Abstract
Hodgkin lymphoma and non-Hodgkin lymphoma are common malignancies in children and are now highly treatable. Imaging plays a major role in diagnosis, staging and response using conventional CT and MRI and metabolic imaging with positron emission tomography (PET)/CT and PET/MRI. Cross-sectional imaging has replaced staging laparotomy and splenectomy by demonstrating abdominal nodal groups and organ involvement. [F-18]2-fluoro-2-deoxyglucose (FDG) PET provides information on bone marrow involvement, and MRI elucidates details of cortical bone and confirmation of bone marrow involvement. The staging system for Hodgkin lymphoma is the Ann Arbor system with Cotswald modifications and is based on imaging, whereas the non-Hodgkin staging system is the St. Jude Classification by Murphy or the more recent revised International Pediatric Non-Hodgkin Lymphoma Staging System (IPNHLSS). Because all pediatric lymphomas are metabolically FDG-avid and identify all nodal, solid organ, cortical bone and bone marrow disease, staging evaluations require FDG PET as PET/CT or PET/MRI in both Hodgkin and non-Hodgkin lymphoma. Both diseases have in common issues of airway compromise at presentation demonstrated by imaging. Differences exist in that Hodgkin lymphoma has several independent poor prognostic factors seen by imaging such as large mediastinal adenopathy, Stage IV disease, systemic symptoms, pleural effusion and pericardial effusion. Non-Hodgkin lymphoma includes more organ involvement such as renal, ovary, central nervous system and skin. Early or interim PET-negative scans are a reliable indicator of improved clinical outcome and optimize risk-adapted therapy and patient management; imaging may not, however, predict who will relapse. A recent multicenter trial has concluded that it is usually sufficient for pediatric lymphoma at staging and interim assessment to evaluate children with PET imaging from skull base to mid-thigh. Various systems of assessment of presence of disease or response are used, including the Deauville visual scale, where avidity is compared to liver; Lugano, which includes size change as part of response; or quantitative PET, which uses standardized uptake values to define more accurate response. Newer methods of immunotherapy can produce challenges in FDG PET evaluation because of inflammatory changes that may not represent disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hodgkin lymphoma was first described by Thomas Hodgkin [1] in 1832 in a series of cases with abnormal lymph node and spleen involvement that did not look like a common infection. Non-Hodgkin lymphoma refers to a long list of more than 60 types of lymphoma that are not Hodgkin lymphoma. This monograph reviews the imaging — basic and metabolic — for pediatric lymphoma (Hodgkin lymphoma and non-Hodgkin lymphoma).
Hodgkin lymphoma — anatomical imaging
Hodgkin lymphoma is a distinct neoplasm of the nodal system and differs even from the other lymphomas. Pediatric Hodgkin lymphoma is most common in adolescence and rare in children younger than 5 years. There are several histological variants, the most common of which is nodular sclerosing Hodgkin lymphoma. Often it exhibits dramatic systemic symptoms, has distinct histology combining inflammation with neoplasm and has high responsiveness to therapy [2]. Historically, in the 1960s the disease was almost uniformly fatal [3]. At that time imaging included primarily plain film findings and lymphangiography where contrast agent was injected into small lymphatics in the dorsum of the feet and followed over hours until it reached retroperitoneal lymph nodes; mesenteric and other nodal groups could not be visualized with this method. Diagnostic laparotomy and splenectomy were performed because the spleen was frequently abnormal and at that time there was no easy method to evaluate its involvement or involvement of nodal groups within the abdomen [4].
The introduction of CT allowed for an entirely new perspective with visualization of multiple nodal groups as well as solid organs. It eliminated the need for diagnostic laparotomy and splenectomy, which had become a standard in diagnosis. The more recent use of MRI has allowed in addition exquisite detail of soft-tissue involvement as well as bone and bone marrow detail, much of which is not apparent on CT. Functional imaging with [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) has become the standard of care in the initial evaluation and management of Hodgkin lymphoma because this tumor is very FDG-PET-avid. There now are excellent cure rates and a variety of therapeutic managements [5].
Disease staging is done using the Ann Arbor staging system with Cotswold modifications [6, 7]. Correct staging is crucial because appropriate management is based on staging, with higher stages requiring more aggressive treatment. Imaging provides the basis for staging. A simplified description of the staging system is that Stage I involves a single group of nodes in one location, Stage II involves two regions of nodes on the same side of the diaphragm, Stage III includes nodal areas on both sides of the diaphragm, and Stage IV involves solid organs such as lung, bone or liver.
Several factors other than imaging have an impact on initial characterization of disease prior to starting therapy. Initially children are evaluated for presence of “B” symptoms, i.e. unexplained recurrent fever ≥38 °C in the preceding month, recurrent drenching sweats in the preceding month, or unexplained weight loss of >10% of baseline body weight in the preceding 6 months. Children who exhibit none of these symptoms are considered to have “A” symptomatology. Those with “B” symptoms have a worse prognosis than those who do not (“A”). Bulk disease has for a long period of time been known to be a poor prognostic factor. Certain findings are associated with poorer event-free survival, as well: namely, large mediastinal adenopathy, Stage IV, low albumin <3.5, and fever [8]. Pleural and pericardial effusions were recently discovered to independently be poor prognostic factors for response [9, 10].
Another distinct component of Hodgkin lymphoma involvement is “E” or extranodal lesions. Some only consider lesions such as pleural or pericardial nodules to be “E” disease, whereas others think that invasion of a solid organ such as lung or bone by adjacent nodal disease is considered “E” disease as well [11]. The literature is not clear or well established on this topic; many of the initial descriptions were made prior to more recent advanced imaging. Extranodal lesions are discussed further under specific solid organ discussions to follow.
The imaging criteria presented here for Hodgkin lymphoma are those used by the Children’s Oncology Group (COG) and the European Network for Pediatric Hodgkin Lymphoma (EuroNet-PHL). The COG, a National Cancer Institute–supported clinical trials group, is the world’s largest organization devoted exclusively to childhood and adolescent cancer research working at more than 200 institutions across North America with some in Australia, New Zealand and Europe. More than 90% of children in the United States with cancer are treated at COG member institutions. The EuroNet-PHL similarly is a European organization with institutions from 22 countries participating in trials. Any major differences in imaging approach between the groups are described below; currently meetings are being held between these groups in an attempt to harmonize the approach to diagnosis, therapy and response in pediatric Hodgkin lymphoma [5]. Hodgkin lymphoma is very radiation-sensitive but because of long-term sequelae in children, more optimal solutions for therapy are being sought.
The imaging armamentarium in current protocols includes a posteroanterior (PA) and lateral chest radiograph, contrast-enhanced CT of neck, chest, abdomen and pelvis, and FDG PET/CT scan from skull base to at least mid-thigh. MRI might be used for more detailed evaluation, particularly with bone and soft-tissue assessment in COG trials, but often it is used as the first imaging study by EuroNet-PHL. MRI might become a more universally used modality in the primary evaluation of nodes; however, pending faster higher-resolution sequences, CT of the chest is still needed to evaluate for lung involvement.
Nodal involvement
Historically, the chest radiograph was a vital component of initial evaluation in Hodgkin lymphoma to determine the presence of mediastinal and hilar adenopathy as well as the presence of lung involvement. Large mediastinal adenopathy even early on was defined as the transverse diameter of the mediastinal mass being ≥1/3 the transverse diameter of the thorax at the level of the diaphragm (Fig. 1). Hilar adenopathy is often also present in Hodgkin lymphoma. If it is confluent with the mediastinal contours on plain radiograph, it also can be included in the measurement for the mediastinal mass. If, however, the soft-tissue planes of the hila are well demarcated and separate from the mediastinum, it is not included. Because large mediastinal adenopathy is considered a poor risk factor, its identification is paramount. This criterion for the presence of large mediastinal adenopathy is still used by COG and other groups in the United States. EuroNet-PHL uses CT or MRI volume measurements of the contiguous mediastinal adenopathy, with large mediastinal adenopathy being ≥200 mL. This is done either by software-based volumetry or multiplanar volume reconstruction on CT or MRI: (AxBxC)÷2 (Fig. 2).
Large mediastinal adenopathy, which is one factor relating to poorer prognosis in Hodgkin lymphoma, is measured in Children’s Oncology Group trials on a posteroanterior upright radiograph of the chest and is positive in this 16-year-old boy if the maximal transverse diameter of the mass is >1/3 the diameter of the thorax at the level of the diaphragm
European Network for Pediatric Hodgkin Lymphoma (EuroNet-PHL), the consortium in Europe responsible for cancer trials in pediatric Hodgkin lymphoma, uses volume of mediastinal masses as the criterion for bulk. This can be performed with CT software, seen here on axial CT (a), and axial (b) and sagittal (c) reformatted images, by measuring lengths in three planes and using the formula for volume (axbxc/2), with 200 cc representing bulk. (Courtesy Dr. Dietrich Stoevesandt, University Clinic Halle [Saale], Halle, Germany)
The presence of airway compromise has important clinical implications, particularly if anesthesia or sedation is necessary for nodal or bone marrow biopsy. This could be at the level of cervical trachea, intrathoracic trachea or major airways distal to the carina (Fig. 3).
Airway compromise can occur at multiple levels, as shown here in coronally reformatted CT images in three people with Hodgkin lymphoma, an 18-year-old man with cervical tracheal narrowing secondary to cervical adenopathy (a), a 13-year-old boy with displacement and narrowing of the thoracic trachea from large mediastinal adenopathy (b), and an 11-year-old girl with narrowing of mainstem and peripheral bronchi by subcarinal and hilar adenopathy (c). Abnormal areas are marked (arrows)
Contrast-enhanced CT or MRI is used to evaluate lymph nodes and organs. Peripheral nodes are considered abnormal if they measure >2 cm in longest dimension. Nodes between 1 cm and 2 cm can be considered abnormal if they are FDG-PET-avid. These criteria are identical for both COG and EuroNet-PHL. Peripheral nodal bulk disease by COG criteria is defined as a nodal mass ≥6 cm in maximum length in any plane (Fig. 4). A cluster of small nodes >6 cm, which can easily be separated spatially by imaging, is not considered bulk disease. EuroNet-PHL defines peripheral bulk as having a volume of the largest contiguous non-mediastinal nodal mass ≥200 mL.
Extramediastinal bulk disease is defined as a nodal mass >6 cm in any plane. In this 10-year-old boy with Hodgkin lymphoma, the mass measures only 4.8 cm in transverse dimension axially (a), but it measures 7.0 cm coronally (b), meeting the qualifications for bulk disease. Many adult trials use 10 cm as the criterion for extramediastinal bulk, but this large size would not be practical in the pediatric age range
Traditionally, decrease in the size of nodes or masses by a specified percentage of the CT measurement was the standard for assessing response to therapy, with early response being 50–60% and late response usually 80%. The actual percentage might have varied slightly by protocol. Since documentation of PET avidity for Hodgkin lymphoma and the widespread use of FDG PET, response is now largely based on decrease in FDG uptake, although some protocols still use size criteria.
Solid organ involvement
Organ involvement by Hodgkin lymphoma constitutes Stage IV disease, with a worse prognosis requiring more intensive therapy. The spleen in Hodgkin lymphoma, however, is considered nodal and not a solid organ, and as such its involvement is categorized as Stage III if there is disease above the diaphragm or Stage II if all disease is below the diaphragm. The designation “S” is sometimes written after the stage, e.g., Stage IIIS, if there is involvement above and below the diaphragm including spleen. Splenic lesions might be a single nodule, multiple nodules, or diffuse micronodular disease, with the nodules being well circumscribed or irregularly marginated (Fig. 5). Splenomegaly alone without any focal imaging abnormality is not considered disease. Similarly, if there is diffuse PET avidity in the spleen greater than liver, this is not considered involvement by Hodgkin lymphoma if there are no CT, MRI or ultrasound correlates of focal involvement; in this circumstance another etiology should be considered. In the past, to be considered a complete response, splenic lesions were required to disappear completely or decrease by at least 50% with chemotherapy, varying with the individual protocol. Now resolution of PET avidity is considered full response.
Splenic lesions, all of which are avid on [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET), can be single and well circumscribed, as in these images. Axial CT images of three boys with Hodgkin lymphoma show (a) a single focus of involvement (arrow) in a 15-year-old; (b) multiple and irregular foci with distortion of the splenic surface (arrow) in an 11-year-old; and (c) diffuse and micronodular disease in a 13-year-old, with an overall mottled pattern comprised of very subtle poorly defined areas of low attenuation shown here with several noted (arrows)
Liver involvement with Hodgkin lymphoma is very uncommon and most often when seen is associated with splenic involvement. The lesions that are FDG-PET-avid are usually well-circumscribed low-attenuation foci on CT (Fig. 6) or T1-hypointense/T2-hyperintense on MRI.
Renal involvement at presentation in the pediatric population is extremely rare, as is pancreas involvement. The gastrointestinal tract is very rarely involved in Hodgkin lymphoma; it is more commonly involved in other forms of lymphoma such as Burkitt lymphoma.
Lung evaluation
Lung involvement is defined as the presence of nodules. Here there is slight variation between COG and EuroNet-PHL. Current COG protocols stipulate that any nodule ≥1 cm that is PET-avid or ≥3 nodules 5–10 mm in size with no PET avidity would be considered disease; if there is a single nodule <1 cm but it is PET-avid, this can be considered positive. For the EuroNet-PHL protocols, >2 foci 2–10 mm are considered involved, whether or not they are PET-avid, or at least one focus ≥10 mm is considered involved. These are all considered Stage IV. With therapy, nodules usually decrease in overall size and most often disappear. Nodules infrequently cavitate with therapy or, rarely, show cavitation at presentation (Fig. 7).
Lung nodules in Hodgkin lymphoma may be multiple and small, as seen on coronal CT image in a 13-year-old boy (arrows, a); or large, as seen on coronal CT image in a 16-year-old girl (arrows,b); or, rarely, cavitated post-therapy or even pre-therapy, as seen on axial CT image in a 13-year-old boy (arrow, c)
The lungs can become involved by direct spread from the mediastinum or other nodal group or extension into the interstitium, designated as “E” or extranodal disease [7]. This feature is not defined in the EuroNet-PHL protocol but is considered in the COG protocols. In this case the lung involvement does not upstage the child to Stage IV, which indicates focal nodular lung disease, but maintains its staging with the added designation of “E”. Another component of “E” disease in the thorax is chest wall or bony thoracic involvement where mediastinal adenopathy might extend into the soft tissues between ribs or the parasternal area, or even invade bone. This might be visible on CT but the extent is better seen with MRI or PET (Fig. 8).
Examples of extranodal disease in Hodgkin lymphoma, which is extension from nodal disease into other organs or tissues. a Axial CT shows extension of nodal disease through the chest wall from a mediastinal mass (M; arrow). b Axial [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) shows extension of nodal disease (arrow) through the chest wall in an 11-year-old girl. c, d Invasion of the manubrium by a mediastinal mass on (c) axial CT (arrow) and (d) sagittal FDG PET (arrow) in another 11-year-old girl
Bone involvement
Bone involvement with Hodgkin lymphoma is the most complex and enigmatic region to evaluate. Focal structural lesions can be lytic, sclerotic or a combination of both. Bone marrow infiltration often shows no abnormality by routine imaging with plain film or CT. Both structural lesions and bone marrow infiltration are considered Stage IV. Bone also can be involved as an “E” lesion where there is erosion of bone by an adjacent mass density (Fig. 8).
Historically, as part of the workup bone marrow biopsies were routinely performed. With the use of FDG PET in recent years, it has been determined that there is very good correlation of FDG PET avidity and bone marrow infiltration. Most adult trials no longer perform bone marrow biopsies but consider three positive foci by FDG PET to be presumptive of disease [12]. The EuroNet-PHL no longer performs bone marrow biopsies and considers two FDG-PET-positive bony foci to be consistent with involvement [13]. COG trials still perform bone marrow biopsies, but even when biopsies are negative for Hodgkin lymphoma but there are a minimum of three FDG-PET-positive bony foci with no associated CT abnormality, the child is considered to have bone marrow infiltration. MRI can be helpful in diagnosing marrow involvement in the absence of CT bony change but with positive PET findings, for instance if there is only a single FDG-PET-avid bony focus.
Focal lesions can be purely lytic or have some sclerotic margins. Often soft-tissue mass is present within the bone or extending outside its margins. This in part might be visualized by CT but is seen more exquisitely by MRI (Fig. 9). Some bony lesions are purely sclerotic or “blastic” in appearance (Fig. 10). These usually are not associated with a soft-tissue mass component.
The locations of bony involvement in order of frequency are thoracolumbar spine, pelvis, rib, femur and sternum [11]. Other extremities and cervical spine are much less common. FDG PET and MRI, however, are sensitive enough that lesions not visualized by older imaging modalities are now easily apparent and alter many prior statistics.
After therapy, very small lytic lesions might show complete healing with no residual lesion seen. Larger lytic and combined lytic with sclerotic rim lesions might show some healing with better-defined margins, but often they do not resolve by imaging even months to years later (Fig. 11). Lesions that are FDG-PET-negative are considered inactive. Totally sclerotic osseous foci might show very little change anatomically over time.
Lytic lesions pre- and post-therapy. a Pre-study imaging in a 15-year-old boy with Hodgkin lymphoma demonstrates a lumber vertebral body lytic lesion on axial CT. b Immediately post chemotherapy the cortical margins are restored and the lytic areas are smaller and better marginated on axial CT, at which time the [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) scan was negative. c Follow-up axial CT exam 15 months after the completion of therapy — at which point there was no evidence of active disease by FDG PET — shows further repair, but some structural abnormality persists
For COG protocols, technetium bone scans are no longer used because their overall usefulness is superseded by FDG PET or MRI. The EuroNet-PHL occasionally uses bone scans if other imaging is not available at a particular institution.
Other extranodal considerations
The Waldeyer ring is a ring of lymphoid tissue in the pharynx composed of palatine tonsils, adenoids and lingual tonsil, with small amounts of additional intervening lymphoid tissue, any of which can be involved by Hodgkin lymphoma. The quality of CT is very detailed, with excellent delineation of soft-tissue planes, often confirming normal tissue. This area, however, presents a distinct issue in the pediatric age because young children, even more than adolescents, often have inflammatory changes in this region associated with common viral or bacterial infections. Both Hodgkin lymphoma and inflammation cause FDG-PET avidity. Other complicating factors are that cervical adenopathy is the most common location of nodes in Hodgkin lymphoma [14] and after chemotherapy children can become less immune-competent and thus more vulnerable to infection, which can cause some difficulty in discerning tumor from other etiologies [15]. Combined COG and EuroNet-PHL harmonization group recommendations to evaluate the Waldeyer ring are forthcoming.
Both pleural and pericardial effusions have been found to be independent poor prognostic factors and thus their identification at the time of diagnosis is significant [10]. Pleural effusion occurs in 7–30% of cases of Hodgkin lymphoma. Statistically, most are chylous and only very few have malignant cells; most resolve with chemotherapy. Very few are associated with pleural nodules. A recent study evaluating 1,423 children and adolescents with intermediate-stage Hodgkin lymphoma [9] showed association with large mediastinal adenopathy, older age, nodular sclerosis histology, stage, slower response to chemotherapy, and increased incidence of relapse. Many with large mediastinal adenopathy demonstrated pleural fluid only at the lung apices. The incidence of pericardial effusion is similar to that of pleural effusion in Hodgkin lymphoma, with reports in the literature of 5–24%, but more pericardial effusions than pleural effusions have malignant cells present [10]. Review of the same 1,423 children also showed association with large mediastinal adenopathy, “B” symptoms, older age, bulk disease, nodular sclerosing histology, and slower response to therapy. One report involving pediatric patients recorded a large percentage with pericardial thickening associated with the effusions [16]. Often, however, anterior mediastinal, hilar and subcarinal adenopathy abut the pericardium, and it can be difficult to establish involvement.
Vascular involvement
Vascular compression can be produced by large mediastinal masses, and because of the high incidence of mediastinal adenopathy in children with moderate- and high-risk Hodgkin lymphoma, its occurrence is common as well. In a study evaluating pleural effusions, it was discovered that because the superior vena cava (SVC) is quite lateral in the superior mediastinum, it is more frequently displaced farther laterally rather than being completely surrounded by mass (62% displaced vs. 38% surrounded) [9]. Although this is a risk for SVC syndrome, it is reported only in small numbers. The left innominate vein, on the other hand, is much more commonly circumferentially surrounded by mass (13% displaced vs. 87% surrounded) [8]. Most in this study had the lumen of both vessels at least mostly restored by end of chemotherapy (Fig. 12), but a small number persisted, some of which had resultant collateral venous circulation [8].
Vascular compression by adenopathy in Hodgkin lymphoma. a Axial contrast-enhanced CT image of an 18-year-old woman shows compression of the superior vena cava (SVC) by mediastinal mass (arrow). b Post-therapy, an axial contrast-enhanced CT image shows that the caliber of the SVC is restored (arrow). c Axial contrast-enhanced CT image of a 17-year-old boy shows left innominate vein is obliterated by surrounding mediastinal adenopathy (arrow). d Post-therapy, an axial contrast-enhanced CT image shows restored vascular patency (arrow)
Non-Hodgkin lymphoma — anatomical imaging
Non-Hodgkin lymphoma, although uncommon in children, newly affects approximately 750–800 children yearly in the United States [17]. This is only about 1% of the occurrence in adults, where in 2015 there were about 72,000 new cases of non-Hodgkin lymphoma (50,000 men and 32,000 women) and 20,000 deaths [18]. There are numerous subtypes of pediatric non-Hodgkin lymphoma. The principal subtypes by incidence are Burkitt and Burkitt-like lymphomas (35–40%), precursor T cell (15–20%), diffuse large B cell (15–20%), and anaplastic large cell lymphoma (15–20%). Indolent lymphomas, common in adults, are rare in children.
In pediatrics non-Hodgkin lymphoma comprises mature B cell non-Hodgkin lymphoma (predominantly Burkitt lymphoma and diffuse large B cell lymphoma [DLBCL]), lymphoblastic lymphoma, anaplastic large cell lymphoma, and post-transplant lymphoproliferative diseases [19]. Non-Hodgkin lymphoma is more common overall, accounting for 90% of all lymphomas. Non-Hodgkin lymphoma is more common than Hodgkin lymphoma in children, representing about 60% of pediatric lymphoma vs. 40% for Hodgkin lymphoma [1].
Staging of non-Hodgkin lymphoma has been based since 1980 on the St. Jude classification by Murphy [20]. This staging system had been devised as a modification of the Hodgkin disease Ann Arbor classification to allow for a diverse group of diseases that encompass non-Hodgkin lymphoma and the increased extranodal involvement. Stage I included a single nodal or extranodal site but excluded thorax and abdomen; Stage II included a single extranodal tumor site with regional involvement, two or more nodal areas on the same side of the diaphragm, two single extranodal tumors with or without regional node involvement on the same side of the diaphragm, or a primary resectable gastrointestinal tumor (ileocecal area usually involved) with or without mesenteric nodes; Stage III included two single extranodal tumors on opposite sides of the diaphragm, two or more nodal areas above and below the diaphragm, primary intrathoracic tumors involving mediastinum, pleura, thymus, intraabdominal disease, paraspinal or epidural tumors, and any of these sites with central nervous system (CNS) or bone marrow involvement; Stage IV included disease in the bone marrow or CNS, with or without other sites of involvement [20].
A revised International Pediatric Non-Hodgkin Lymphoma Staging System (IPNHLSS) was published in 2015 to account for new findings of organ involvement that include mucosal sites, skin, bone, ovary and kidney, and to account for advances in imaging techniques [21]. Stage I now includes a single site, nodal or extranodal, but also designates whether there is skin or bone involved; Stage II now excludes multiorgan involvement irrespective of position relative to diaphragm; Stage III includes two or more extranodal sites irrespective of position relative to diaphragm, designation as to skin, bone, lymph node, ovary or kidney involvement, and intrathoracic and spinal tumor; Stage IV includes any of the previous sites with CNS or bone marrow disease and is dependent on their method of confirmation. For bone marrow involvement this includes more detection methods to include bone marrow morphology, immunohistochemical or flow cytometry methods, cytogenetic or fluorescence in situ hybridization (FISH) analysis, and polymerase chain reaction (PCR)-based molecular methods. For CNS involvement this includes the use of advanced MR and CT imaging [21].
There is a histologic arbitrary distinction between non-Hodgkin lymphoma and leukemia based on percentage of infiltrated malignant cells identified on bone marrow aspirate. The diagnosis is non-Hodgkin lymphoma when marrow aspirate shows 5–25% marrow involvement, and it is acute leukemia when there is more than 25% malignant cells identified [17].
Distinguishing Hodgkin from non-Hodgkin lymphoma on anatomical imaging
Non-Hodgkin lymphoma usually presents as aggressive disseminated disease or as a rapidly growing mass. Vascular compression of the SVC or inferior vena cava (IVC) might be present. Other presentations could include acute airway compression, spinal cord compression (Fig. 13), or pericardial tamponade. Non-Hodgkin lymphoma more than Hodgkin lymphoma can have a gastrointestinal presentation that includes intussusception or intestinal obstruction (Fig. 14). The radiologic manifestations suggest non-Hodgkin lymphoma diagnosis more than Hodgkin lymphoma if these are present. Initial evaluation for non-Hodgkin lymphoma includes contrast-enhanced CT chest, abdomen, and pelvis or similar-site MRI. Optimized contrast-enhanced PET/CT or PET/MRI are then the study of choice for staging and response assessment [17, 22].
Spinal cord involvement in a 7-year-old boy who presented with back pain and leg and foot weakness. a Sagittal T2-W MR image shows a homogeneously enhancing extradural mass from T5 to T10 in the thoracic spinal canal; it was biopsy proven Burkitt non-Hodgkin lymphoma. b–d Axial [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) MRI T1-W post contrast (b), FDG PET (c) and fused PET/MRI (d) show metabolic FDG activity in the thoracic spinal mass (arrows). e Whole-body coronal FDG PET maximum-intensity projection image also shows uptake in the thoracic spinal mass as well as a large right jugulodigastric node (arrow)
Gastrointestinal involvement in a 14-year-old boy. a The boy presented with large left axillary nodal mass on coronal CT (arrow). b Coronal [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET)/CT image shows, in addition to axillary nodal uptake (arrow), diffuse abdominal disease with bowel wall thickening. There was intense FDG activity at staging caused by Burkitt non-Hodgkin lymphoma (chevrons in a and b)
Rapidly enlarging neck and mediastinal adenopathy is a typical presentation for large B cell lymphoma. T cell lymphoblastic lymphoma more commonly presents with large mediastinal mass. Bone and skin involvement are more common in large B cell lymphoma as compared to T cell lymphoblastic lymphoma.
While most of the imaging criteria described for Hodgkin lymphoma can be applied for non-Hodgkin lymphoma, two important life-threatening conditions occur more commonly with non-Hodgkin lymphoma and might be anticipated by their imaging presentation. These include airway obstruction from large mediastinal mass and tumor lysis syndrome caused by large burden of disease.
In the presence of large mediastinal mass, the risk of airway compromise with the child in the supine position must be considered when planning diagnostic evaluation. Modification of cross-sectional imaging (i.e. the child lying on his or her side) and possible biopsy (upright position) that are non-sedated or with minimal anesthesia might be required to get needed staging evaluation. In a study by Anghelescu et al. [23] of 118 pediatric patients with large mediastinal masses, 9.4% had an anesthetic-related complication that was predicted by the features of orthopnea, upper body edema, great vessel compression and mainstem bronchus compression. In another study, by Ng et al. [24], that included among their cases 23 children with non-Hodgkin lymphoma and mediastinal mass, 15% of non-Hodgkin lymphoma patients had anesthesia-related complications including 2 deaths. The most significant risk factor was tracheal or bronchial compression as assessed by chest radiograph or CT scan. The entire team including oncology, anesthesia and radiology needs to be aware of potential risks in these children and work together to determine how best to obtain the needed evaluations while minimizing risk to the child (Fig. 15).
Anesthetic complications in a 15-year-old boy who presented with neck swelling and cough. a, b Anteroposterior (a) and lateral (b) chest radiographs identify a large anterior mediastinal mass. c Subsequent contrast-enhanced axial CT scan shows the large mass to be encasing vessels in the upper mediastinum and significantly narrowing the airway (arrow). The diagnosis was T cell lymphoblastic lymphoma. Critical anesthetic complication occurred at time of line placement
Tumor lysis syndrome is caused by the breakdown of malignant cells and is characterized by hyperuricemia, hyperphosphatemia, hyperkalemia and hypocalcemia. Clinical sequelae can include acute kidney injury, cardiac arrhythmias, seizures and death. Alavi et al. [25] described the increased likelihood of tumor lysis syndrome in people with non-Hodgkin lymphoma on chemotherapy who had renal involvement. CT and ultrasound were the imaging methods to determine renal involvement [25].
The main use of imaging in lymphoma now relates to the use of metabolic imaging for staging and response assessment and this is discussed in the following sections for both Hodgkin lymphoma and non-Hodgkin lymphoma.
Metabolic imaging of Hodgkin disease
Role of [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) in Hodgkin lymphoma
FDG PET has been used effectively for staging and treatment response assessment in Hodgkin lymphoma because of the disease’s inherent status as a highly FDG-avid lymphoma type. FDG PET has been invaluable as a noninvasive functional imaging tool to assess sites of viable lymphoma versus treated disease, allowing for increased sensitivity for staging sites of lymphoma as well as allowing for risk-adapted therapy to optimize patient management and improve clinical outcomes for children with Hodgkin lymphoma [26].
Early negative scan as a reliable indicator for therapy response
Children with Hodgkin lymphoma who have a negative FDG PET in response assessment have been shown to have excellent prognosis, with an early negative FDG PET providing a reliable indicator for therapy response. However, there is poor positive predictive value in early and late chemotherapy response assessment by FDG PET in pediatric Hodgkin lymphoma for predicting disease relapse [27,28,29].
An early retrospective pediatric oncology study reported the value of negative FDG PET during early interim therapy and at end of chemotherapy, and concluded that a negative PET scan is more representative of disease remission than a positive PET is diagnostic of relapsed Hodgkin lymphoma [29]. The first reported prospective study, by Furth et al. [28], in pediatric patients with Hodgkin lymphoma assessing interim post-cycle 2 of chemotherapy and end-of-chemotherapy course FDG PET response reported that children with a negative FDG PET scan had an excellent prognosis while those with a positive FDG PET scan had an increased risk for relapse. Interim PET had high sensitivity (100%) and high negative predictive value (NPV; 100%), with moderate specificity (68%), low positive predictive value (PPV; 14%) and overall accuracy of 70% [28]. Specificity for predicting relapse was significantly higher at interim FDG PET compared to conventional imaging modalities. Similar findings were also reported at end-of-chemotherapy FDG PET (sensitivity 100%, specificity 78%, PPV, 25%, NPV 100%, accuracy 79%), also with significantly high specificity for predicting relapse compared to conventional imaging [28]. In this study, FDG PET assessment was performed visually and a PET-positive scan was considered to have focal increased FDG uptake above reference surrounding tissue or increased uptake greater than mediastinal blood pool activity [28].
Evolution of visual response criteria to Deauville score
There has been an evolution over time of visual FDG PET criteria to more consistently and reliably determine an FDG-PET-positive or -negative signal to represent sites of viable or treated lymphoma (Table 1) [27, 30,31,32,33,34,35]. The most stringent early criteria established a negative PET as FDG uptake above local background at sites of previously FDG-avid lymphoma, whereas all other uptake, including faint residual uptake, was considered positive by PET [34]. The revised International Working Group (IWG) criteria proposed in 2007 used mediastinal blood pool as reference FDG PET activity and incorporated the size of the mass PET partial-volume artifact in which smaller lesions under-represent the level of FDG uptake. In the IWG criteria, if a mass is ≥2 cm in greatest transaxial diameter, it is considered PET-positive if FDG uptake is greater than mediastinal blood pool activity. If a mass is <2 cm in greatest transaxial diameter, then a positive PET lesion is considered above that of surrounding background [31, 32]. The Gallamini criteria, also proposed in 2007, proposed positive PET uptake as focal FDG uptake with clearly increased activity relative to the mediastinal blood pool, and maximum standardized uptake value (SUVmax) greater than 3.5, regardless of the size of the residual mass [30]. The Deauville criteria in 2009 (originally called London criteria) established a 5-point visual scale using both a reference physiological liver and mediastinal blood pool activity [36, 37]. The Deauville criteria are scored as follows: 1 is no uptake above surround background, 2 is ≤ mediastinal blood pool, 3 is > mediastinal blood pool and ≤ physiological liver activity, 4 is moderately increased compared to physiological liver activity, and 5 is markedly increased compared to physiological liver activity (Figs. 16 and 17) [33]. A study by Le Roux et al. [34] comparing outcome based on these different visual FDG PET criteria confirmed the high NPV of interim-chemotherapy PET for predicting treatment response in Hodgkin lymphoma, recommending the use of Gallamini and Deauville (a.k.a. London) criteria. The 5-point Deauville criteria are now incorporated as the visual FDG PET assessment score in the Lugano criteria (see Lugano criteria section) [27, 35].
Example of Hodgkin lymphoma [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) complete metabolic response to interim therapy with Deauville score. a Baseline pre-therapy coronal FDG PET/CT shows Deauville 5, intense FDG uptake in mediastinal and abdominal retroperitoneal lymphadenopathy (arrows). b Post-cycle 2 coronal PET/CT shows Deauville 2, a favorable response with no abnormal FDG uptake at previous FDG-avid sites of mediastinal and abdominal retroperitoneal lymphadenopathy
Example of Hodgkin lymphoma [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) partial metabolic response to interim therapy with Deauville score. a Baseline pre-therapy coronal FDG PET/CT shows Deauville 5, intense FDG uptake in mediastinal and abdominal retroperitoneal lymphadenopathy (arrows). b Post-cycle 2 coronal and transaxial FDG PET/CT shows residual Deauville 5 retroperitoneal lymphadenopathy (arrows), compatible with residual lymphoma refractory to therapy
Application of Deauville criteria for response assessment
The Deauville score threshold for determining a positive or negative FDG PET site can vary depending on the clinical application, either de-escalation to avoid undertreatment or escalation to avoid overtreatment [27]. A reference liver FDG PET threshold can be used to consider FDG uptake at sites higher than liver to be PET-positive for lymphoma (PET-positive Deauville score of 4 or 5, versus PET-negative Deauville score of 1, 2 or 3); this can be used in a conservative interpretation and might be appropriate for interim chemotherapy PET response assessment. Alternatively, a more sensitive interpretation, which might be more appropriate for end of chemotherapy, could use mediastinal blood pool as a reference threshold to consider FDG uptake in sites higher than mediastinum uptake as PET-positive for lymphoma (PET-positive Deauville scores 3, 4 or 5 versus PET-negative Deauville 1 or 2).
The application of the Deauville criteria as an easy and relatively reproducible method for interim-therapy FDG PET response assessment was confirmed in a study assessing inter-reader concordance among four centers in a multicenter clinical trial composed of two experienced readers from each center [38]. From a total of 50 patients with baseline and interim FDG PET/CT imaging, there was agreement at all four centers in 44 patients with a conservative reading (PET-positive Deauville score of 4 or 5 versus PET-negative Deauville score of 1, 2 or 3), with agreement reached in 46 patients after discussion of their Deauville score among their sites [38]. With the sensitive reading (PET-positive Deauville score of 3, 4 or 5 versus PET-negative Deauville score of 1 or 2), there was agreement at all four centers on 41 patients, which increased to 44 patients after discussion [38]. The level of agreement among all four centers using non-weighted kappa statistics was very good for a conservative reading at 0.85 (95% confidence interval [CI] 0.74–0.96) and good for a sensitive reading at 0.79 (95% CI 0.67–0.90) [38].
A more recent study, however, reported that the inter-reader reliability of the 5-point Deauville scale was poor, with the greatest inter-reader variability in differentiating a Deauville score of 2 versus 3 [39]. This was not unexpected because of the very narrow range of difference in FDG PET uptake between reference mediastinal blood pool and liver activity differentiating a Deauville score of 2 versus 3, resulting in subjective visual variation. This study assessed baseline and interim therapy FDG PET/CT scans in 100 randomly selected pediatric patients with Hodgkin lymphoma from a multi-institutional clinical trial; these patients were reviewed by five expert readers for retrospective analysis [39]. This study confirmed that a binary conservative reading (PET-positive Deauville score of 4 or 5 versus PET-negative Deauville score of 1, 2 or 3) is the most reliable criterion for clinical decision-making, with a pairwise kappa statistic of 0.559 (range: 0.428–0.710), compared to a kappa statistic of 0.358 (range, 0.189–0.514) for the binary sensitive reading (PET-positive Deauville score of 3, 4 or 5 versus PET-negative Deauville score of 1 or 2) [39].
Lugano criteria
The most recent updated consensus of the International Conference on Malignant Lymphomas Imaging Working Group published the Lugano criteria in 2014 [35], with accompanying detailed imaging recommendations [27]. The 5-point Deauville score is now incorporated in the Lugano criteria as the visual FDG PET assessment score in the Lugano criteria for FDG-avid lymphoma histologies, which include Hodgkin lymphoma. In summary, it recommends use of FDG PET/CT for response assessment in FDG-avid histologies including Hodgkin lymphoma.
In FDG-avid histologies, response assessment is made using FDG PET/CT based on FDG PET regardless of size of residual mass anatomically. For lymph nodes and extralymphatic soft-tissue sites, a complete metabolic response, even with a persistent mass, is considered a complete remission (Deauville scores of 1, 2 or 3), where lesions with a Deauville score of 3 may or may not be considered positive depending on the clinical scenario (example: PET-negative in a child with good prognosis using a conservative read; PET-positive in a child who is at higher risk, to avoid inadequate treatment). A partial metabolic response has reduced FDG PET uptake compared with baseline regardless of size of residual mass (Deauville scores of 4 or 5). Stable disease or no response shows no significant metabolic change on FDG PET (Deauville scores of 4 or 5). Progressive disease shows increase in intensity of FDG PET uptake from baseline or new FDG-avid foci consistent with lymphoma at interim or end-of-treatment assessment.
In low-variable FDG-avid lymphoma histologies, the Lugano criteria recommend CT-based size-response criteria using the sum of the product of the perpendicular diameters (SPD). Partial response requires a decrease by more than 50% in the SPD of up to six representative nodes or extranodal lesions. Progressive disease by CT criteria requires an increase in the product of the perpendicular diameters of a single node by 50%. FDG PET/CT is recommended for routine staging of FDG-avid histologies as the gold standard. However, surveillance scans after remission are discouraged, especially for FDG-avid histologies such as Hodgkin lymphoma, although a repeat study might be considered after an equivocal finding on the post-therapy PET/CT.
Reticuloendothelial and lymphoid FDG PET uptake can be problematic in children with Hodgkin lymphoma to differentiate between normal physiological reactive uptake and lymphoma disease involvement. It is recognized that in tonsillar Waldeyer ring and extranodal sites with high physiological uptake, or with diffuse activity within spleen or bone marrow, FDG PET uptake might be greater than both normal mediastinum and liver because of inherent physiological background activity. In these circumstances, a complete metabolic response is recommended by the Lugano criteria as “inferred if uptake at sites of initial involvement is less than surrounding normal tissue, even if the tissue has high physiologic uptake” [27].
A multifocal pattern of FDG PET bone marrow activity has been found to be a more sensitive and specific noninvasive imaging finding for detecting bone marrow lymphomatous involvement in Hodgkin lymphoma compared to standard pelvic bone marrow biopsies [13, 40]. A study by Purz et al. [13] in 2011 compared bone marrow biopsy with FDG PET at initial staging in 175 pediatric patients with newly diagnosed classic Hodgkin lymphoma. The authors compared the performance of FDG PET/CT to that of routine bone marrow biopsies, MRI or CT, and FDG PET response to follow-up PET/CT during or at the end of chemotherapy. They concluded that routine bone marrow biopsy could be replaced with FDG PET/CT and recommended the use of more than two foci of FDG uptake in bone marrow to be considered positive for bone marrow lymphomatous involvement, and a targeted bone marrow biopsy be performed for two or fewer FDG-avid skeletal/bone marrow sites for confirmation as clinically indicated [13].
A systematic review and meta-analysis on the diagnostic performance of FDG PET/CT for detecting bone marrow involvement at initial staging in Hodgkin lymphoma concluded that FDG PET/CT might be an appropriate method to replace routine bone marrow biopsies [40]. Nine studies with a total of 955 patients met the study criteria from a search of the published literature; these studies were deemed to have overall moderate methodological quality, limited by use of various indirect tests as references standards (example: pelvic bone marrow biopsies, FDG PET/CT follow-up, MRI) in the absence of histological bone marrow biopsy confirmation at the site of FDG-avid focus and the absence of large-scale prospective validation studies. The combined pooled estimates for sensitivity and specificity of FDG PET/CT for detecting Hodgkin lymphoma bone marrow involvement was high at 96.9% (95% CI: 93.0% to 99.0%) and 99.7% (95% CI: 98.9% to 100%), respectively. Performance based on a weighted symmetrical summary receiver operating characteristic (ROC) area under the curve was also robust at 0.9860.
Quantitative positron emission tomography (PET) response
A quantitative PET lymphoma assessment might be preferable to the current visual Deauville criteria to improve inter-reader variability and repeatability for PET-based response assessment in pediatric Hodgkin lymphoma. A proposed quantitative PET extension of the visual Deauville score method would incorporate use of lymphoma- and reference-liver-based standardized uptake values (SUV) parameters by Hasenclever et al. [41]. They proposed use of quantitative PET, which uses a ratio of quantitative PET-defined lymphoma SUVpeak (average maximum SUV in four voxels, involving the maximum SUV voxel and three highest adjacent voxels), which for clarification is different from the PET Response Criteria in Solid Tumors (PERCIST) proposed SUVpeak, to a liver SUVmean (a cuboid volume of interest with a volume of 30 mL positioned in the right liver lobe) [42]. When quantitative PET is compared to Deauville scores, the borderlines between Deauville scores 3, 4 and 5 were determined to correspond to quantitative PET values of 0.95, 1.3 and 2.0, respectively, which could replace visual Deauville scores. The use of quantitative PET or other quantitative SUV-based PET criteria for Hodgkin disease detection and response assessment might be limited for widescale multisite clinical research applications because of concerns of inherent variability and repeatability of lymphoma and normal reference liver semi-quantitative PET among PET/CT scanners and scans, allowing for precision but relatively low accuracy, with correspondence and discussion in the literature [43,44,45].
Quantitative FDG-PET-based metabolic volume measurements might provide additional prognostic value in lymphomas (Fig. 18). Kanoun et al. [46] reported intriguing and promising initial data for use of FDG-PET-based total metabolic tumor volume in 59 consecutive people with baseline initial staging FDG PET/CT scans prior to treatment. Both baseline total metabolic tumor volume and change in tumor SUVmax from baseline to interim PET (ΔSUVmaxPET0–2) were determined to be independent predictors of progression-free survival in multivariate analysis, whereas CT-based bulk tumor (single tumor ≥10 cm) did not reach significance. They proposed using a combined baseline total metabolic tumor volume and change in tumor SUVmax from baseline to interim PET to identify, and potentially stratify, subsets of Hodgkin patients with different predicted outcomes [46].
Example of [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) segmentation of intensely FDG-avid Hodgkin lymphoma in the mediastinum and lower neck. Top row: CT in transaxial, sagittal and coronal views. Middle row: PET in transaxial, sagittal and coronal views. Bottom row: fused PET/CT in transaxial, sagittal and coronal views. Colors denote different tumor FDG PET using different segmentation thresholds
Immunotherapy and [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET) response assessment
With the use of immune-based therapies in Hodgkin lymphoma, Cheson et al. [47] in 2016 proposed a refinement of the Lugano lymphoma response criteria: the lymphoma response to immunomodulatory therapy criteria (LYRIC). The LYRIC criteria introduced an indeterminate-response category to account for inflammatory infiltrates and potential flare/pseudoprogression with use of immune-based therapies. The authors recommended biopsy or repeat imaging to reclassify response as true progression versus pseudoprogression using FDG PET/CT [47]. The three imaging patterns that were proposed to constitute an indeterminate response are: (1) indeterminate response 1, an increase in the sum of the product of the diameters of up to six measurable lesions by at least 50% in the first 12 weeks of therapy without clinical deterioration; (2) indeterminate response 2, new lesions or growth of one or more lesions by at least 50% without overall progression (example: <50% increase in sum of the product of the diameters of up to six lesions at any time during treatment); (3) indeterminate response 3, an increase in FDG uptake by one or more lesions without a concomitant increase in lesion size meeting the criteria for progression as described [47].
Although the use of interim FDG PET response assessment should be applied with caution because of concerns of a flare/pseudoprogression secondary to an inflammatory response to immune-based therapies, a recent study by Castello et al. [48] provided evidence for the validity of applying visual Deauville criteria and quantitative FDG PET in this setting. They evaluated 43 people with refractory or relapsed Hodgkin lymphoma at a single institution who received an FDG PET/CT at baseline before immunotherapy and at 8 weeks (early) and 17 weeks (interim) after administration of a PD-1 (programmed cell death protein) immune checkpoint inhibitor (nivolumab or pembrolizumab) [48]. Imaging was reviewed by two expert nuclear medicine physicians according to Deauville score criteria using the Lugano criteria and also taking into consideration the concept of indeterminate response established by the LYRIC criteria [48]. Best overall response outcome was assessed at a median follow-up of 19 months (range 2–34 months). Both early and interim FDG PET assessments using the Deauville criteria (negative-PET Deauville scores of 1, 2 or 3; positive-PET Deauville scores of 4 or 5) and change in SUVmax could be used to differentiate responders versus non-responders [48]. Interim assessment also showed a statistically significant positive correlation to early assessment with both Deauville score and change in SUVmax. Additionally, the Deauville criteria could differentiate between response groups, and this was statistically significantly greater in people with progressive disease (P=0.008). The authors did not report a significant difference between response cohorts when applying the LYRIC criteria; three of four people with progressive disease had an indeterminate response type 1 (indeterminate response 1; increase in tumor burden without clinical deterioration), which was confirmed as tumor progression, and the fourth was noted to be true progression [48]. This study, albeit a small single-center study, raises the possibility of using both visual and quantitative FDG PET/CT assessment for early immune checkpoint inhibitor therapy for interim response assessment, and possibly at an earlier 8-week therapy time-point to facilitate patient management decisions in the setting of immune-related adverse events.
Metabolic imaging of non-Hodgkin lymphoma
The role of FDG PET and FDG PET/CT has been studied in pediatric patients with non-Hodgkin lymphoma but less extensively than in adults and less extensively than in pediatric Hodgkin lymphoma. Uslu et al. [49] performed a comprehensive literature assessment of the use of FDG PET in pediatric patients with lymphomas. Of 22 studies reported, 9 had children with Hodgkin lymphoma only, 12 had children with both Hodgkin lymphoma and non-Hodgkin lymphoma, and one study [50] involved non-Hodgkin lymphoma only. At diagnosis, FDG PET and FDG PET/CT for staging had sensitivities of 95–100% [49].
It is generally accepted that FDG PET/CT and contrast-enhanced diagnostic CT provide overlapping but complementary disease information. Protocols vary from institution to institution depending on local preferences. Options include performing the studies on different machines (for example, the contrast-enhanced CT on a standalone CT), on the same machine in sequence (for example, performing a contrast-enhanced CT followed by separate PET/CT), or on the same machine as a PET/contrast-enhanced CT, using the contrast-enhanced CT for attenuation correction [51, 52]. Each has minor advantages and disadvantages, but with contemporary scanners, very high-quality examinations are expected with these approaches, with trivial differences in dosimetry. In addition, we routinely recommend propranolol administration to reduce metabolically active fat, which can considerably complicate the interpretation of images of the neck and thorax [53]. PET/MRI might prove to be a suitable alternative, allowing for high-quality structural images along with the PET, resulting in patient dose reduction from lack of attenuation correction CT and the ability to lower the FDG dose because of the relatively long MRI acquisition.
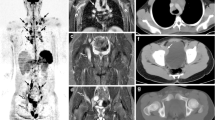
Non-Hodgkin lymphoma is considerably more likely than Hodgkin lymphoma to involve bones. Therefore, at diagnosis, we perform whole-body scans (head to toes). Figure 19 illustrates an example of the importance of whole-body imaging at staging in pediatric patients with non-Hodgkin lymphoma. For follow-up studies in children who did not have bone involvement at diagnosis, we perform limited studies head to mid thighs [54].
Value of whole-body imaging, here in an 8-year-old boy with new diagnosis of Burkitt lymphoma. a Maximum-intensity projection (MIP) image from referred [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET)/CT shows widespread soft-tissue involvement, including the right testicle. The lower extremities were not imaged. b MIP image from FDG PET/CT scan obtained 1 week later shows a triangular area of markedly elevated uptake in the left distal femur. c–f Images obtained approximately 10 days after beginning of chemotherapy. Transverse PET image through the distal femurs (c) shows markedly elevated uptake in the left femur, with normal uptake in the right femur. Axial CT attenuation correction (d), bone window, at the same level as (c) is unremarkable. Axial fusion image (e) demonstrates uptake within the left distal femur and suggests soft-tissue involvement medial to the femur. Coronal MRI (f) shows marked signal abnormality within the left distal femur corresponding with PET findings and confirming tumor involvement (also suggesting that the extraosseous activity seen on PET/CT fusion images was due to motion artifact). g Whole-body MIP shows that most of the sites of abnormal uptake on the initial study are no longer apparent, although abnormal uptake persists in the left distal femur. h Whole-body MIP 2 months after beginning of therapy shows that each of the sites of abnormal activity seen at diagnosis has resolved. The left distal femur appears as an area of diminished uptake compared to the uninvolved right femur, consistent with successful eradication of lymphoma in the left distal femur
In some children, early follow-up studies show poor tumor uptake (Fig. 20). Sharp et al. [55] described transiently altered FDG uptake in 6 of 11 children with lymphoblastic lymphoma. Uptake was increased in the face and other superficial soft tissue about 1 month following the initiation of chemotherapy, with no tumor uptake in 5 of the 6. The abnormal pattern, including low hepatic uptake, subsequently resolved, and in 2 children uptake in lymphoma returned. This pattern was associated with very high doses of glucocorticoids. Caution is warranted in interpreting such studies because tumor uptake might be temporarily suppressed.
Poor early uptake in an 8-year-old boy with T cell lymphoblastic lymphoma. The boy underwent 12 days of treatment, including high-dose glucocorticoids (likely the cause of the altered biodistribution), before [F-18]2-fluoro-2-deoxyglucose (FDG) positron emission tomography (PET)/CT scan. a Axial contrast-enhanced CT scan pre-treatment shows large mediastinal mass. b Anterior maximum-intensity projection image from FDG PET/CT obtained 12 days after beginning of chemotherapy shows no definite tumor uptake but widespread background activity. c FDG PET transverse image through the mediastinum shows no substantial uptake in the residual mediastinal mass. d Axial fusion FDG PET/CT image, same level as (c), demonstrates no substantial uptake in the residual mediastinal mass. Uptake in axillary fat is present
Riad et al. [56] described the role of PET/CT in malignant pediatric lymphoma. Of 152 children, 35 had non-Hodgkin lymphoma. Data were not reported separately for non-Hodgkin lymphoma, but the authors did conclude that FDG PET/CT was useful for staging, early assessment of treatment response, and for accurate characterization of residual masses following therapy [56].
In a large series of adults with DLBCL (n=99), Vishnu et al. [57] reported that PET/CT and bone marrow aspiration/biopsy were largely concordant for the presence or absence of bone marrow involvement at diagnosis. Of the 85 people with concordant findings, 73 had both negative PET/CT and negative bone marrow aspiration/biopsy, and 12 had both positive PET/CT and positive bone marrow aspiration/biopsy [57]. In the 14 adults with discordant results, 12 were positive by PET and negative on bone marrow aspiration/biopsy [57]. Two had positive bone marrow aspiration/biopsy and negative PET findings. The positive predictive value of PET with regard to bone marrow involvement by bone marrow aspiration/biopsy was (12)/(12+12)=50%; the negative predictive value was quite high, (73)/(73+2)=97% [57]. The authors cautioned that the findings should be confirmed in a prospective study [57].
Karantanis et al. [58] reported the findings on 15 people with Burkitt lymphoma. Six of the patients were 17 years or younger. The authors concluded that FDG PET and FDG PET/CT are sensitive for detecting viable disease in people with Burkitt lymphoma, and that high uptake resolves with successful treatment [58].
Bailly et al. [59] found FDG PET valuable for prognosis and management of children with Burkitt lymphoma after induction chemotherapy. Nineteen children (21 scans; 1 child had 3 scans) were studied after initial chemotherapy for response evaluation [59]. FDG PET was negative in 15 children, and conventional imaging was negative in 9 of these [59]. Six children had negative FDG scans but positive conventional imaging because of residual masses; these lesions were resected and necrosis, but no tumor, was identified [59]. One child with both conventional imaging and PET studies that were negative had early relapse of disease 3 months after therapy. Six children had positive FDG findings; four of those had concordant conventional imaging studies, only one of which actually represented residual non-Hodgkin lymphoma; the other three were caused by inflammatory findings [59]. In the remaining two children with positive FDG but negative conventional imaging, follow-up showed disease progression [59]. The authors concluded that in children with Burkitt lymphoma who have residual masses without FDG uptake following chemotherapy, resection and biopsy can be safely avoided [59]. Thus, scan findings are most useful when negative, leading to a negative predictive value of 93% [59]. The positive predictive value was only 50% because of false-positive findings [59]. Otto et al. [60] reported a patient with non-Hodgkin lymphoma, diffuse large cell, who had a xanthomatous pseudotumor that mimicked persistent disease following treatment.
Adams and Kwee [61] conducted a meta-analysis of articles in which biopsy was performed for lesions with FDG uptake during or following treatment for non-Hodgkin lymphoma or Hodgkin lymphoma. In non-Hodgkin lymphoma, the pooled false-positive rate for interim FDG PET was 83%. For end of treatment, the pooled false-positive proportion of FDG PET was nearly 1/3 [61]. The majority of false-positive findings were caused by inflammation [61]. These authors called for re-evaluation of the role of interim and end-of-treatment FDG PET in the management of people with lymphoma [61].
Bakhshi et al. [50] reported the use of FDG PET/CT in 34 pediatric patients (age range 4–16 years, median 10.2 years) with non-lymphoblastic non-Hodgkin lymphoma. At diagnosis, 112 disease sites were located by both PET/CT and contrast-enhanced CT, and 18 additional sites by FDG PET/CT, while contrast-enhanced CT identified 2 sites of disease that PET/CT did not [50]. FDG PET/CT identified bone marrow involvement in 4 of 4 children, with involvement confirmed on bone marrow biopsy [50]. In 5 children, disease was up-staged; no children had their disease down-staged using FDG PET/CT [50].
In a study of 115 pediatric patients with non-Hodgkin lymphoma (100 children with Burkitt lymphoma, 87%; 15 children with DLBCL, 13%), Abdel Rahman et al. [62] reported the results of 152 PET scans. Comparing PET and CT at diagnosis prior to chemotherapy, for evaluation of response, and during follow-up, the sensitivity, specificity, positive predictive value and negative predictive value for FDG PET were approximately 90%, 85%, 46% and 98%; for CT, they were 63%, 57%, 18% and 92% [62]. Findings were similar for both groups of children. The greatest strength of either technique lies in its negative predictive value, enabling confidence in concluding that residual masses following therapy do not represent active neoplasm [61].
Additional data regarding the use of FDG PET/CT are expected from group-wide trials of therapy in non-Hodgkin lymphoma, including the Children’s Oncology Group Trial ANHL12P1 and a randomized Phase 2 trial of brentuximab vedotin (SGN35, NSC# 749710) or crizotinib (NSC# 749005, commercially labeled), in combination with FDG PET and PET/CT in non-Hodgkin lymphoma.
Chemotherapy trials for people with newly diagnosed anaplastic large cell lymphoma (IND# 117117) indicate that FDG PET is highly recommended at initial staging, and following Courses 2, 4 and 6 of chemotherapy. As part of the criteria for complete response or complete response unconfirmed, PET, if previously obtained, must be negative at the time of restaging. FDG uptake in bone lesions might persist and does not preclude complete metabolic response unconfirmed.
The Children’s Oncology Group Trial Phase III randomized trial investigating bortezomib (NSC# 681239; IND# 58443) on a modified augmented Berlin Frankfurt Munster (ABFM) backbone in newly diagnosed T cell lymphoblastic leukemia and T cell lymphoblastic lymphoma (AALL1231) [63] includes a recommendation for PET scans at diagnosis, and requires one in children who have residual masses following treatment. Somewhat surprisingly, this protocol expresses a preference for bone scanning in children who have bone symptoms, although FDG PET can be used as a substitute [63].
In a St. Jude Children’s Research Hospital protocol, Mature B Cell Lymphoma and Leukemia Study III, an exploratory aim is to describe the relationship between treatment outcome and clinical, biological and radiologic features, including day 7 PET. Additional PET scans are obtained at diagnosis, following induction therapy, following consolidation treatment, and subsequently as clinically indicated.
In an International Atomic Energy Agency (IAEA) multicenter trial of 250 pediatric patients with Hodgkin lymphoma or non-Hodgkin lymphoma from multiple socioeconomic populations, the use of interim PET evaluation was found to be the best predictor of event-free survival and overall survival [64]. The study found that Lugano-positive (Deauville 4–5-positive) interim PET was independently associated with increased overall mortality (hazard ratio [HR]=8.96; P<0.001) and was associated with more events in Hodgkin lymphoma (HR=9.77; P<0.001) than in non-Hodgkin lymphoma (HR 2.41; P=0.10) [64]. This same multicenter trial also concluded that in most children with lymphoma, routine “eyes to thighs” field-of-view imaging is more than adequate to stage and monitor disease [65].
Imaging to include anatomical and metabolic imaging is now the standard of care to help the referring clinician provide risk-adapted therapy to children with diagnosis of Hodgkin and non-Hodgkin lymphomas. Many of the approaches to imaging described here have or will be incorporated into clinical trials and treatment plans for the care of children.
References
Hodgkin (1832) On some morbid appearances of the absorbent glands and spleen. Med Chir Trans 17:68–114
Hoppe RT, Mauch PT, Armitage JO et al (2007) Hodgkin lymphoma. Lippincott Williams & Wilkins, Philadelphia
Guermazi A (2004) Radiological imaging in hematological malignancies. Springer-Verlag, Berlin
Gossman A (2007) Anatomic imaging. In: Hoppe RT, Mauch PT, Armitage JO et al (eds) Hodgkin lymphoma. Lippincott Williams & Wilkins, Philadelphia
Flerlage JE, Kelly KM, Beishuizen A et al (2017) Staging evaluation and response criteria harmonization (SEARCH) for childhood, adolescent and young adult Hodgkin lymphoma (CAYAHL): methodology statement. Pediatr Blood Cancer 64
Lister TA, Crowther D, Sutcliffe SB et al (1989) Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 7:1630–1636
Hodgson DC, Gospodarowicz MK (2007) Clinical evaluation and staging of Hodgkin lymphoma. In: Hoppe RT, Mauch PT, Armitage JO et al (eds) Hodgkin lymphoma. Lippincott Williams & Wilkins, Philadelphia, pp 124–128
Schwartz CL, Chen L, McCarten K et al (2017) Childhood Hodgkin International Prognostic Score (CHIPS) predicts event-free survival in Hodgkin lymphoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 64
McCarten KM, Metzger ML, Drachtman RA et al (2018) Significance of pleural effusion at diagnosis in pediatric Hodgkin lymphoma: a report from Children's Oncology Group protocol AHOD0031. Pediatr Radiol 48:1736–1744
Marks LJ, McCarten KM, Pei Q et al (2018) Pericardial effusion in Hodgkin lymphoma: a report from the Children's Oncology Group AHOD0031 protocol. Blood 132:1208–1211
Guermazi A, Brice P, de Kerviler EE et al (2001) Extranodal Hodgkin disease: spectrum of disease. Radiographics 21:161–179
Adams HJ, Kwee TC, Fijnheer R et al (2015) Bone marrow FDG-PET/CT in Hodgkin lymphoma revisited: do imaging and pathology match? Ann Nucl Med 29:132–137
Purz S, Mauz-Korholz C, Korholz D et al (2011) [18F]Fluorodeoxyglucose positron emission tomography for detection of bone marrow involvement in children and adolescents with Hodgkin's lymphoma. J Clin Oncol 29:3523–3528
Guillerman P, Parker BR (2004) Pediatric lymphoma. In: Guermazi A (ed) Radiological imaging in hematological malignancies. Springer-Verlag, Berlin, p 256
Del Rocio Estrada-Sanchez G, Altamirano-Ley J, Ochoa-Carrillo FJ (2007) Normal variants and frequent pitfalls with (18)FDG PET/CT study. Cir Cir 75:491–497
Bashir H, Hudson MM, Kaste SC et al (2007) Pericardial involvement at diagnosis in pediatric Hodgkin lymphoma patients. Pediatr Blood Cancer 49:666–671
Allen CE, Kamdar KY, Bollard CM (2016) Malignant non-Hodgkin lymphomas in childhood. In: Pizzo PA, Poplack DG, Adamson PC et al (eds) Principles and practice of pediatric oncology. Wolters Kluwer, Philadelphia, p xxiv
Evens AM, Winter JN, Gordon LI et al (2015) Non-Hodgkin lymphoma. Cancer Network website. http://www.cancernetwork.com/cancer-management/non-hodgkin-lymphoma. Accessed 27 Aug 2019
Allen CE, Kelly KM, Bollard CM (2015) Pediatric lymphomas and histiocytic disorders of childhood. Pediatr Clin N Am 62:139–165
Murphy SB (1980) Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin Oncol 7:332–339
Rosolen A, Perkins SL, Pinkerton CR et al (2015) Revised international pediatric non-Hodgkin lymphoma staging system. J Clin Oncol 33:2112–2118
Bakhshi S, Bhethanabhotla S, Kumar R et al (2017) Posttreatment PET/CT rather than interim PET/CT using Deauville criteria predicts outcome in pediatric Hodgkin lymphoma: a prospective study comparing PET/CT with conventional imaging. J Nucl Med 58:577–583
Anghelescu DL, Burgoyne LL, Liu T et al (2007) Clinical and diagnostic imaging findings predict anesthetic complications in children presenting with malignant mediastinal masses. Paediatr Anaesth 17:1090–1098
Ng A, Bennett J, Bromley P et al (2007) Anaesthetic outcome and predictive risk factors in children with mediastinal tumours. Pediatr Blood Cancer 48:160–164
Alavi S, Arzanian MT, Abbasian MR, Ashena Z (2006) Tumor lysis syndrome in children with non-Hodgkin lymphoma. Pediatr Hematol Oncol 23:65–70
Barrington SF, Johnson PWM (2017) (18)F-FDG PET/CT in lymphoma: has imaging-directed personalized medicine become a reality? J Nucl Med 58:1539–1544
Barrington SF, Mikhaeel NG, Kostakoglu L et al (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048–3058
Furth C, Steffen IG, Amthauer H et al (2009) Early and late therapy response assessment with [18F]fluorodeoxyglucose positron emission tomography in pediatric Hodgkin's lymphoma: analysis of a prospective multicenter trial. J Clin Oncol 27:4385–4391
Meany HJ, Gidvani VK, Minniti CP (2007) Utility of PET scans to predict disease relapse in pediatric patients with Hodgkin lymphoma. Pediatr Blood Cancer 48:399–402
Gallamini A, Hutchings M, Rigacci L et al (2007) Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 25:3746–3752
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Juweid ME, Stroobants S, Hoekstra OS et al (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 25:571–578
Gallamini A, Fiore F, Sorasio R, Meignan M (2009) Interim positron emission tomography scan in Hodgkin lymphoma: definitions, interpretation rules, and clinical validation. Leuk Lymphoma 50:1761–1764
Le Roux PY, Gastinne T, Le Gouill S et al (2011) Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging 38:1064–1071
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
Horning SJ, Juweid ME, Schoder H et al (2010) Interim positron emission tomography scans in diffuse large B-cell lymphoma: an independent expert nuclear medicine evaluation of the Eastern Cooperative Oncology Group E3404 study. Blood 115:775–777
Meignan M, Gallamini A, Meignan M et al (2009) Report on the first international workshop on interim-PET-scan in lymphoma. Leuk Lymphoma 50:1257–1260
Barrington SF, Qian W, Somer EJ et al (2010) Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 37:1824–1833
Kluge R, Chavdarova L, Hoffmann M et al (2016) Inter-reader reliability of early FDG-PET/CT response assessment using the Deauville scale after 2 cycles of intensive chemotherapy (OEPA) in Hodgkin's lymphoma. PLoS One 11:e0149072
Adams HJ, Kwee TC, de Keizer B et al (2014) Systematic review and meta-analysis on the diagnostic performance of FDG-PET/CT in detecting bone marrow involvement in newly diagnosed Hodgkin lymphoma: is bone marrow biopsy still necessary? Ann Oncol 25:921–927
Hasenclever D, Kurch L, Mauz-Korholz C et al (2014) qPET — a quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur J Nucl Med Mol Imaging 41:1301–1308
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50:122s–150s
Weber WA, Gatsonis CA, Mozley PD et al (2015) Repeatability of 18F-FDG PET/CT in advanced non-small cell lung cancer: prospective assessment in 2 multicenter trials. J Nucl Med 56:1137–1143
Laffon E, Marthan R (2014) Interim FDG PET scans in lymphoma: SUV measurement error may impair qPET methodology. Eur J Nucl Med Mol Imaging 41:2154
Kluge R, Barrington S, Kurch L, Hasenclever D (2017) Reply to: Laffon and Marthan "FDG PET for therapy monitoring in Hodgkin's and non-Hodgkin's lymphomas: qPET versus rPET". Eur J Nucl Med Mol Imaging 44:2331–2332
Kanoun S, Rossi C, Berriolo-Riedinger A et al (2014) Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 41:1735–1743
Cheson BD, Ansell S, Schwartz L et al (2016) Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 128:2489–2496
Castello A, Grizzi F, Qehajaj D et al (2018) (18)F-FDG PET/CT for response assessment in Hodgkin lymphoma undergoing immunotherapy with checkpoint inhibitors. Leuk Lymphoma 60:367–375
Uslu L, Donig J, Link M et al (2015) Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med 56:274–286
Bakhshi S, Radhakrishnan V, Sharma P et al (2012) Pediatric nonlymphoblastic non-Hodgkin lymphoma: baseline, interim, and posttreatment PET/CT versus contrast-enhanced CT for evaluation — a prospective study. Radiology 262:956–968
Brady SL, Shulkin BL (2017) Dose optimization: a review of CT imaging for PET attenuation correction. Clin Transl Imaging 5:359–371
Parisi MT, Bermo MS, Alessio AM et al (2017) Optimization of pediatric PET/CT. Semin Nucl Med 47:258–274
Wong KK, Brady SL, Doubrovin M et al (2018) Propranolol decreases 18F-fluorodeoxyglucose uptake in brown adipose tissue on pediatric oncology PET/CT. J Nucl Med 59:310
Sammer MB, Shulkin BL, Alessio A, Parisi MT (2011) Role of limited whole-body PET/CT in pediatric lymphoma. AJR Am J Roentgenol 196:1047–1055
Sharp SE, Gelfand MJ, Absalon MJ (2012) Altered FDG uptake patterns in pediatric lymphoblastic lymphoma patients receiving induction chemotherapy that includes very high dose corticosteroids. Pediatr Radiol 42:331–336
Riad R, Omar W, Kotb M et al (2010) Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imaging 37:319–329
Vishnu P, Wingerson A, Lee M et al (2017) Utility of bone marrow biopsy and aspirate for staging of diffuse large B cell lymphoma in the era of positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-deoxyglucose integrated with computed tomography. Clin Lymphoma Myeloma Leuk 17:631–636
Karantanis D, Durski JM, Lowe VJ et al (2010) 18F-FDG PET and PET/CT in Burkitt's lymphoma. Eur J Radiol 75:e68–e73
Bailly C, Eugene T, Couec ML et al (2014) Prognostic value and clinical impact of (18)FDG-PET in the management of children with Burkitt lymphoma after induction chemotherapy. Front Med 1:54
Otto M, Shulkin BL, Kundu M et al (2012) Histiocyte-rich xanthomatous pseudotumor mimicking relapse on positron emission tomography imaging in an adolescent with primary mediastinal diffuse large B-cell lymphoma. J Pediatr Hematol Oncol 34:232–235
Adams HJA, Kwee TC (2016) Proportion of false-positive lesions at interim and end-of-treatment FDG-PET in lymphoma as determined by histology: systematic review and meta-analysis. Eur J Radiol 85:1963–1970
Abdel Rahman H, Sedky M, Hamoda A et al (2016) Role of FDG-PET scan in the management of pediatric mature B cell non-Hodgkin’s lymphoma. CCHE experience. J Egypt Natl Canc Inst 28:95–99
Children’s Oncology Group (2015) AALL1231: a phase III randomized trial investigating Bortezomib on a modified augmented BFM (ABFM) backbone in newly diagnosed T-lymphoblastic leukemia (T-ALL) and T-lymphoblastic lymphoma (T-LLy). https://www.childrensoncologygroup.org/aall1231. Accessed 25 Apr 2019
Nadel H, Etchebehere E, Cerci J et al (2018) Validation of standardized interpretation criteria for early response evaluation with (18)FDG-PET/CT in pediatric lymphoma — a report on an IAEA multicenter prospective study. Eur J Nucl Med Mol Imaging 45:S187–S187
Cerci JJ, Etchebehere EC, Nadel H et al (2019) Is true whole-body (18)F-FDG PET/CT required in pediatric lymphoma? An IAEA multicenter prospective study. J Nucl Med 60:1087–s1093
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McCarten, K.M., Nadel, H.R., Shulkin, B.L. et al. Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr Radiol 49, 1545–1564 (2019). https://doi.org/10.1007/s00247-019-04529-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04529-8