Abstract
The use of cross-sectional imaging in the pediatric population continues to rise, particularly the use of MRI. Limiting motion artifact requires cooperative subjects who do not move during imaging, so there has been an increase in the need for pediatric sedation or anesthesia. Over the last decade, concern has increased that exposure to anesthesia might be associated with long-term cognitive deficits. In this review we report current understanding of the effects of anesthesia on the pediatric population, with special focus on long-term developmental and cognitive outcomes, and suggest how radiologists can use new technologies or imaging strategies to mitigate or minimize these potential risks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
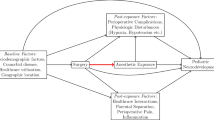
Use of cross-sectional imaging in the pediatric population continues to rise, particularly the use of MRI [1, 2]. The myriad benefits of cross-sectional imaging include precise identification of anatomy in pre-surgical planning; sensitive evaluation for life-threatening injury in trauma; detection and accurate diagnosis of disease, including systemic disease spanning multiple body systems; and follow-up evaluation of surgical repair, interval healing, disease progression and treatment effect. Achieving diagnostic-quality imaging requires image optimization across multiple realms, including limiting interference from imaging artifact. Limiting motion artifact requires cooperative subjects who can hold still on command; thus there has been a concomitant increase in the need for pediatric sedation or anesthesia to fulfill this demand. In a 12-year study at one university medical center, researchers found a growth rate in pediatric CT and MRI of 8.1% with an essentially equal 8.5% increase in anesthesia care during imaging [3]. In practicality, the clinical requirements in the pediatric population necessitate levels of sedation that most commonly fit the definitions of deep sedation or general anesthesia. In this review we use the terms “sedation” and “anesthesia” to refer to the use of pharmacological agents to ensure adequate patient conditions for MRI.
When approached in a systematic manner, modern pediatric anesthesia care for children undergoing imaging appears to be extraordinarily safe [4]. However over the last decade concern has increased that exposure to anesthesia might be associated with long-term cognitive deficits [5]. As a result, strategies that reduce anesthesia exposure at an early age could have significant clinical value [6]. In this review we report current understanding of the effects of anesthesia on the pediatric population, with special focus on long-term developmental and cognitive outcomes.
Sedation medications and adverse events
Pharmacological strategies for achieving immobility during imaging encompass a spectrum of interventions from oral anxiolytics to general anesthesia with intubation. However, younger children typically require the use of general anesthesia in order to produce acceptable image quality. Currently in the United States, anesthesia for cross-sectional imaging is primarily the purview of the anesthesiologists or specially trained designees, including nurses [7, 8]. A structured approach includes appropriate use of medications (with a particular emphasis on limiting side effects) and efficient anesthesia administration [9].
The ideal sedation agent for use in pediatric imaging is easy to administer, fast-acting, predictable, rapidly reversible and well-tolerated. The most common classes of medication used for imaging procedures historically have been benzodiazepines, opioid analgesics, intravenous anesthetics (propofol, ketamine), inhalational anesthetics (e.g., sevoflurane), selective alpha-2 agonists (dexmedetomidine), barbiturates (chiefly pentobarbital) and hypnotics (e.g., chloral hydrate). These medications have been thoroughly reviewed [10, 11]. Much of the literature on these agents’ use in children focuses on optimization of agent and dose [12,13,14,15]. Longer-acting agents such as chloral hydrate and pentobarbital have fallen out of favor. In recent years, propofol has been the predominant agent of choice, occasionally supplemented by a benzodiazepine such as midazolam [4].
A sizeable body of literature has examined the rate of adverse events occurring during pediatric sedation. These studies have found a wide range in the incidence of adverse events, varying from <1% to >10% [16,17,18]. Although the incidence of adverse events in some of these studies was quite high, they included a wide range of practices including the use of older anesthetic agents such as pentobarbital.
More recent data indicate that when approached in a systematic and well-organized manner, anesthesia for MRI is extremely safe. The Pediatric Sedation Research Consortium collected data on adverse events in pediatric sedations performed outside the operating room, of which greater than 60% of the studied sedations occurred for imaging. Their analysis included data pertaining to more than 30,000 sedations at 26 international sites. They found a complication rate of 5.3%, with oxygen desaturations below 90% for at least 30 s comprising nearly half of all reported events [4]. Despite this relatively high adverse event rate, it was notable that serious adverse events were rare, and there were no reported deaths. The authors concluded that the low rate of serious events was attributable to the use of a highly motivated and organized pediatric sedation service and that safety was related to the ability to effectively manage less serious events.
In other studies, developmental disability [19] and underlying respiratory illness [20] were found to confer additional risk for adverse event during sedation for imaging, whereas the rate of adverse events remained independent of patient gender, age and weight across multiple series. A systematic review of 118 case reports of adverse sedation events in pediatrics concluded that drug overdoses and drug–drug interactions were the most frequent root causes of adverse events, particularly when three or more agents were used concurrently, regardless of agent used or route of administration [21].
Anesthesia and neurotoxicity
Over the last two decades, concern has mounted that exposure to all currently available anesthetic agents (including sedatives) might be neurotoxic in young animals during periods of synaptogenesis and rapid central nervous system (CNS) cell growth. Animal studies comprise the preponderance of literature evaluating direct neurotoxicity of sedation medications [22, 23]. Studies of rodent and non-human primate models have revealed immediate neurotoxic effects including accelerated apoptosis and increased neuronal cell death [24, 25]. In addition to neuronal cells, the supporting stroma of the CNS is also affected. Evidence of concomitant oligodendrocyte cell death introduces a plausible mechanism by which even brief exposure to anesthetic agents could lead to long-term structural CNS changes [26]. These neurotoxic effects have been observed in animal models exposed to inhaled and intravenous anesthetics with actions on both the γ-aminobutyrate (GABA) and N-methyl-D-aspartate (NMDA) receptors. In addition to histological findings, animal models consistently exhibit evidence of cognitive deficits, primarily in the domains of learning and memory. Anesthesia-exposed rats exhibit deficiencies in performance in water maze testing as well as recollection memory [27]. In addition to deficits in memory, specific patterns of behavior might be adversely affected by early anesthesia exposure. Female rats exposed to general anesthesia at an early age appear to develop sexually normally and produce healthy offspring [28]. However, these rats exhibit extremely poor maternal nurturing behavior and their offspring suffer high mortality rates from maternal neglect [29, 30].
Translation of these results to human populations poses significant challenges. There are species-specific differences in development, including the timetable of brain maturation and the window of peak vulnerability to anesthetic exposure [31, 32]. In addition, it is difficult to compare anesthetic regimens between laboratory protocols and clinical anesthesia conditions in human infants. Despite these limitations, it is generally well accepted that early exposure to anesthesia causes permanent structural and functional changes in the CNS of laboratory animals. However, whether early anesthesia exposure causes meaningful harm in humans remains uncertain [33, 34].
Long-term effects: neurodevelopmental or cognitive risks?
Evaluation of anesthesia-related neurotoxicity in the human pediatric population is predominated by observational case–control and retrospective cohort study designs [35, 36]. The vast majority of these studies evaluate cognitive or neurodevelopmental outcomes in children exposed to surgical anesthesia as compared to age-matched non-exposed cohorts [37,38,39,40,41].
Some studies reveal no identifiable link between anesthesia exposure and negative neurodevelopmental or cognitive outcomes. For example, a decade-long cohort study in the Netherlands followed more than 1,100 monozygotic twin pairs with varying histories of early anesthesia exposure. Although there were greater rates of cognitive problems in concordant twin pairs in which both twins had early exposure to anesthesia compared to unexposed concordant pairs, they identified no statistical difference in the rates of learning disabilities between the 71 discordant twin pairs in which only one twin had early anesthesia exposure [42]. This study of monozygotic twins controlled for many confounding variables including genetic variability, birth history including prematurity, and in utero toxic exposures; however interpretation of this paper is limited by a lack of details regarding anesthetic administration. A recent large database study from Denmark found no statistically significant difference in long-term academic performance between children with and without a history of exposure to anesthesia and surgery at an early age [43]. A retrospective study of children who underwent urological surgical procedures prior to 6 years of age similarly showed no statistically significant difference in the rates of behavioral disturbances in children operated on before or after age 24 months [44].
Other studies have revealed differences in development and cognition between anesthesia-exposed and non-exposed cohorts. A Mayo Clinic study of greater than 5,000 children in Olmstead County, MN, found an increased risk of learning disabilities in children with two or more anesthesia exposures prior to the age of 4 years [45]. An additional study of children from the same population confirmed repeated early anesthesia exposures as a risk factor for learning disability later in childhood [46]. In another study, Ing et al. [47] found significant domain-related language deficits and other cognitive deficits in a cohort of exposed children in Australia.
Lack of an identifiable phenotype of anesthetic neurotoxicity has also limited human research. Of particular interest to radiologists, MRI and fMRI studies are beginning to shed light on this issue. Taghon et al. [48] demonstrated that fMRI might be a useful tool in the investigation of anesthetic neurotoxicity by demonstrating activation differences in the cerebellum, cingulate gyrus and paracentral lobule between groups of exposed and unexposed children. Backeljauw et al. [49] found decreased performance intelligence quotient (IQ) as well as structural CNS changes in the form of lower-gray-matter density in the occipital cortex and cerebellum. An objective model of potential anesthetic neurotoxicity would be of enormous value. Such a model would help to determine whether there are permanent changes in the human CNS after exposure in clinical scenarios. In addition, an objective phenotype would aid in understanding potential mechanisms of neurotoxicity as well as provide a template to judge the efficacy of potential mitigating strategies or antidotes.
An oft-cited criticism of the existing clinical studies of anesthesia and neurotoxicity, irrespective of study result, is that by comparing populations that have had anesthesia during surgery to those that have not had anesthesia, the surgical exposure itself serves as an undeniable confounder. It is possible that either the need for surgery, the surgical experience, per se, or demographic characteristics of groups of children requiring early infant surgery might influence later tests of cognitive function. Multiple approaches to address this limitation are underway. In Iowa, a study by Block et al. [50] found marked decreases in elementary school test performance in children who had received general anesthesia for pyloromyotomy, hernia repair or circumcision. At our own institution, a study of 265 children who underwent spinal anesthesia for the same procedures revealed no relationship between infant spinal anesthesia and very poor elementary school performance [51]. Currently multiple international centers are engaged in a similar study under the General Anesthesia Spinal Anesthesia (GAS) group — a prospective, randomized trial comparing cognitive outcomes in infants undergoing inguinal hernia repair under either general anesthesia or spinal anesthesia [52]. At the 2-year interval, the GAS study revealed no association between 1 hour of sevoflurane and adverse neurodevelopmental outcomes compared with infants who underwent the same surgical procedure with spinal anesthesia [53, 54].
Conclusion and next steps
While the diagnostic and prognostic benefits of cross-sectional imaging in the pediatric population are robust, the cost–benefit analysis of using sedation to acquire diagnostic imaging remains complex. The clinical relevance to pediatric populations of anesthetic agent-related neurotoxicity in the form of accelerated neuronal cell death seen in rodent and non-human primate models is uncertain [55]. Furthermore, as astutely delineated by Flick and Warner [56], the observational studies on infant and early childhood exposure to anesthesia and negative neurodevelopmental outcomes later in life are inherently challenging to interpret. These concerns are addressed in consensus statements from the U.S. Food & Drug Administration and other stakeholders including the American Academy of Pediatrics and the American Pediatric Surgical Association [57, 58]. Radiologists who perform scans that require the use of anesthetic and sedative drugs should familiarize themselves with these statements.
Inherent to the discussion of limiting the potential harmful effects of anesthesia on the developing brain are the non-pharmacologic approaches to management of pediatric patients during image acquisition and their relative effectiveness. Imaging simulation and play preparation have been in use for more than 20 years [59, 60] and demonstrate continued success [61, 62]. Stimulation-reduction techniques and feeding neonates and infants immediately prior to MRI have proved effective [63], with a growing evidence base [64]. Cincinnati Children’s Hospital Medical Center demonstrated measurable success reducing sedation of pediatric patients undergoing cross-sectional imaging, by using varied strategies including child-life specialists, video and audio technologies, and moving light shows projected within the imaging suites [65]. In their initial study of children under 7 years of age, the Cincinnati team observed statistically significant decreases in sedation utilization of 34.6% for MRI and 44.9% for CT [65].
Some children undoubtedly require the use of anesthesia to provide suitable conditions for high-quality scans. At present time, our understanding of the potential neurotoxic effects of anesthetic drugs is not sufficient to allow recommendation of one agent vs. another (e.g., inhalational anesthesia vs. propofol). The vast majority of the commonly used anesthetic agents in children have been shown to be of concern in animal models. However, alpha-2 agents, including dexmedetomidine, are not thought to be neurotoxic, and dexmedetomidine might even be neuroprotective in animals [66]. Dexmedetomidine has been successfully used for MRI; however it has a higher failure rate than propofol, has been associated with motion artifact, and has a longer recovery time [67].
Recent advances in image acquisition techniques, including dramatic improvements in isotropic 3-D imaging with the ability to reconstruct images in orthogonal planes, offer pediatric radiologists the opportunity to re-evaluate MR protocols and potentially realize significant time savings without image degradation, and substantially reduce anesthesia times. In addition, development of MR sequences that are less motion-sensitive might allow less anesthesia to be employed for certain clinical indications.
Whereas exposure to some form of anesthesia for surgery is obligatory, in radiology we have the opportunity to apply tools and technologies to systematically reduce rates of pediatric anesthesia exposure. Regardless of what the future demonstrates about correlations between early exposure to anesthesia and neurotoxicity and neurodevelopmental and cognitive impairments, it is not difficult to agree that unnecessary anesthesia exposure, like unnecessary radiation, is a thing to be avoided. We support the priority adoption of an ALARA (dose as low as reasonably achievable) principle for anesthesia such that in the acquisition of pediatric imaging we would apply a multifaceted approach to reduce imaging sedation as much as is reasonably achievable.
References
Gessner KE, Tomlinson C, Jaramillo D et al (2008) Is awareness about CT and radiation exposure effecting imaging utilization at large children’s hospitals? http://archive.rsna.org/2008/6021223.html. Accessed 01 September 2016
Huang Y, Li L, Monteleone M et al (2016) Use of anesthesia for imaging studies and interventional procedures in children. J Neurosurg Anesthesiol 28:400–404
Wachtel RE, Dexter F, Dow AJ (2009) Growth rates in pediatric diagnostic imaging and sedation. Anesth Analg 108:1616–1621
Cravero JP, Blike GT, Beach M et al (2006) Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: report from the pediatric sedation research consortium. Pediatrics 118:1087–1096
Mellon RD, Simone AF, Rappaport BA (2007) Use of anesthetic agents in neonates and young children. Anesth Analg 104:509–520
Davidson AJ (2011) Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatr Anaesth 21:716–721
Slovis TL (2011) Sedation and anesthesia issues in pediatric imaging. Pediatr Radiol 41:S514–S516
Ruess L, O’Connor SC, Mikita CP et al (2002) Sedation for pediatric diagnostic imaging: use of pediatric and nursing resources as an alternative to a radiology department sedation team. Pediatr Radiol 32:505–510
Macias CG, Chumpitazi CE (2011) Sedation and anesthesia for CT: emerging issues for providing high-quality care. Pediatr Radiol 41:S517–S522
Arlachov Y, Ganatra RH (2012) Sedation/anesthesia in pediatric radiology. Br J Radiol 85:e1018–e1031
Starkey E, Sammons HM (2011) Sedation for radiological imaging. Arch Dis Child Educ Pract Ed 96:101–106
Malviya S, Voepel-Lewis T, Tait AR et al (2004) Pentobarbital vs chloral hydrate for sedation of children undergoing MRI: efficacy and recovery characteristics. Paediatr Anaesth 14:589–595
Siddappa R, Riggins J, Kariyanna S et al (2010) High-dose dexmedetomidine sedation for pediatric MRI. Paediatr Anaesth 21:153–158
Mallory MD, Baxter AL, Kost SI et al (2009) Propofol vs pentobarbital for sedation of children undergoing magnetic resonance imaging: results from the pediatric sedation research consortium. Paediatr Anaesth 19:601–611
Baxter AL, Mallory MD, Spandorfer PR et al (2007) Etomidate versus pentobarbital for computed tomography sedations. Pediatr Emerg Care 23:690–695
Cutler KO, Bush AJ, Godambe SA et al (2007) The use of a pediatric emergency medicine-staffed sedation service during imaging: a retrospective analysis. Am J Emerg Med 25:654–661
Malviya S, Voepel-Lewis T, Eldevik OP et al (2000) Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth 84:743–748
Newman DH, Azer MM, Pitetti RD et al (2003) When is a patient safe for discharge after procedural sedation? The timing of adverse effect events in 1,367 pediatric procedural sedations. Ann Emerg Med 42:627–635
Kannikeswaran N, Mahajan PV, Sethuraman U et al (2009) Sedation medication received and adverse events related to sedation for brain MRI in children with and without developmental disabilities. Paediatr Anaesth 19:250–256
Sanborn PA, Michna E, Zurakowski D et al (2005) Adverse cardiovascular and respiratory events during sedation of pediatric patients for imaging examinations. Radiology 237:288–294
Coté CJ, Karl HW, Notterman DA et al (2000) Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics 106:633–644
Loepke AW, Soriano SG (2008) An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg 106:1681–1707
Sanders RD, Hassell J, Davidson AJ et al (2013) Impact of anaesthetics and surgery on neurodevelopment: an update. Br J Anaesth 110:i53–i72
Creeley C, Dikranian K, Dissen G et al (2013) Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth 110:i29–i38
Satomoto M, Satoh Y, Terui K et al (2009) Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology 110:628–637
Brambrink AM, Evers AS, Avidan MS et al (2010) Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 112:834–841
Stratmann G, Lee J, Sall JW et al (2014) Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology 39:2275–2287
Takaenoki Y, Satoh Y, Araki Y et al (2014) Neonatal exposure to sevoflurane in mice causes deficits in maternal behavior later in adulthood. Anesthesiology 120:403–415
Jevtovic-Todorovic V, Hartman RE, Izumi Y et al (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882
Hansen T (2015) Anesthesia-related neurotoxicity and the developing animal brain is not a significant problem in children. Paediatr Anaesth 25:65–72
Anand KJS (2007) Anesthetic neurotoxicity in newborns: should we change clinical practice? Anesthesiology 107:2–4
Pound P, Bracken MB (2014) Is animal research sufficiently evidence based to be a cornerstone of biomedical research? BMJ 348:g3387
Hays SR, Deshpande JK (2013) Newly postulated neurodevelopmental risks of pediatric anesthesia: theories that could rock our world. J Urol 189:1222–1228
Vutskits L, Xie Z (2016) Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci 17:705–717
Hansen TG, Flick R (2009) Anesthetic effects on the developing brain: insights from epidemiology. Anesthesiology 110:1–3
Hansen TG (2013) Neurotoxicity, general anesthesia and the developing brain: what have we learned from the human studies so far? Curr Anesthesiol Rep 3:175–183
DiMaggio C, Sun LS, Kakavouli A et al (2009) A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol 21:286–291
DiMaggio C, Sun LS, Li G (2011) Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg 113:1143–1151
Walker K, Halliday R, Holland AJA et al (2010) Early developmental outcome of infants with infantile hypertrophic pyloric stenosis. J Pediatr Surg 45:2369–2372
Hansen TG, Pedersen JK, Henneberg SW et al (2013) Educational outcome in adolescence following pyloric stenosis repair before 3 months of age: a nationwide cohort study. Paediatr Anaesth 23:883–890
Bong CL, Allen JC, Kim JTS (2013) The effects of exposure to general anesthesia in infancy on academic performance at age 12. Anesth Analg 117:1419–1428
Bartels M, Althoff RR, Boomsma DI (2009) Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet 12:246–253
Hansen TG, Pedersen JK, Henneberg SW et al (2011) Academic performance in adolescence after inguinal hernia repair in infancy. Anesthesiology 114:1076–1085
Kalkman CJ, Peelen L, Moons KG et al (2009) Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology 110:805–812
Wilder RT, Flick RP, Sprung J et al (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804
Flick RP, Katusic SK, Colligan RC et al (2011) Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128:e1053–e1061
Ing C, DiMaggio C, Whitehouse A et al (2012) Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics 130:e476–e485
Taghon TA, Masunga AN, Small RH et al (2015) A comparison of functional magnetic resonance imaging findings in children with and without a history of early exposure to general anesthesia. Paediatr Anaesth 25:239–246
Backeljauw B, Holland SK, Altaye M et al (2015) Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics 136:e1–12
Block RI, Thomas JJ, Bayman EO et al (2012) Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology 117:494–503
Williams RK, Black IH, Howard DB et al (2014) Cognitive outcome after spinal anesthesia and surgery during infancy. Anesth Analg 119:651–660
Nemergut ME, Crow S, Flick RP (2014) Cognitive outcomes after infant spinal anesthesia: the other side of the coin. Anesth Analg 119:514–515
Davidson AJ, Disma N, de Graaff JC et al (2016) Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 387:239–250
Chinn GA, Sasaki Russel JM, Sall JW (2016) Is a short anesthetic exposure in children safe? Time will tell: a focused commentary of the GAS and PANDA trials. Ann Transl Med 4:408–413
Jevtovic-Todorovic V, Absalom AR, Blomgren K et al (2013) Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg seminar. Br J Anaesth 111:143–151
Flick RP, Warner DO (2012) A users’ guide to interpreting observational studies of pediatric anesthetic neurotoxicity. Anesthesiology 117:459–462
SmartTots (2012) Consensus statement on the use of anesthetics and sedatives in children. http://www.asdahq.org/sites/default/files/Smart%20Tots%20Consensus%20Statement%202012.pdf. Accessed 28 Mar 2017
SmartTots (2015) Consensus statement on the use of anesthetic and sedative drugs in infants and toddlers. http://smarttots.org/about/consensus-statement/. Accessed 28 Mar 2017
Rosenberg DR, Sweeney JA, Gillen JS et al (1997) Magnetic resonance imaging of children without sedation: preparation with simulation. J Am Acad Child Adolesc Psychiatry 36:853–859
Sury MRJ, Harker H, Begent J et al (2005) The management of infants and children for painless imaging. Clin Radiol 60:731–741
de Bie HMA, Boersma M, Wattjes MP et al (2010) Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur J Pediatr 169:1079–1085
Hallowell LM, Stewart SE, de Amorem e Silva CT et al (2008) Reviewing the process of preparing children for MRI. Pediatr Radiol 38:271–279
Gale C, Jeffries S, Logan KM et al (2013) Avoiding sedation in research MRI and spectroscopy in infants: our approach, success rate and prevalence of incidental findings. Arch Dis Child Fetal Neonatal Ed 98:F267–F268
Edwards AD, Arthurs OJ (2011) Paediatric MRI under sedation: is it necessary? What is the evidence for the alternatives? Pediatr Radiol 41:1353–1364
Khan JJ, Donnelly LF, Koch BL et al (2007) A program to decrease the need for pediatric sedation for CT and MRI. Appl Radiol 36:30–33
Sanders RD, Xu J, Shu Y et al (2009) Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology 110:1077–1085
Mason KP, Zurakowski D, Zgleszewski SE et al (2008) High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth 18:403–411
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Barton, K., Nickerson, J.P., Higgins, T. et al. Pediatric anesthesia and neurotoxicity: what the radiologist needs to know. Pediatr Radiol 48, 31–36 (2018). https://doi.org/10.1007/s00247-017-3871-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3871-4