of water-soluble contrast agent [29]. Using CEUS to confirm needle placement obviates the need for a fluoroscopy suite, allowing needle placement to be performed at the bedside. This is expected to facilitate increased access to this technique and could improve procedure times.
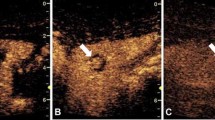
When treating lymphatic flow disorders, the presence or absence of thoracic duct patency is important. For instance, if the thoracic duct is obstructed, the patient might be a candidate for lympho-venous anastomosis [34]. Given that dynamic contrast-enhanced MR lymphangiography cannot definitively determine patency because of the dilution of lymphatic gadolinium-based contrast agent as it enters the venous system, thoracic duct patency is most often confirmed with a lymphangiogram under fluoroscopy. Our group has used CEUS to determine patency of the thoracic duct outlet following direct lymph node injection in children by visualizing lymphatic contrast agent entering the venous system at the left or right venous angle (Figs. 2 and 3) [11]. Using this technique, thoracic duct patency can be assessed at the bedside and without the use of ionizing radiation.
Contrast-enhanced ultrasound (CEUS) for determining thoracic duct patency in a 6-month-old boy who presented with bilateral chylothorax. Transverse CEUS of the right-side neck, with dual display of contrast (left) and gray-scale (right) images, reveals passage of contrast agent (arrowhead) into the right internal jugular vein (arrows), confirming thoracic duct patency. If the thoracic duct had been obstructed, no contrast agent would be seen in this portion of the venous system
Contrast-enhanced ultrasound (CEUS) for determining thoracic duct patency in a 2-month-old boy with chylothorax. Transverse contrast-enhanced ultrasound (CEUS) of the left-side neck, with dual display of the contrast (left) and gray-scale (right), reveals intranodal lymphatic US contrast agent opacifying the left internal jugular vein (arrows), consistent with a patent thoracic duct
Intraoperative contrast-enhanced ultrasound
Intraoperative CEUS is an emerging application that has been used for various organs and pathologies to improve diagnosis and surgical management. In the case of liver lesions, intraoperative CEUS has improved visualization and characterization at the time of resection [35, 36]. Intraoperative visualization of new lesions not detected on pre-surgical planning can alter the surgical approach in real time by identifying new target lesions for resection [15]. Furthermore, studies have reported a strong correlation between CEUS enhancement parameters and the histological grade of resected hepatocellular carcinoma, providing further evidence of its utility in lesion characterization [37].
Similarly, CEUS has been applied to improve visualization and characterization of brain tumors [38,39,40,41]. In the operating room, CEUS has been used to assist neurosurgeons in determining the border between viable intracranial tumor and normal adjacent brain parenchyma [16, 39]. In addition, CEUS enhancement patterns such as perfusion kinetics have been correlated to different histological grades of cerebral gliomas [42]. While histological analysis remains the gold standard, CEUS can be helpful in guiding the region for biopsy, potentially improving the accuracy of the final histological diagnosis. CEUS-based real-time visualization of the feeding arteries and venous drainages in higher-grade tumors could also change intraoperative management by identifying vascular structures at high risk for bleeding complications or the need for more aggressive tumor margins.
Other exploratory uses of intraoperative CEUS include assessment of femoral head perfusion in developmental dysplasia of the hip and perfusion of free-flap transplanted tissues in the operating room to assess viability [14, 43]. The use of CEUS to confirm the viability of surgically reduced or implanted organs and tissues prior to leaving the operating room can prevent repeat surgeries from complications such as avascular necrosis of the femoral head or implanted tissues. Finally, intraoperative contrast-enhanced VUS has also been used to evaluate the treatment of reflux in children to maximize the effectiveness of endoscopic bulking agents, e.g., Deflux injection therapy. Real-time intraoperative assessment of residual reflux following Deflux injection allows for repeated injections to improve the success of the endoscopic treatment [44].
Therapeutic applications
Emerging and evolving therapeutic applications include drug delivery and assessment of treatment response. Prior review articles have extensively covered their status [18, 19, 45,46,47]. Here we briefly discuss molecular imaging and drug delivery applications.
Molecular imaging
Ultrasound contrast agent can be used to target molecular markers of pathological processes, e.g., vascular endothelial growth factor receptor 2 (VEGFR2) in tumor angiogenesis. The contrast agent’s shell can be conjugated to small molecules (e.g., peptides) and antibodies, allowing the targeting and imaging of specific cell surface receptors [47,48,49]. This technique has been investigated for evaluating cardiovascular disease and tumor imaging (Fig. 4) [50,51,52,53,54,55]. In preclinical studies, targeted microbubbles have been used to image specific tumors and their response to anti-angiogenic therapy [17, 19, 20, 46, 56]. In pediatrics, anti-angiogenic treatment monitoring of neuroblastomas could be a promising application for US contrast imaging [57, 58].
Molecular imaging. a, b Representative contrast-enhanced US images depict vascular endothelial growth factor receptor (VEGFR2) targeted microbubble imaging of a control mouse hindlimb (a). Compare (a) to an MC38 colon carcinoma tumor implanted in a mouse hindlimb (b), which demonstrates marked enhancement of the hindlimb tumor. Images courtesy of Alexander Klibanov from the University of Virginia
Drug delivery
The therapeutic index of drugs can be increased by localized delivery at the disease location. Microbubble-based drug delivery systems rely on acoustic manipulation of the agent for local release. Conventionally, a low mechanical index (MI<0.3) is used for diagnostic CEUS because it causes stable microbubble oscillation that allows continuous imaging. In therapeutic applications, a high mechanical index (MI>0.8) is typically used to destroy the microbubbles and release the drug payload for local delivery. The high US pressures induce localized microbubble inertial cavitation and fluid microjetting that can lead to reversible cellular sonoporation (i.e. the formation of nanopores in the membrane) as well as cellular tight junction disruption, facilitating drug delivery to the extravascular space. Microbubble-based drug delivery and release is generally implemented in one of the following ways: (1) co-injection of the UCA and free drug, accompanied by site-specific insonation, or (2) loading or conjugating the drug to the microbubble shell and scanning at the desired location. Microbubble accumulation at the site can be enhanced by attaching targeting ligands to surface of the microbubble. This allows receptor binding and therefore targeted imaging and drug delivery.
Many studies have demonstrated the potential applications of drug delivery. While most have been conducted in pre-clinical animal models or in vitro, phase I trials have been performed in adults with inoperable pancreatic cancer using the chemotherapeutic drug gemcitabine [17, 19, 49, 59]. In addition to altering the composition of the lipid shell of the microbubbles, an active body of research has focused on understanding the influence of acoustic parameters on permeability and intracellular delivery of therapies [45, 48]. Further investigation of the acoustic cavitation processes is required to fully understand the potential of these microbubble-based agents for drug delivery applications. While the higher mechanical indices often utilized in these processes are unlikely to exceed levels observed with B-mode and color Doppler imaging, cavitation-related bioeffects to normal tissues have been observed that must be considered when developing an appropriate safety profile for these innovative techniques [60]. Using microbubbles to achieve drug delivery could play an important role in the treatment of pediatric brain tumors, wherein safe and selective opening of the blood–brain barrier is desirable for maximizing the therapeutic effect.
Conclusion
Contrast-enhanced US offers many benefits in pediatric patients, with potential applications including 3-D/4-D technologies, lymphatic imaging, intraoperative tumor characterization, tissue viability assessment, treatment efficacy improvement in VUR, molecular imaging and targeted drug delivery. Although many of these applications are early in development, most of the literature is on in vitro studies and initial investigations are in adults, future translation and use of these innovative technologies in children appears to be on the horizon.
References
Coleman JL, Navid F, Furman WL, McCarville MB (2014) Safety of ultrasound contrast agents in the pediatric oncologic population: a single-institution experience. AJR Am J Roentgenol 202:966–970
Hwang M, Piskunowicz M, Darge K (2019) Advanced ultrasound techniques for pediatric imaging. Pediatrics 143:e20182609
Laugesen NG, Nolsoe CP, Rosenberg J (2017) Clinical applications of contrast-enhanced ultrasound in the pediatric work-up of focal liver lesions and blunt abdominal trauma: a systematic review. Ultrasound Int Open 3:E2–E7
McCarville MB (2011) Contrast-enhanced sonography in pediatrics. Pediatr Radiol 41:238–242
Xu H-X (2009) Contrast-enhanced ultrasound: the evolving applications. World J Radiol 1:15–24
Yusuf GT, Sellars ME, Deganello A et al (2017) Retrospective analysis of the safety and cost implications of pediatric contrast-enhanced ultrasound at a single center. AJR Am J Roentgenol 208:446–452
Hwang M, De Jong RM, Herman S et al (2017) Novel contrast-enhanced ultrasound evaluation in neonatal hypoxic ischemic injury: clinical application and future directions. J Ultrasound Med 36:2379–2386
Lu Y, Liu B, Zheng Y et al (2018) Application of real-time three-dimensional contrast-enhanced ultrasound using SonoVue for the evaluation of focal liver lesions: a prospective single-center study. Am J Transl Res 10:1469–1480
Lee JC, Yan K, Lee SK et al (2017) Focal liver lesions: real-time 3-dimensional contrast-enhanced ultrasonography compared with 2-dimensional contrast-enhanced ultrasonography and magnetic resonance imaging. J Ultrasound Med 36:2015–2026
Wang Y, Jing X, Ding J (2016) Clinical value of dynamic 3-dimensional contrast-enhanced ultrasound imaging for the assessment of hepatocellular carcinoma ablation. Clin Imaging 40:402–406
Mejia EJ, Otero HJ, Smith CL et al (2020) Use of contrast-enhanced ultrasound to determine thoracic duct patency. J Vasc Interv Radiol 31:1670–1674
Nadolski GJ, Ponce-Dorrego MD, Darge K et al (2018) Validation of the position of injection needles with contrast-enhanced ultrasound for dynamic contract-enhanced MR lymphangiography. J Vasc Interv Radiol 29:1028–1030
Sever A, Broillet A, Schneider M et al (2010) Dynamic visualization of lymphatic channels and sentinel lymph nodes using enhanced ultrasound in a swine model. J Ultrasound Med 29:1699–1704
Back SJ, Chauvin NA, Ntoulia A et al (2019) Intraoperative contrast-enhanced ultrasound imaging of femoral head perfusion in developmental dysplasia of the hip: a feasibility study. J Ultrasound Med 39:247–257
Pace C, Nardone V, Roma S et al (2019) Evaluation of contrast-enhanced intraoperative ultrasound in the detection and management of liver lesions in patients with hepatocellular carcinoma. J Oncol 2019:6089340
Prada F, Perin A, Martegani A et al (2014) Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery 74:542–552
Chowdhury SM, Lee T, Willmann JK (2017) Ultrasound-guided drug delivery in cancer. Ultrasonography 36:171–184
Kiessling F, Fokong S, Bzyl J et al (2014) Recent advances in molecular, multimodal and theranostic ultrasound imaging. Adv Drug Deliv Rev 72:15–27
Yang F, Chen Z-Y, Lin Y (2013) Advancement of targeted ultrasound contrast agents and their applications in molecular imaging and targeted therapy. Curr Pharm Des 19:1516–1527
Sorace AG, Saini R, Mahoney M, Hoyt K (2012) Molecular ultrasound imaging using a targeted contrast agent for assessing early tumor response to antiangiogenic therapy. J Ultrasound Med 31:1543–1550
Woźniak MM, Osemlak P, Ntoulia A et al (2018) 3D/4D contrast-enhanced urosonography (ceVUS) in children — is it superior to the 2D technique? J Ultrason 18:120–125
Woźniak MM, Pawelec A, Wieczorek AP et al (2013) 2D/3D/4D contrast-enhanced voiding urosonography in the diagnosis and monitoring of treatment of vesicoureteral reflux in children — can it replace voiding cystourethrography? J Ultrason 13:394–407
Woźniak MM, Wieczorek AP, Pawelec A et al (2016) Two-dimensional (2D), three-dimensional static (3D) and real-time (4D) contrast enhanced voiding urosonography (ceVUS) versus voiding cystourethrography (VCUG) in children with vesicoureteral reflux. Eur J Radiol 85:1238–1245
Luo W, Numata K, Morimoto M et al (2010) Differentiation of focal liver lesions using three-dimensional ultrasonography: retrospective and prospective studies. World J Gastroenterol 16:2109–2119
Xu HX, De Lu M, Xie XH et al (2010) Treatment response evaluation with three-dimensional contrast-enhanced ultrasound for liver cancer after local therapies. Eur J Radiol 76:81–88
Dori Y, Keller MS, Rome JJ et al (2016) Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation 133:1160–1170
Itkin M, Piccoli DA, Nadolski G et al (2017) Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol 69:2929–2937
Chavhan GB, Amaral JG, Temple M, Itkin M (2017) MR lymphangiography in children: technique and potential applications. Radiographics 37:1775–1790
Dori Y (2016) Novel lymphatic imaging techniques. Tech Vasc Interv Radiol 19:255–261
Cox K, Taylor-Phillips S, Sharma N et al (2018) Enhanced pre-operative axillary staging using intradermal microbubbles and contrast-enhanced ultrasound to detect and biopsy sentinel lymph nodes in breast cancer: a potential replacement for axillary surgery. Br J Radiol 91:20170626
Goldberg BB, Merton DA, Liu J-B et al (2004) Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US. Radiology 230:727–734
Sever AR, Mills P, Jones SE et al (2011) Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol 196:251–256
Zhao J, Zhang J, Zhu QL et al (2018) The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: a prospective study. Eur Radiol 28:1654–1661
Weissler JM, Cho EH, Koltz PF et al (2018) Lymphovenous anastomosis for the treatment of chylothorax in infants: a novel microsurgical approach to a devastating problem. Plast Reconstr Surg 141:1502–1507
Da Silva NPB, Hornung M, Beyer LP et al (2019) Intraoperative shear wave elastography vs. contrast-enhanced ultrasound for the characterization and differentiation of focal liver lesions to optimize liver tumor surgery. Ultraschall Med 40:205–211
Leen E, Ceccotti P, Moug SJ et al (2006) Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg 243:236–240
Arita J, Hasegawa K, Takahashi M et al (2011) Correlation between contrast-enhanced intraoperative ultrasound using sonazoid and histologic grade of resected hepatocellular carcinoma. AJR Am J Roentgenol 196:1314–1321
Engelhardt M, Hansen C, Eyding J et al (2007) Feasibility of contrast-enhanced sonography during resection of cerebral tumours: initial results of a prospective study. Ultrasound Med Biol 33:571–575
He W, Jiang X, Wang S et al (2008) Intraoperative contrast-enhanced ultrasound for brain tumors. Clin Imaging 32:419–424
Kanno H, Ozawa Y, Sakata K et al (2005) Intraoperative power Doppler ultrasonography with a contrast-enhancing agent for intracranial tumors. J Neurosurg 102:295–301
Lekht I, Brauner N, Bakhsheshian J et al (2016) Versatile utilization of real-time intraoperative contrast-enhanced ultrasound in cranial neurosurgery: technical note and retrospective case series. Neurosurg Focus 40:E6
Prada F, Mattei L, Del Bene M et al (2014) Intraoperative cerebral glioma characterization with contrast enhanced ultrasound. Biomed Res Int 2014:484261
Prantl L, Pfister K, Kubale R et al (2007) Value of high resolution ultrasound and contrast enhanced US pulse inversion imaging for the evaluation of the vascular integrity of free-flap grafts. Clin Hemorheol Microcirc 36:203–216
Woźniak MM, Osemlak P, Pawelec A et al (2014) Intraoperative contrast-enhanced urosonography during endoscopic treatment of vesicoureteral reflux in children. Pediatr Radiol 44:1093–1100
Yeh C-K, Kang S-T (2012) Ultrasound microbubble contrast agents for diagnostic and therapeutic applications: current status and future design. Biom J 35:125–139
Kiessling F, Bzyl J, Fokong S et al (2012) Targeted ultrasound imaging of cancer: an emerging technology on its way to clinics. Curr Pharm Des 18:2184–2199
Unnikrishnan S, Klibanov AL (2012) Microbubbles as ultrasound contrast agents for molecular imaging: preparation and application. AJR Am J Roentgenol 199:292–299
Ferrara KW, Borden MA, Zhang H (2009) Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res 42:881–892
Klibanov AL (2006) Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Investig Radiol 41:354–362
Deshpande N, Ren Y, Foygel K et al (2011) Tumor angiogenic marker expression levels during tumor growth: longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology 258:804–811
Kaufmann BA, Lindner JR (2007) Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol 18:11–16
Kaufmann BA, Sanders JM, Davis C et al (2007) Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation 116:276–284
Korpanty G, Carbon JG, Grayburn PA et al (2007) Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res 13:323–330
Lindner JR (2009) Contrast ultrasound molecular imaging of inflammation in cardiovascular disease. Cardiovasc Res 84:182–189
Pysz MA, Gambhir SS, Willmann JK (2010) Molecular imaging: current status and emerging strategies. Clin Radiol 65:500–516
Wang H, Lutz AM, Hristov D et al (2017) Intra-animal comparison between three-dimensional molecularly targeted us and three-dimensional dynamic contrast-enhanced us for early antiangiogenic treatment assessment in colon cancer. Radiology 282:443–452
Tadeo I, Bueno G, Berbegall AP et al (2016) Vascular patterns provide therapeutic targets in aggressive neuroblastic tumors. Oncotarget 7:19935–19947
Zormpas-Petridis K, Jerome NP, Blackledge MD et al (2019) MRI imaging of the hemodynamic vasculature of neuroblastoma predicts response to antiangiogenic treatment. Cancer Res 79:2978–2991
Dimcevski G, Kotopoulis S, Bjånes T et al (2016) A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release 243:172–181
Pitt WG, Husseini G, Staples BJ (2004) Ultrasonic drug delivery — a general review. Expert Opin Drug Deliv 1:37–56
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Yusuf has received speaker fees from Siemens and Bracco.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Online Supplementary Material 1
Coronal four-dimensional (4-D) contrast-enhanced voiding urosonography (VUS) cine clip of the left kidney in a 7-month-old boy demonstrates the dynamic process of vesicoureteral reflux and pelvicalyceal dilation. (MP4 9,067 kb)
Rights and permissions
About this article
Cite this article
Didier, R.A., Biko, D.M., Hwang, M. et al. Emerging contrast-enhanced ultrasound applications in children. Pediatr Radiol 51, 2418–2424 (2021). https://doi.org/10.1007/s00247-021-05045-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05045-4