Abstract
Background
Developmental dysplasia of the hip (DDH) is known to result in smaller femoral head size in toddlers; however, alterations in femoral head size and growth have not been documented in infants.
Objective
To determine with ultrasound (US) whether femoral head size and growth are altered in infants (younger than 1 year of age) with severe DDH.
Materials and methods
We identified all patients at our tertiary care children’s hospital from 2002 to 2014 who underwent US for DDH. We included studies with at least one hip with severe DDH, defined as <25% coverage of the femoral head, and excluded teratological DDH. We constructed a control group of randomized patients with normal US studies. Two pediatric radiologists blinded to diagnosis measured bilateral femoral head diameter. Inter-reader variability and femoral head diameter difference between dislocated and contralateral normal femoral heads were evaluated. Mean femoral head diameters were compared across types of hip joint; femoral head growth rates per month were calculated.
Results
Thirty-seven children with DDH (28 female) were identified (median age: 33 days). The control group contained 75 children (47 female) with a median age of 47 days. Fifty-three of the 74 hips in the study group had severe DDH. Twenty-four children with DDH had completely dislocated hips (nine bilateral, five with contralateral severe subluxations). Thirteen other children had severe subluxation, two bilaterally. There was good inter-reader agreement in the normal femoral head group and moderate agreement in the severe DDH group. In the study group, severe DDH femoral head diameter was significantly smaller than their contralateral normal hip. Severe DDH femoral head diameter was significantly smaller than normal femoral head diameter in the control group. The severe DDH femoral head growth rate was slightly less but not significantly slower than normal femoral head growth rate in the study group.
Conclusion
On US during infancy, femoral head size is significantly reduced in severe cases of DDH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Developmental dysplasia of the hip (DDH) affects approximately 1.5 of every 1,000 Caucasian Americans and is less frequent among African Americans [1,2,3]. DDH is comprised of a spectrum of abnormalities, ranging from laxity of the joint and mild subluxation to fixed dislocation. In the United States, hip ultrasound (US) is selectively performed in infants with risk factors, such as family history of DDH, breech presentation and inconclusive physical examination [4]. While there are some worldwide variations in the approach to DDH on US, diagnosis is generally based on both the configuration of the acetabular fossa and the position of the femoral head with and without stress maneuvers [5, 6].
DDH has a multifactorial pathogenesis that is not completely understood. One theory proposes that ligamentous laxity predisposes the developing hip to mechanical forces that cause the femoral head to move outside the acetabulum. Another approach emphasizes the shallow configuration of the acetabulum as the primary abnormality [5]. Unstable or dislocated hips often have progressive dysplasia, and resolution without intervention is unlikely after the age of 6 months [7].
The diagnosis of DDH in newborns in the United States is primarily based on physical examination demonstrating unstable, subluxated or dislocated hips [8]. Imaging is used selectively when there is equivocal physical examination or in children with high risk of DDH [9]. Anatomical imaging findings are based on morphological dysplastic changes of the acetabular fossa. While acetabular changes are well-documented in DDH cases, little is known regarding morphological changes of the femoral head during the first several months of life in children with DDH. A recent study in walking-age children with DDH showed the femoral head is dysplastic and usually aspherical, suggesting that DDH results in morphological changes in the femoral head in addition to the acetabular changes [10]. Femoral head changes later reported as sequelae of DDH include flattening of the femoral head ossification, a valgus neck-shaft angle and excess femoral anteversion [10,11,12].
We have anecdotally noted that the femoral head is small and deformed in the majority of pelvic magnetic resonance imaging (MRI) performed in children used to assess reduction after treatment of femoral head dislocation. This leads to the question of whether significant differences in femoral head size can be noted earlier with US. Abnormally small femoral head size in children with severe DDH could provide insight into the complex pathogenesis of DDH, which involves both deformed acetabular fossa and femoral heads.
This present study wishes to assess if, based on hip US, femoral head size and growth rate are altered in infants (younger than 1 year of age) with severe DDH compared to their normal counterparts.
Materials and methods
Patient selection
Institutional review board approval with waiver of informed consent was obtained for this retrospective study. Using the radiology information system, we identified all hip US studies from 2002 to 2014 performed to evaluate for DDH in infants younger than 1 year of age. The study group included all patients with a US study demonstrating severe DDH (defined as either complete dislocation or severe subluxation with α-angle <43° and less than 25% coverage of the femoral head) in at least one hip. Femoral head coverage was assessed on the coronal views using the bony iliac line at rest. Patients with teratological DDH and history of prematurity were excluded. We also identified a control group of randomized normal studies from the same time period; twice as many controls as DDH patients were included in our evaluation. The electronic medical record was then used to gather demographic information, underlying medical abnormalities, clinical indication for the US and treatment. Age of patients was recorded as postnatal age in days. In DDH patients with more than one US, we included the first and last examination in the study group.
For each US examination, each hip was categorized by a pediatric radiologist as normal, severe dysplasia (α-angle <43° with coverage of the femoral head ≤25% or dislocated femoral head), and other/immature/DDH with femoral head coverage >25%.
Femoral head measurement
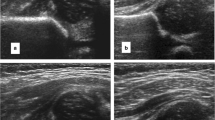
The DDH and control group US exams were then placed in a randomized order, without indication of which group the patient belonged to. Two pediatric radiologists (reader 1 [M.R.W.] with 9 years of experience and reader 2 [B.K.] with 19 years of experience) independently reviewed each US exam on the PACS system, and measured the maximum diameter of both femoral heads. Each reader measured each femoral head in both planes and recorded the maximal diameter (since a measurement of diameter could be falsely low but not falsely high). Figure 1 shows an example of how measurements were performed.
A 28-day-old boy with bilateral hip clicks. Coronal view hip US shows complete dislocation of the right femoral head (a, average diameter of 13.9 mm [double-headed arrow]) and normal left hip (b, average diameter of 16.6 mm [double-headed arrow]) with 51% (d/D) coverage of the femoral head (c). d distance of the femoral head covered by the acetabular bony roof. D diameter of the femoral head
Statistical analysis
Descriptive statistics (medians and interquartile ranges [IQR], ranges, frequencies) were calculated for patient demographics, clinical and treatment information, and US results. To compare baseline demographic and clinical characteristics of patients between study and control groups, chi-square and Fisher exact tests were used for analysis of categorical variables, and the Mann-Whitney-Wilcoxon test was used for continuous variables.
Inter-reader variability was evaluated for the two readers’ femoral head measurements using intraclass correlation coefficient (ICC) and Bland-Altman analysis with limits of agreement. The ICC was calculated as the between-sample variable divided by the sum of the between-sample variable and the within-sample variance. ICC >0.75 indicates good agreement, 0.50–0.75 moderate agreement and <0.50 poor agreement [13].
The mean femoral head diameters measured by reader 1 and reader 2 were calculated and then plotted against the difference of the two evaluations (difference = reader 1 – reader 2). Horizontal lines were drawn at the mean difference and at the 95% limits of agreement, which are defined as the mean difference +/− 1.96 times the standard deviation (SD) of the differences.
An independent t-test comparison was used to evaluate femoral head size differences between the study and control groups. A paired t-test was used to evaluate femoral head size difference in patients with one severe DDH hip and one normal hip.
As we included more than one US result for the same patient, a generalized linear mixed model was adopted to adjust for the inherent correlation among repeated outcome measures on each subject. The normality assumption of the model was satisfied. We used this approach to investigate the main effects of the type of hip (normal or severe DDH) and age, and their interaction in relation to femoral head size by US, while controlling gender. The rate of femoral head growth for different hip types (study group severe DDH, study group normal, control group normal) was also assessed. Actual femoral head diameter by age for each hip type was then plotted. A trend line depicted expected femoral head size by US over time. All statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Study population
The study group (severe DDH of at least one hip) included 37 children (28 female), with a median age of 33 days (IQR: 15–42 days). The control group (bilateral normal hips) included 75 children (47 female), with a median age of 47 days (IQR: 39–78 days). The indication for US varied significantly between the study and control groups. More children in the study group had abnormal physical examination indicating DDH than in the control group (study group: 16/37 patients [43.2%] vs. control group: 9/75 patients [12.0%], P<0.01). About a quarter of children in the study group had a diagnosis of dislocated hip before US was performed, while no one in the control group did (study group: 9/37 patients [24.3%] vs. control group: 0/75 patients [0%], P<0.01). In contrast, more children in the control group underwent US for screening following breech presentation (study group: 10/37 patients [27%] vs. control group: 36/75 patients [48.0%], P<0.01) (Table 1).
Of the study group children, 64.9% (24/37) had completely dislocated hips (9 bilaterally, 5 with contralateral severe subluxation). Of that group, 35.1% (13/37) had severe subluxation (2 bilaterally). Overall, there were 53 hip joints with severe DDH. Of the patients in the study group, 81.1% (30/37) had follow-up US. The last follow-up study was performed at an age range from 1 month to 12.4 months (average: 3.6 months). The control group did not have follow-up studies.
Femoral head diameter
Generalized linear mixed model was used to test the main effects: type of hip (normal or severe DDH) and age, and their interaction with femoral head diameter, while controlling gender. Our results showed that at an average age of 2.18 months across gender, the average femoral head diameter was 17.4 mm for normal hips in the control group and 17.2 mm for normal contralateral hips in the study group. There was no difference (P=0.39) in femoral head diameter between normal hips in the study and control groups. However, the femoral head diameters in DDH hips were 2.3 mm smaller (13.3%, 2.3 mm/17.2 mm) than the normal contralateral hips in the study group (P<0.01), and 2.5 mm smaller (14.4%, 2.5 mm/17.4 mm) than normal hips in the control group (P<0.01), respectively.
Our results also showed that the femoral head growth rate was 0.5 mm per month (P<0.01) in the study group of hips with severe DDH, while at 0.6 mm per month (P<0.01) for contralateral normal hips in the study group and normal hips in the control group. However, the growth rate of hips with severe DDH in the study group was not significantly different compared to their contralateral normal hips (difference: −0.1 mm, P=0.16), or normal hips in control group (difference: −0.1 mm, P=0.11) (Fig. 2).
Scatter plot of actual measurement of femoral head (FH) diameter (mm) by US and age (months) by type of hip (severe developmental dysplasia of the hip [DDH] femoral heads, contralateral normal femoral heads and normal control femoral heads). Y-axis is the average femoral head diameter measurement by the two readers. Individual regression lines are shown for each group
The overall mean difference in femoral head diameter between reader 1 and reader 2 was 0.2 mm (95% CI: -1.4–1.7 mm). The inter-reader agreement was moderate (ICC=0.62). A Bland-Altman plot of femoral head diameters shows no systematic variation between readers. The data showed a smaller mean difference with better agreement between readers in the control group (mean difference: 0.1 mm, 95% CI: -1.26–1.51, ICC=0.80) compared with the study group (mean difference: 0.2 mm, 95% CI: -1.66–2.07, ICC=0.50); no systematic variation was seen on Bland-Altman plot in either group (Fig. 3).
Treatment of DDH hips
Most children in the study group 31/37 (83.8%) were treated initially with a Pavlik harness. Closed reduction was performed in 21/37 (56.7%) of patients. Open reduction was necessary in 14/37 (37.8%) of patients who failed to respond to closed reduction; more advanced surgery included femoral osteotomy in 3/37 (8.1%) and pelvic osteotomy in 5/37 (13.5%).
Discussion
Research in newborns with DDH has mostly discussed the morphological changes in the acetabulum. Only a few studies have evaluated the morphological changes of the femoral head in DDH. Two of these studies were in adults with DDH prior to hip arthroplasty [14, 15]. These studies demonstrated decreased height of the center of the femoral head, short femoral neck and increased anteversion. Another study in adults [16] found a difference in femoral morphology in the presence of deficient acetabular coverage (i.e. with DDH) versus excessive acetabular coverage. The patients with deficient acetabula had elliptical rather than spherical femoral heads, decreased epiphyseal height, less extension of the epiphysis toward the femoral neck and decreased head-neck offset. Clohisy et al. [17] noted similar findings in adults previously treated with periacetabular osteotomy. Another study in adults with acetabular dysplasia demonstrated that these patients had significantly deformed femoral heads [18].
There are only a few studies evaluating femoral head morphology in children with a late diagnosis of DDH. These studies demonstrated aspherical femoral head morphology [11] and small dysplastic femoral head before surgery with some developing coxa magna after open reduction [19].
Our study is unique because it evaluates changes in femoral head size and growth in infancy. It is well-known that DDH causes delay in the appearance of the ossification center of the femoral head and asymmetrically smaller size, which can last for 6–12 months [20]. However, the ossification center does not represent the true diameter of the femoral head. Our data show that in the first few months of life there is a significant decrease in size (mean difference: 2.29–2.51 mm, P<0.01) of the femoral head in hips with severe DDH. The femoral head growth rate was 0.51 mm per month in the study group of hips with severe DDH versus 0.64 mm per month for contralateral normal hips in the study group and normal hips in the control group, though the difference was not statistically significant.
We demonstrated that the femoral head in severe DDH was significantly smaller than either the femoral heads in the control group or the normal contralateral hip. In addition, there was no significant difference in the size and growth between the femoral heads in the control group and the study group contralateral normal hips. This excludes potentially confounding variables such as underlying age, gender or technical factors.
There are some similarities between DDH and posterior subluxation of the humeral head in infants with brachial plexus injury. Both situations involve the cartilaginous epiphysis moving outside the joint during development. Using US to evaluate the shoulder in these infants, Poyhia et al. [21] found that all patients with permanent brachial plexus birth injury had reduced humeral head growth. In addition, the size reduction was most pronounced in patients with posterior subluxation, with a mean size difference of 7% in the humeral head and 18% in the ossification center at 1 year of age. This suggests that subluxation may alter growth of the humeral head similar to what we demonstrated with the femoral head. It is well-known that normal position of the femoral head is essential for normal growth and shape of the acetabular fossa. We hypothesize that normal position of the femoral head is also important for the growth and development of the femoral head.
In our study, we found that agreement between the two readers was good (ICC: 0.80) in the control group and only moderate in the study group (ICC: 0.50). We believe this results from better depiction of the femoral head when it is normal in shape (more spherical), size and location.
A larger prospective study would be helpful to determine whether the growth rate is less in severe DDH hips and whether measuring femoral head size can serve as an additional prognostic factor. Perhaps future research using 3-D US could help ensure measurement of the maximal diameter of the femoral head and whether it is spherical or eccentric. This could help assess whether femoral head morphology is another prognostic indicator.
Our study has several limitations. One main limitation is its retrospective nature. The ultrasounds (when originally performed) were focusing on acetabulum morphology, femoral head position and femoral head stability/laxity (rather than focusing on measuring femoral head diameter). Therefore, we had to work with the images that were available when assessing femoral head diameter. Though we believe the femoral heads were adequately demonstrated, it is possible that none of the saved images was a true depiction of the femoral head diameter, and the measurement could be more of a chord than a diameter and, therefore, falsely low. It is possible that this effect may have had a greater impact on the abnormal hips than the normal hips since the abnormal hips would be more likely to not be centered in the acetabulum. In addition, the definition of severe DDH that included patients with dislocated hips and less than 25% of coverage of the femoral head is arbitrary, and the effect of less severe forms of DDH on femoral head size was not evaluated. The small number of patients and the short follow-up period also preclude a meaningful evaluation of how smaller femoral heads might predict success/failure of treatment or clinical outcome. The small number of patients may have affected our ability to show a statistically significant difference in femoral head growth rate.
Conclusion
We found that femoral head size is reduced in infants with severe DDH. While the femoral head growth rate of infants with severe DDH is slightly slower, the difference is not statistically significant.
References
American Academy of Pediatrics (2000) Clinical practice guideline: early detection of developmental dysplasia of the hip. Committee on quality improvement, subcommittee on developmental dysplasia of the hip. Pediatrics 105:896–905
Guille JT, Pizzutillo PD, MacEwen GD (2000) Development dysplasia of the hip from birth to six months. J Am Acad Orthop Surg 8:232–242
Loder RT, Skopelja EN (2011) The epidemiology and demographics of hip dysplasia. ISRN Orthop 2011:238607
Kotlarsky P, Haber R, Bialik V et al (2015) Developmental dysplasia of the hip: What has changed in the last 20 years? World J Orthop 6:886–901
Clarke NM, Harcke HT, McHugh P et al (1985) Real-time ultrasound in the diagnosis of congenital dislocation and dysplasia of the hip. J Bone Joint Surg (Br) 67:406–412
Graf R (1980) The diagnosis of congenital hip-joint dislocation by the ultrasonic combound treatment. Arch Orthop Trauma Surg 97:117–133
Vitale MG, Skaggs DL (2001) Developmental dysplasia of the hip from six months to four years of age. J Am Acad Orthop Surg 9:401–411
Shipman SA, Helfand M, Moyer VA et al (2006) Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics 117:e557–e576
Vane AG, Gwynne Jones DP, Dunbar JD et al (2005) The diagnosis and management of neonatal hip instability: results of a clinical and targeted ultrasound screening program. J Pediatr Orthop 25:292–295
Sankar WN, Neubuerger CO, Moseley CF (2010) Femoral head sphericity in untreated developmental dislocation of the hip. J Pediatr Orthop 30:558–561
Fabry G, MacEwen GD, Shands AR Jr (1973) Torsion of the femur. A follow-up study in normal and abnormal conditions. J Bone Joint Surg Am 55:1726–1738
Shefelbine SJ, Carter DR (2004) Mechanobiological predictions of growth front morphology in developmental hip dysplasia. J Orthop Res 22:346–352
Portney LG, Watkins MP (2000) Foundations of clinical research. Applications to practice, 2nd edn. Upper Saddle River, Prentice Hall Health
Crowe JF, Mani VJ, Ranawat CS (1979) Total hip replacement in congenital dislocation and dysplasia of the hip. J Bone Joint Surg Am 61:15–23
Sugano N, Noble PC, Kamaric E et al (1998) The morphology of the femur in developmental dysplasia of the hip. J Bone Joint Surg (Br) 80:711–719
Steppacher SD, Tannast M, Werlen S et al (2008) Femoral morphology differs between deficient and excessive acetabular coverage. Clin Orthop Relat Res 466:782–790
Clohisy JC, Nunley RM, Carlisle JC et al (2009) Incidence and characteristics of femoral deformities in the dysplastic hip. Clin Orthop Relat Res 467:128–134
Okano K, Yamaguchi K, Ninomiya Y et al (2013) Femoral head deformity and severity of acetabular dysplasia of the hip. Bone Joint J 95-B:1192–1196
O'Brien T, Salter RB (1985) Femoral head size in congenital dislocation of the hip. J Pediatr Orthop 5:299–301
Harcke HT, Lee MS, Sinning L et al (1986) Ossification center of the infant hip: sonographic and radiographic correlation. AJR Am J Roentgenol 147:317–321
Poyhia TH, Lamminen AE, Peltonen JI et al (2010) Brachial plexus birth injury: US screening for glenohumeral joint instability. Radiology 254:253–260
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Wanner, M.R., Loder, R.T., Jennings, S.G. et al. Changes in femoral head size and growth rate in young children with severe developmental dysplasia of the hip. Pediatr Radiol 47, 1787–1792 (2017). https://doi.org/10.1007/s00247-017-3938-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3938-2