Abstract
Background
Some iterative reconstruction algorithms are useful for reducing the radiation dose in pediatric cardiac CT. A new iterative reconstruction algorithm (forward-projected model-based iterative reconstruction solution) has been developed, but its usefulness for radiation dose reduction in pediatric cardiac CT is unknown.
Objective
To investigate the effect of the new algorithm on CT image quality and on radiation dose in pediatric cardiac CT.
Materials and methods
We obtained phantom data at six dose levels, as well as pediatric cardiac CT data, and reconstructed CT images using filtered back projection, adaptive iterative dose reduction 3-D (AIDR 3-D) and the new algorithm. We evaluated phantom image quality using physical assessment. Four radiologists performed visual evaluation of cardiac CT image quality.
Results
In the phantom study, the new algorithm effectively suppressed noise in the low-dose range and moderately generated modulation transfer function, yielding a higher signal-to-noise ratio compared with filtered back projection or AIDR 3-D. When clinical cardiac CT was performed, images obtained by the new method had less perceived image noise and better tissue contrast at similar resolution compared with AIDR 3-D images.
Conclusion
The new algorithm reduced image noise at moderate resolution in low-dose CT scans and improved the perceived quality of cardiac CT images to some extent. This new algorithm might be superior to AIDR 3-D for radiation dose reduction in pediatric cardiac CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac CT has become a standard technique for assessing congenital heart disease [1, 2]. Although cardiac CT provides detailed information on anatomical structures, it exposes patients to ionising radiation [3, 4], which is a concern in children. CT has been associated with an increased incidence of leukemia [5] and thyroid cancer [6].
Lowering the radiation dose, however, results in lower image quality, and this is related to a decrease in diagnostic accuracy. Low-dose images can be improved with reconstruction techniques. Filtered back projection (FBP) is currently the most widespread image reconstruction technique. It is a simple and rapid reconstruction method, but it has disadvantages with respect to poor image quality when low-dose CT is performed [7]. In contrast, iterative reconstruction techniques can be used to improve the quality of low-dose CT images [8]. Various iterative reconstruction algorithms have been developed [9] and introduced clinically for head [10], chest [11], abdominal [12] and cardiac [13] CT. They can be classified into hybrid-iterative reconstruction and full-iterative reconstruction techniques. Hybrid-iterative reconstruction techniques, which only use backward projection, include adaptive iterative dose reduction 3-D (AIDR 3-D; Toshiba Medical Systems, Tochigi, Japan); adaptive statistical iterative reconstruction (ASIR; GE Healthcare, Buchinghamshire, UK), and sinogram affirmed iterative reconstruction (SAFIRE; Siemens Healthcare, Forchheim, Germany). Full-iterative reconstruction techniques are based on both forward and backward projection, and two algorithms have been developed — Veo (GE Healthcare) and iterative model reconstruction (IMR; Philips Healthcare, Best, the Netherlands).
A new full-iterative reconstruction algorithm, forward-projected model-based iterative reconstruction solution (Toshiba Medical Systems), was recently released and has attracted particular interest for reducing CT radiation doses. However, its effects on the quality of low-dose CT are not fully understood. If the new algorithm provides sufficient-quality CT images at lower dose, it is likely to be useful in pediatric cardiac CT.
Therefore we assessed the effect of the new algorithm on image quality and radiation dose in pediatric cardiac CT by grading phantom and clinical cardiac CT images reconstructed by the new algorithm both objectively and qualitatively in comparison with reconstructions of the same datasets using filtered back projection and AIDR 3-D.
Materials and methods
Data acquisition and image reconstruction
A 320-row detector CT system (Aquilion ONE/ViSION; Toshiba Medical Systems, Tochigi, Japan) was used in this study. Imaging parameters are listed in Table 1. The cylindrical water phantom (200 mm in diameter) and Catphan phantom (CTP401/404; Phantom Laboratories, Salem, NY) were scanned for physical assessment of image noise and image resolution, respectively.
We collected raw data of 20 children undergoing contrast-enhanced cardiac CT for retrospective visual evaluation. The Ethical Committee of Shimane University School of Medicine approved this study and waived the requirement for informed consent for this retrospective evaluation. The patients were children younger than 1 year with congenital heart disease. The children received iohexol (Omnipaque; Daiichi Sankyo, Tokyo, Japan) at a dose of 480 mg iodine/kg as the contrast agent for cardiac CT. Iohexol was prepared as a 120-mg iodine/ml solution in saline and was administered intravenously at 1.0–1.5 ml/s with an automatic injector (Dual Shot GX7; Nemoto Kyorindo, Tokyo, Japan).
AIDR 3-D has four selectable noise reduction levels (weak, mild, standard and strong) and the new algorithm has three (cardiac mild, cardiac standard, and cardiac strong). In our preliminary assessment, AIDR 3-D in the mild mode and the new algorithm in the cardiac standard mode achieved the best perceived quality of cardiac CT images. Therefore, in the present study all phantom and cardiac CT images were reconstructed with a 0.5-mm slice thickness and 0.5-mm slice interval by using filtered back projection, AIDR 3-D in the mild mode, and the new algorithm in the cardiac standard mode. The FC04 kernel was applied for filtered back projection and AIDR 3-D images.
Standard deviation of Hounsfield units
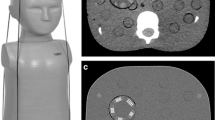
The cylindrical water phantom was scanned and CT images were reconstructed. Five circular regions of interest (ROIs, each 200 mm2) were positioned on the middle slice (Fig. 1). The standard deviation (SD) of the number of Hounsfield units (HU) in each ROI was measured as the variability of the noise signal (noise standard deviation) using ImageJ software (version 1.50b, National Institutes of Health, Bethesda, MD).
Phantom image used for physical assessment of image noise and image resolution. a Five circular regions of interest (ROIs) are positioned on the water phantom image, and the standard deviation of the Hounsfield units (HU) value is calculated. Four peripheral ROIs are located 50 mm outside the central ROI. b Noise power spectra are obtained by the radial frequency method from a square ROI positioned on the water phantom image. c Image of the Catphan phantom, which includes 990 HU Teflon (arrow). The modulation transfer function was obtained by the circular edge method from the square ROI surrounding the Teflon
Noise power spectrum
The noise power spectrum NPS quantifies the frequency characteristics of the noise signal. Higher-frequency noise is fine-grained, so its removal improves the perceived granularity of CT images. In this study, the noise power spectrum was measured by the radial frequency method [14]. Briefly, the cylindrical water phantom was scanned and CT images were reconstructed. Then the noise power spectrum curve was obtained from a central ROI (256 × 256 pixels) positioned on an integrated image of 300 CT slices using ImageJ software (Fig. 1).
Modulation transfer function
The modulation transfer function (MTF) is an indicator of image resolution, with a higher MTF increasing the perceived sharpness of CT images. Image resolution is often specified by the frequency of the 10% MTF value. In this study, the MTF was analyzed by the circular edge method [15] using image analysis software (CT measure version 0.96a; Japanese Society of CT Technology, Hiroshima, Japan). Briefly, a Catphan phantom was scanned and a disk-shaped object (Teflon, 990 HU) on the image of the phantom was surrounded by a square ROI (Fig. 1). The edge of the object was analyzed to determine the edge-spread function, which was differentiated to obtain the line-spread function. Then an object-specific MTF was generated by Fourier transformation of the line-spread function. Data from 50 CT scans were used to create clear MTF curves.
Signal-to-noise ratio
The signal-to-noise ratio (SNR) was calculated at a CTDIvol of 6 mGy using the results of modulation transfer function and noise power spectrum analyses. SNR was defined as a function of spatial frequency (f) and was calculated with the following formulae:
where CS is the contrast scale, μ water and μ air are the linear attenuation coefficients of water and air, respectively, and CT water and CT air are the HU values of water and air, respectively.
Visual evaluation of pediatric cardiac CT images
The effect of the new algorithm on pediatric cardiac CT images was visually assessed by the Scheffe-Nakaya paired comparison method, in which there is no need to consider the order effect [16]. Three kinds of cardiac CT images were reconstructed for each child using the three algorithms. Then a pair of images was randomly selected and displayed on a high-resolution monitor (RadiForce RX220; EIZO, Ishikawa, Japan). Four radiologists (with 17, 16, 6, and 6 years of experience in diagnostic CT imaging) performed comparison of granularity, contrast, and resolution by using five grades (scored from −2 to +2 points: −2 for much worse, −1 for moderately worse, 0 for similar, +1 for moderately better, and +2 for much better). Then we calculated the mean preference scores for each parameter and compared the three algorithms.
Statistical analysis
Differences of the noise standard deviation and preference score among the three algorithms were determined by the Tukey test and yardstick analysis [17], respectively, with P < 0.05 being considered significant. In Fig. 2 the asterisk (*) and hashtag (#) indicate significant differences between the new algorithm and filtered back projection, and the new algorithm and AIDR 3-D, respectively.
Dose-dependent changes of the standard deviation of the Hounsfield units value. The standard deviation of Hounsfield units in circular regions of interest was determined as an indicator of image noise. The noise standard deviation of filtered back projection (FBP) images shows an increase along with reduction of the CT dose index volume (CTDIvol). However, the forward-projected model-based iterative reconstruction solution (the new algorithm) strongly suppresses the increase of noise standard deviation in the low-dose range, and it is more effective than adaptive iterative dose reduction 3-D (AIDR 3-D). Data represent the mean ± standard deviation from measurements made in five regions of interest. Asterisks (*) and hashtags (#) indicate significant differences (P < 0.05) between the new algorithm and filtered back projection, and the new algorithm and AIDR 3-D, respectively
Results
Effect of the new algorithm on noise
In order to investigate the effect of the new algorithm on image noise, we measured the noise standard deviation and noise power spectrum. As demonstrated in Fig. 2, the noise standard deviation of filtered back projection images showed a dramatic increase in noise with CTDIvol reduction. Use of the AIDR 3-D algorithm moderately inhibited the increase of noise standard deviation in the low-dose range. With the AIDR 3-D algorithm, noise standard deviation values were significantly lower at a CTDIvol of 2 mGy (P < 0.001), 3 mGy (P < 0.001), 4 mGy (P < 0.01) and 6 mGy (P < 0.05) as compared with the filtered back projection algorithm. However the new algorithm inhibited the increase of noise standard deviation more effectively. At all CTDIvol levels, noise standard deviation values were significantly lower on images obtained with the new algorithm as compared with filtered back projection images (P < 0.001) and AIDR 3-D images (P < 0.01 at 14 mGy and P < 0.001 at 2 mGy, 3 mGy, 4 mGy, 6 mGy and 11 mGy).
The noise power spectrum of filtered back projection and AIDR 3-D images increased along with reduction of CTDIvol. However, the images obtained with the new algorithm showed an almost constant noise power spectrum at spatial frequencies higher than 0.4 cycles/mm. Furthermore, at the same CTDIvol the new algorithm provided the lowest noise power spectrum values among the three algorithms (Fig. 3).
Dose-dependent changes of the noise power spectrum (NPS) curve. a Filtered back projection (FBP), (b) adaptive iterative dose reduction 3-D (AIDR 3-D) and (c) forward-projected model-based iterative reconstruction solution (new algorithm). The noise power spectrum of filtered back projection and AIDR 3-D increased as the level of radiation exposure decreased, while the new algorithm prevented an increase of noise power spectrum in the low-dose range. d Comparison of noise power spectrum at a CT dose index volume (CTDIvol) of 6 mGy. At the same CTDIvol, the new algorithm achieves the lowest noise power spectrum among the three algorithms
Effect of the new algorithm on spatial resolution
We measured modulation transfer function to investigate the effect of the new algorithm on spatial resolution. As can be seen in Fig. 4, the modulation transfer function of AIDR 3-D images showed a slight decline compared with that of filtered back projection images. In contrast, modulation transfer function was higher with the images obtained with the new algorithm than in filtered back projection images. The spatial frequency at 10% modulation transfer function was 0.75 for filtered back projection, 0.73 for AIDR 3-D and 0.96 for the new algorithm.
Modulation transfer function (MTF) curves obtained at a CT dose index volume of 6 mGy. The influence of the forward-projected model-based iterative reconstruction solution (new algorithm) on image resolution is examined by measuring the modulation transfer function. The new algorithm improves image resolution compared with filtered back projection (FBP). In contrast, use of adaptive iterative dose reduction 3-D (AIDR 3-D) results in a slight decrease of image resolution
Effect of the new algorithm on signal-to-noise ratio
SNR was calculated as a comprehensive index of image quality. Filtered back projection and AIDR 3-D images showed almost the same SNR values over the entire spatial frequency range, while the images obtained with the new algorithm had the highest SNR values at spatial frequencies above 0.2 cycles/mm (Fig. 5).
Signal-to-noise ratio (SNR) curves obtained at a CT dose index volume of 6 mGy. The forward-projected model-based iterative reconstruction solution (new algorithm) achieves the highest signal-to-noise ratio value over the entire spatial frequency range. Signal-to-noise ratio curves are similar for adaptive iterative dose reduction 3-D (AIDR 3-D) and filtered back projection (FBP) images
Effect of the new algorithm on cardiac CT image quality
We visually evaluated the quality of clinical images to assess the possibility of reducing radiation doses by using the new algorithm in pediatric cardiac CT. While numerous streak artifacts were observed on filtered back projection and AIDR 3-D images, the new algorithm effectively prevented streak artifacts and sharpened the microarchitecture such as tiny blood vessels in the lungs.
According to Scheffe-Nakaya paired comparison, filtered back projection, AIDR 3-D and the images obtained with the new algorithm had similar preference scores for resolution. Although AIDR 3-D images had a slightly higher preference score for granularity compared with filtered back projection images, there was no significant difference. However, the images obtained with the new algorithm had a significantly higher preference score for granularity compared with both the filtered back projection images (P < 0.05) and the AIDR 3-D images (P < 0.05). Furthermore, there was a significantly higher preference score for contrast in the images obtained with the new algorithm as compared with filtered back projection images (P < 0.05) and AIDR 3-D images (P < 0.05), although the actual differences were small (Fig. 6).
Axial CT images in a 1-year-old girl with a single right ventricle and common atrioventricular valve regurgitation. Images were obtained at a CT dose index volume of 6.7 mGy and were reconstructed by using (a) filtered back projection (FBP), (b) adaptive iterative dose reduction 3-D (AIDR 3-D) and (c) forward-projected model-based iterative reconstruction solution (new algorithm). In (c), tiny blood vessels in lungs are sharply defined (arrow) and there are fewer streak artifacts (arrowheads) in images obtained with the new algorithm. d Preference scores for granularity, contrast and resolution. Compared with FBP and AIDR 3-D images, the new algorithm provides better granularity and contrast with moderate resolution. Asterisks (*) and hashtags (#) indicate significant differences (P < 0.05) between the new algorithm and FBP, and the new algorithm and AIDR 3-D, respectively
Discussion
In the present study, the new algorithm achieved higher-quality low-dose CT images compared with the filtered back projection and AIDR 3-D algorithms. In the phantom study, images obtained with the new algorithm had lower noise levels and higher spatial resolution, resulting in a higher SNR. Visual evaluation of pediatric cardiac CT images provided further evidence that the new algorithm produces high-quality images, because the proposed algorithm significantly improved the granularity and contrast of cardiac CT images as compared with AIDR 3-D images. Moreover, streak artifacts were effectively prevented by the new algorithm. These findings suggest that the new algorithm could allow further radiation dose reduction in pediatric cardiac CT.
Although details of the reconstruction process employed by the new algorithm have not been disclosed, it is thought to provide low-noise and high-resolution CT images by taking into account the optics model in addition to the statistical noise model. The noise level is one of the most important determinants of image quality. It is well known that the filtered back projection algorithm strongly enhances the noise of radiation dose-reduced CT images [7]. In fact, FBP-reconstructed phantom images showed considerable noise in the lower-dose range. The AIDR 3-D algorithm was designed to allow performance of lower-dose CT by using noise-suppression processes involving the statistical noise model, scanner model and projection noise estimation. Tomizawa et al. [18] reported that AIDR 3-D coronary CT angiography images that were obtained with 40% reduction of tube current maintained similar image quality to standard-dose filtered back projection images, with 22% reduction of radiation exposure [18]. Similar results have been reported with other hybrid iterative reconstruction algorithms such as Iterative Reconstruction in Image Space (IRIS; Siemens Healthcare) [19] and ASIR [20]. In this study, AIDR 3-D inhibited the elevation of noise standard deviation and noise power spectrum values in the low-dose range, but it was less effective than the new algorithm. This indicates that the new algorithm has an advantage in noise suppression compared with AIDR 3-D.
Noise suppression is important for creating high-quality images but is associated with loss of spatial resolution. Depending on the iterative reconstruction level, kernel or imaging conditions, use of hybrid-iterative reconstruction algorithms often results in a decline of modulation transfer function [21]. In our phantom study performed at a CTDIvol of 6 mGy, AIDR 3-D suppressed image noise with slight loss of spatial resolution compared with filtered back projection. However, the new algorithm strongly reduced noise with an increase of modulation transfer function, resulting in a higher SNR. These results suggest that the new algorithm could suppress noise without loss of spatial resolution. In fact, visual evaluation confirmed excellent granularity and moderate resolution of cardiac CT images obtained with the new algorithm. The microarchitecture, such as tiny blood vessels in the lungs, was sharply defined by the new algorithm. Other full-iterative reconstruction algorithms like iterative model reconstruction [21, 22] and Veo [21, 23] have been implemented to improve the spatial resolution of low-dose CT scans. It seems that the new algorithm has similar effects to these full-iterative reconstruction algorithms.
At our hospital, AIDR 3-D in the mild mode has routinely been used for dose reduction in pediatric cardiac CT, resulting in a mean CTDIvol of 6 mGy, which is nearly 50% less than the diagnostic reference level for pediatric chest CT in Japan [24]. In the present study, the new algorithm achieved significantly higher-quality cardiac CT images as compared with AIDR 3-D. According to the observers, the cardiac CT images obtained with the new algorithm exhibited better granularity and contrast through improved suppression of noise, suggesting that use of this algorithm would allow further radiation dose reduction in pediatric cardiac CT. Even at the same dose level, it could be clinically advantageous by providing better image quality as compared with AIDR 3-D. However, image reconstruction takes longer with our new algorithm, so it may be unsuitable for use in emergencies.
This study has several limitations. First, it was difficult to estimate the dose reduction capacity of the new algorithm for pediatric cardiac CT under our experimental conditions. Second, we did not investigate the influence of the new algorithm on diagnostic accuracy. Although we demonstrated that further radiation dose reduction might be possible, it is not clear whether the new algorithm had a negative effect on the diagnostic accuracy of lower-dose pediatric cardiac CT. More detailed studies are necessary for practical application of our new algorithm according to the “as low as reasonably achievable” concept [25]. Finally, we did not assess the quality of cardiac CT and phantom images reconstructed by our new algorithm using other modes (cardiac mild and cardiac strong). It has been reported that using the iterative reconstruction technique with higher reconstruction levels creates unfamiliar noise textures and could lead to reduced diagnostic accuracy [26–28]. Further studies are required to make a detailed assessment of the effects of our new algorithm on image quality.
Conclusion
Forward-projected model-based iterative reconstruction solution suppressed noise in radiation-dose-reduced CT images more effectively than AIDR 3-D, without the loss of spatial resolution, and the new algorithm might therefore enable further reduction of the radiation dose required for pediatric cardiac CT. Although the perceived image quality of cardiac CT images obtained with the new algorithm was similar to that of filtered back projection and AIDR 3-D images, the new algorithm achieved somewhat lower perceived noise and improved perceived image contrast.
References
Juan CC, Hwang B, Lee PC et al (2011) Diagnostic application of multidetector-row computed tomographic coronary angiography to assess coronary abnormalities in pediatric patients: comparison with invasive coronary angiography. Pediatr Neonatol 52:208–213
Kulkarni A, Hsu HH, Ou P et al (2016) Computed tomography in congenital heart disease: clinical applications and technical considerations. Echocardiography 33:629–640
Hausleiter J, Meyer T, Hermann F et al (2009) Estimated radiation dose associated with cardiac CT angiography. JAMA 301:500–507
Dill T, Deetjen A, Ekinci O et al (2008) Radiation dose exposure in multislice computed tomography of the coronaries in comparison with conventional coronary angiography. Int J Cardiol 124:307–311
Miglioretti DL, Johnson E, Williams A et al (2013) The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 167:700–707
Su YP, Niu HW, Chen JB et al (2014) Radiation dose in the thyroid and the thyroid cancer risk attributable to CT scans for pediatric patients in one general hospital of China. Int J Environ Res Public Health 11:2793–2803
Naoum C, Blanke P, Leipsic J (2015) Iterative reconstruction in cardiac CT. J Cardiovasc Comput Tomogr 9:255–263
Beister M, Kolditz D, Kalender WA (2012) Iterative reconstruction methods in X-ray CT. Phys Med 28:94–108
Padole A, Ali Khawaja RD, Kalra MK et al (2015) CT radiation dose and iterative reconstruction techniques. AJR Am J Roentgenol 204:W384–W392
McKnight CD, Watcharotone K, Ibrahim M et al (2014) Adaptive statistical iterative reconstruction: reducing dose while preserving image quality in the pediatric head CT examination. Pediatr Radiol 44:997–1003
Gay F, Pavia Y, Pierrat N et al (2014) Dose reduction with adaptive statistical iterative reconstruction for paediatric CT: phantom study and clinical experience on chest and abdomen CT. Eur Radiol 24:102–111
Kalra MK, Woisetschläger M, Dahlström N et al (2012) Radiation dose reduction with sinogram affirmed iterative reconstruction technique for abdominal computed tomography. J Comput Assist Tomogr 36:339–346
Chen MY, Steigner ML, Leung SW et al (2013) Simulated 50% radiation dose reduction in coronary CT angiography using adaptive iterative dose reduction in three-dimensions (AIDR3D). Int J Cardiovasc Imaging 29:1167–1175
Kijewski MF, Judy PF (1987) The noise power spectrum of CT images. Phys Med Biol 32:565–575
Richard S, Husarik DB, Yadava G et al (2012) Toward task-based assessment of CT performance: system and object MTF across different reconstruction algorithms. Med Phys 39:4115–4122
Nikkagiren kannoukensa iinkai (1999) [Kannoukensa handbook]. Nikkagiren, Tokyo
Scheffe H (1952) An analysis of variance for paired comparisons. J Am Stat Assoc 47:381–400
Tomizawa N, Nojo T, Akahane M et al (2012) Adaptive iterative dose reduction in coronary CT angiography using 320-row CT: assessment of radiation dose reduction and image quality. J Cardiovasc Comput Tomogr 6:318–324
Renker M, Ramachandra A, Schoepf UJ et al (2011) Iterative image reconstruction techniques: applications for cardiac CT. J Cardiovasc Comput Tomogr 5:225–230
Tumur O, Soon K, Brown F et al (2013) New scanning technique using adaptive statistical iterative reconstruction (ASIR) significantly reduced the radiation dose of cardiac CT. J Med Imaging Radiat Oncol 57:292–296
Löve A, Olsson ML, Siemund R et al (2013) Six iterative reconstruction algorithms in brain CT: a phantom study on image quality at different radiation dose levels. Br J Radiol 86:20130388
Suzuki S, Haruyama T, Morita H et al (2014) Initial performance evaluation of iterative model reconstruction in abdominal computed tomography. J Comput Assist Tomogr 38:408–414
Miévillea FA, Gudinchetb F, Brunellec F et al (2013) Iterative reconstruction methods in two different MDCT scanners: physical metrics and 4-alternative forced-choice detectability experiments — a phantom approach. Phys Med 29:99–110
Japan Network for Research and Information on Medical Exposures (2015) Diagnostic reference levels based on latest surveys in Japan — Japan DRLs 2015. http://www.radher.jp/J-RIME/report/DRLhoukokusyoEng.pdf. Accessed 26 Feb 2016
International Commission on Radiological Protection (2007) The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP 37:1–332
Kligerman S, Mehta D, Farnadesh M et al (2013) Use of a hybrid iterative reconstruction technique to reduce image noise and improve image quality in obese patients undergoing computed tomographic pulmonary angiography. J Thorac Imaging 28:49–59
Nelson RC, Feuerlein S, Boll DT (2011) New iterative reconstruction techniques for cardiovascular computed tomography: how do they work, and what are the advantages and disadvantages? J Cardiovasc Comput Tomogr 5:286–292
Khawaja RD, Singh S, Otrakji A et al (2015) Dose reduction in pediatric abdominal CT: use of iterative reconstruction techniques across different CT platforms. Pediatr Radiol 45:1046–1055
Acknowledgments
We are grateful to the staff of the Departments of Radiology at Shimane University Hospital and Shimane University School of Medicine for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Nishiyama, Y., Tada, K., Nishiyama, Y. et al. Effect of the forward-projected model-based iterative reconstruction solution algorithm on image quality and radiation dose in pediatric cardiac computed tomography. Pediatr Radiol 46, 1663–1670 (2016). https://doi.org/10.1007/s00247-016-3676-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3676-x