Abstract
Background
Undifferentiated embryonal sarcoma of the liver is a rare malignant mesenchymal tumour occurring mostly in children ages 6–10 years. The discrepancy between its solid appearance on US and cystic-like appearance on CT has been described.
Objective
To study the imaging particularities and similarities among our cases of undifferentiated embryonal sarcoma and to report the errors in initial diagnoses.
Materials and methods
We conducted a retrospective study of 15 children with undifferentiated embryonal sarcoma diagnosed or referred to our hospital during 1997–2015 and analysed the clinical, biological and imaging data.
Results
We identified eight boys and seven girls ages 9 months to 14 years. Ten children presented with abdominal pain. Alpha-fetoprotein was slightly increased in one. Initial US and CT had been performed for all, while additional MRI had been done in two children. Initial CT demonstrated a hypoattenuated mass in all. Rupture was seen in five and intratumoural bleeding in seven children. Tumour volumes reduced during neoadjuvant chemotherapy in 10 children.
Conclusion
Undifferentiated embryonal sarcoma might be suggested in a non-secreting unifocal tumour with well-defined borders, fluid-filled spaces on US, hypoattenuation and serpiginous vessels on CT, and if there are signs of internal bleeding or rupture on CT or MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
First described by Stocker and Ishak [1] in 1978, undifferentiated embryonal sarcoma of the liver is a rare malignant mesenchymal tumour occurring mostly in children ages 6 years to 10 years. Cases of younger children and adults have also been encountered. It was historically reported as having a poor prognosis, but current literature shows that a combination of surgery, pre- and post-surgical chemotherapy, and sometimes radiotherapy can substantially improve clinical outcome in these children [2–5]. The imaging characteristics of this lesion have been scarcely described and there has been an emphasis on the discrepancy between the solid aspect on US and the cystic-like appearance on CT. Although the diagnosis of this non-secreting tumour is based on anatomopathology, recent articles [6, 7] have addressed possible pitfalls in diagnosis.
Our goal was to review the different imaging aspects of undifferentiated embryonal sarcoma encountered at our hospital over the last 18 years and to look for the imaging features that support the diagnosis of undifferentiated embryonal sarcoma.
Materials and methods
We retrospectively included undifferentiated embryonal sarcoma cases that were diagnosed or referred and treated between 1997 and 2015 in our hospital, which is a tertiary centre for liver surgery in children. Children were identified in the database of malignant hepatic tumours diagnosed in our department and treated by our surgical team.
This retrospective study was conducted without the need for internal review board approval. Some of our patients were also included in the surgical series of Wildhaber et al. [6] and Merli et al. [7].
Our review was based on a thorough analysis of the relevant clinical, biological and imaging data of each child. We first determined the most frequent clinical manifestations, looking for the blood level of alpha-fetoprotein and for biological signs of anemia. We also collected medical, surgical treatment and follow-up data for each child. We studied the different imaging aspects of the tumour, including contours, dimensions, aspect, enhancement, signs of bleeding and rupture. Imaging of all studies was analysed retrospectively by two senior radiologists from our department: a clinical fellow (FG: more than 6 years of experience) and a senior consultant (DP: more than 20 years of experience) who is specialised in liver imaging.
The terms “hypoechoic”, “hyperechoic” and “anechoic” were defined by comparing tumour structure with the unaffected surrounding liver. The term “cystic” was used to describe a well-defined fluid-filled space. In CT, “low-attenuation” was used to characterise the part of the tumour that appears hypoattenuated in comparison to the unaffected surrounding liver. In MRI, the terms “hyperintense” and “hypointense” were used to characterise the tumour signal by comparing it with the signal of the unaffected surrounding liver.
The clinical fellow estimated tumour volume (V) in five children, using the equation that calculates the volume of ellipsoid structures: 4/3 × π × radius 1 × radius 2 × radius 3. However in early cases, where only films were available for review, only the largest diameter on axial images was measured.
In our series we defined two types of tumour rupture: the discontinuity of the tumour border, which was sometimes accompanied by intratumoural haemorrhage, was defined as a “subcapsular rupture”. If this aspect was accompanied by intraperitoneal free fluid, it was considered to be an “intraperitoneal rupture”. Staging was done by means of CT scans at the time of diagnosis.
At our institution, the treatment scheme for undifferentiated embryonal sarcoma followed the malignant mesenchymal tumours protocol (SIOP-MMT95) before 2005 and the rhabdomyosarcoma 2005-EpSSG protocol from 2005 onwards [7]. These protocols included preoperative biopsy, neoadjuvant chemotherapy, surgery, and adjuvant chemotherapy with or without associated radiotherapy, depending on the degree of resection.
Results
Our population consisted of 15 children (8 boys) ages 9 months to 14 years, with a median age of 9 years. Thirteen of the 15 US examinations performed at diagnosis were available for review. Every child had been assessed with an initial abdominal and pelvic CT to determine the precise extent of the liver mass and to evaluate possible spread of the disease outside the liver. Additional MRI had been performed in two cases at diagnosis and in one before surgery.
Nine children presented with abdominal pain. A routine follow-up for Beckwith–Wiedemann syndrome led to the discovery of the mass in another child. Fever was documented in three children and anemia in four (Table 1). Alpha-fetoprotein value was normal in 14 children; it was slightly increased (20 ng/ml) in our youngest patient, who was 9 months old (case 6).
The tumour was unifocal in all studies and located in the right liver in 14 cases. According to the PRETEXT staging system [8], nine tumours were classified as PRETEXT II, four as PRETEXT III and two as PRETEXT I.
The largest initial tumour diameter was estimated to be between 6 cm and 21 cm, with a mean value of 13.5 cm.
Initial US characteristics could be assessed in 13 children (Table 2). Nine lesions were predominantly hyperechoic, two were predominantly anechoic. Cystic spaces were present in 10 observations, emerging from all three types of echoic appearance (Fig. 1).
Varied US appearances of undifferentiated embryonal sarcoma of the liver. a Image in a 5-year-old boy (case 10) who presented with pain and fever. Transverse US image demonstrates a round hyperechoic right liver lesion (black arrows). Notice the presence of several anechoic fluid-filled spaces (white arrows). b Image in a 4-year-old girl (case 9) who presented with a painless abdominal mass. Transverse US image shows an exophytic mass of the liver (delimitated by callipers) with a predominantly hypoechoic content (black arrowheads) compared to the surrounding normal hepatic tissue (not shown on the image), with several septa within (white arrowheads). Notice the two anechoic spaces (white arrows), which correspond to fluid-filled spaces. c Images in a 7-year-old boy (case 11) with Beckwith-Wiedemann who presented for a surveillance scan. A transverse US image demonstrates a liver mass (delimitated between callipers) with predominantly anechoic content (arrowheads) delimitated by septa (white arrows). Notice the presence of a solid hyperechoic component (black arrows)
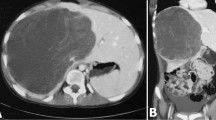
Initial CT scans demonstrated well-defined borders in all studies (Fig. 2). The lesions had a predominantly low- attenuating appearance in all cases. Nodular solid components of lesions in nine children (Fig. 3) and intratumoural septations in 14 children demonstrated mild progressive enhancement. Serpiginous vessels were observed in the tumour at the arterial phase after contrast enhancement in nine children at initial studies (Fig. 4) and at recurrence of the tumour in one other child.
Nodular components on CT in a 6-year-old girl (case 8) with undifferentiated embryonal sarcoma of the liver who presented with abdominal pain. a Axial non-enhanced CT demonstrates a predominantly hypoattenuated mass (arrow) compared with the normal liver (not shown on the image), with a peripheral nodular component (blue circle; density of 31 Hounsfield units [HU]), slightly denser than the rest of the lesion. b Axial enhanced CT of the mass (white arrow) during portal phase at the same level as in (a) shows the enhancement of the previously described peripheral nodule (blue circle; density 60 HU) and also of the anterior surrounding portion (black arrow). The rest of the mass remains hypoattenuated compared to the normal liver (not shown in this image). c Coronal reconstruction of the enhanced CT during portal venous phase demonstrates the predominantly exophytic development of the hypoattenuating mass (arrowhead) from the inferior surface of the 3rd and 4th segments. Notice the irregular cranial margin of the tumour (arrows)
Serpiginous vessels on enhanced CT in a 5-year-old boy (case 10) with undifferentiated embryonal sarcoma of the liver. Axial enhanced CT during arterial phase (thin maximum-intensity projection) shows numerous small vessels with a sinuous trajectory within the mass (arrowheads). Notice once more the hypoattenuated aspect of the mass (arrow) compared with the normal density of the surrounding liver
Venous thrombus was not found in any of the observations but the lack of visibility of one or several liver veins, mostly because of vascular compression by the mass, was noted in some instances. None of the children had adenopathy or secondary lesions.
MRI (performed in only two children), showed a hyperintense heterogeneous signal with cystic components on T2-weighted images (Figs. 5 and 6) and a hypointense signal of the mass on T1-weighted images (Fig. 5). Bleeding resulted in hyperintense intratumoural zones on T1-weighted images; these zones were hypointense on T2-weighted images. This was observed on both MRIs, with fluid-fluid levels present in one of the children (Figs. 5 and 6). Both children displayed progressive enhancement, with the strongest signal seen on late acquisitions.
MRI in an 11-year-old girl (case 2) with undifferentiated embryonal sarcoma of the liver who presented with abdominal pain and anaemia. a Axial non-enhanced T1-weighed (repetition time/echo time [TR/TE] = 155/1.5 ms) gradient echo with fat saturation demonstrates a right liver mass (arrow) with predominantly hypointense signal compared to the signal of the normal surrounding liver. Notice the lack of visibility of the lateral margin of the mass resulting from peripheral rupture with a liquid collection under the liver capsule, partly haemorrhagic (white arrowhead). Also notice intratumoural haemorrhage (black arrowhead). b Axial non-enhanced T2-weighed (TR/TE = 6,923/90 ms) fast spin echo MRI with fat saturation demonstrates that the mass (arrow) is predominantly hyperintense compared to the signal of the normal surrounding liver. As in (a), notice the lack of visibility of the lateral margin of the mass from the peripheral rupture, with the previously described liquid collection under the liver capsule (white arrowhead). The intratumoural haemorrhagic zone (black arrowhead) has low signal compared to the rest of the mass. c Coronal non-enhanced T2-weighed fast spin echo (TR/TE = 5,667/89 ms) shows once more the predominantly high signal mass (arrow) with poor definition of the lateral margin caused by rupture and subcapsular collection (white arrowheads), and intratumoural haemorrhage (black arrowhead). d Coronal enhanced T1-weighed (TR/TE = 155/1.4 ms) gradient echo with fat saturation shows heterogeneous enhancement of the mass (black arrowhead) when compared to the surrounding normal liver. The mass contains nonenhancing zones (white arrowhead). The lateral margin of the mass is interrupted by the known rupture; the subcapsular collection (white arrow) does not enhance, as seen by the predominantly low signal compared to the enhancing portion of the tumour and the normal liver
MRI in a 9-year-old girl (case 7) with undifferentiated embryonal sarcoma of the liver who presented with abdominal pain. Axial T2-weighed (TR/TE = 7,826/86 ms) spin echo MRI with variable phase encoding direction shows a right liver tumour (arrowhead) with relatively high signal intensity. Notice the small fluid-fluid level (arrow), the dependent component of which is hypointense, consistent with haemorrhage. TE echo time, TR repetition time
Patient 14 underwent MRI after chemotherapy but prior to surgery. His MRI showed a predominantly hypointense/hyperintense area on T1-weighted images and T2-weighted images, respectively, alongside total lack of enhancement.
The tumour was ruptured in five children (Fig. 5) and intratumoural bleeding was documented in seven children, with three having fluid-fluid levels (Fig. 6).
In 10 of the children, diagnosis was made by US-guided biopsy; CT biopsy was performed in 1 child. Tumour resection provided the anatomopathological result in three children. For patient 6, who had a mixed tumour consisting of cystic mesenchymal hamartoma and undifferentiated embryonal sarcoma, the initial diagnosis provided by US biopsy was cystic mesenchymal hamartoma, the final diagnosis being provided by surgical material (Fig. 7).
Mixed tumour in a 9-month-old boy (case 6) who presented with an abdominal mass. a Axial enhanced CT during portal phase through the upper part of the right liver mass (mesenchymal hamartoma, white arrowheads) shows the hypoattenuated content of the lesion compared to the surrounding normal liver. Notice the septations (black arrowheads) within the mass and a solid nodular component (arrow). b Axial enhanced CT during portal venous phase through the lower part of the right liver mass (undifferentiated embryonal sarcoma; white arrowheads) demonstrates heterogeneous content and enhancement and hyperattenuated dots (black arrowheads) consistent calcifications
The initial diagnosis was wrong in four children, and cystic mesenchymal hamartoma components were present in one child (case 6). Imaging interpretation was responsible for two of these errors (initial diagnosis of liver haematoma for case 5 and cystic lymphangioma for case 9) (Fig. 8). Histopathological analyses of percutaneous liver biopsies were responsible for two other errors, cystic mesenchymal hamartoma for case 11 and hepatoblastoma for case 2 (Fig. 5) (Table 1).
Diagnostic pitfalls in a 4-year-old girl (case 9) with undifferentiated embryonal sarcoma of the liver. a Axial enhanced CT during portal venous phase shows a hypoattenuated liver mass (thick arrows) within the lower part of the liver. Because of the presence of multiple fluid-fluid levels, with hyperattenuated lower levels (arrows) consistent with bleeding and separated by septations (white arrowhead), the initial erroneous imaging diagnosis was cystic lymphangioma. b Coronal enhanced CT during the portal venous phase demonstrates the hypoattenuated liver mass (arrow) within the lower part of the liver, with a predominant extrahepatic development
Prior to initial treatment, the tumour increased rapidly in size in two patients. In case 8, the tumour increased by 60% over 4 days (Fig. 9) after which it was treated by chemotherapy. In case 2, the tumour increased by about 510% within the first month after discovery of the mass and the child exhibited signs of Budd-Chiari syndrome from the compression of the inferior vena cava. Thus, emergency surgery was performed on this child prior to any chemotherapy.
Tumour evolution in a 6-year-old girl (case 8) with undifferentiated embryonal sarcoma of the liver (same girl as in Fig. 3). a–c Evolution of the tumour in coronal reconstructions of portal venous phase contrast-enhanced CT. a At diagnosis the hypoattenuated liver mass (arrow) in the lower part of the liver with extrahepatic development has greatest diameter of 17 cm (red calliper). b Four days after diagnosis there has been spontaneous increase of the mass caused by haemorrhage. Its greatest diameter now measures almost 20 cm (red calliper). Free intraperitoneal fluid (arrowhead) is seen from rupture of the tumour. c After 3 months of chemotherapy the mass (arrow) shows a significant decrease in size (greatest diameter about 13 cm, red calliper), with homogeneous hypoattenuated content and the appearance of capsular enhancement (arrowhead)
In the 10 cases where chemotherapy was administered prior to surgery, a decrease in tumour size was observed (Fig. 9). During the time between initial diagnosis or the start of the chemotherapy, and the moment of surgical treatment, we calculated that the decrease in tumour volume ranged 48–86% in the cases in which the volume could be assessed.
Following initial chemotherapy imaging also demonstrated a change in the appearance of the lesions, with three of them becoming homogeneously more hypoattenuated on CT, still with no evident contrast enhancement (cases 8, 10, 12) (Fig. 9).
All tumours were surgically removed. An emergency liver transplant had to be performed because of liver insufficiency after tumour removal for patient 11. In 13 children, surgery was performed at least 1 month after initial diagnosis (mean interval of 4.5 months).
Five children underwent postsurgical radiotherapy while the 14 non-transplanted children had postoperative chemotherapy (Table 1).
Tumour recurrence was only seen in one patient (case 9) 3.5 years after initial diagnosis. All patients are alive at the time of this report.
Discussion
Undifferentiated embryonal sarcoma is a rare malignant tumour of mesenchymal origin; it is the third most common primary malignant tumour of the liver in childhood, after hepatoblastoma and hepatocellular carcinoma. As in other series, most of our patients were ages 6–10 years at diagnosis, with a median age of 9 years; however our youngest patient was only 9 months old. The youngest reported patient with undifferentiated embryonal sarcoma in literature was 4 months [3].
Alpha fetoprotein, usually not increased in this type of tumour [2–4, 9] can be exceptionally elevated, as was found in one patient from the series published by Ismail et al. [3]; this patient had a serum alpha fetoprotein level of 3,410 UI/ml (normal value <5 IU/ml). We also found a slight increase of alpha fetoprotein in patient 6 (as alpha fetoprotein is expected to reach adult levels after 8 months of age) [10], who had a mixed type of tumour combining cystic mesenchymal hamartoma and undifferentiated embryonal sarcoma; this could be explained by the presence of the cystic mesenchymal hamartoma component of the mass [6].
Anemia from mass bleeding, a feature not discussed in previous studies, was documented in four of the patients studied here.
The mass is said to appear on US as a solid heterogeneous tumour, mostly hyperechoic [11–14]; however in the series presented here, anechoic cyst-like spaces were also observed in most cases. Moreover in two studies the lesion was predominantly anechoic and composed of fluid-filled spaces separated by septa, simulating a benign tumour.
On CT, the lesion appears as a solitary and well-defined predominantly hypoattenuated mass, which gives the false impression of a cystic appearance [11, 14]. However, solid nodules and septations were also present in the majority of our cases, showing progressive enhancement. Serpiginous vessels within the tumour were not emphasised in the literature but were observed in 10 studies here. We consider these vessels to be of an arterial origin because they appeared during the early enhanced acquisition. In one child, these vessels were not present at the initial diagnosis but appeared when the disease recurred 3.5 years later. These vessels were observed in most of the cases studied here, so we think the presence of these serpiginous vessels is an important finding in diagnosing undifferentiated embryonal sarcoma when the radiologist is confronted with a hypoattenuated cystic-like tumour appearance on CT.
Bleeding, defined in CT as foci of slightly higher attenuation [11, 12] and as zones having a high signal on T1-weighted MR images and low signal on T2-weighted MR images [11, 14] or as fluid-fluid levels in both CT and MR images, was observed in nearly half of the children studied here.
The discrepancy between the predominant solid-like appearance on US and the cyst-like appearance on CT, which has been emphasised in the literature [14], could be explained by the myxoid component and the bleeding areas, which are usually hyperechoic on US.
The possibility of spontaneous rupture of this type of neoplasm [14] is a very important feature of this tumour because of the risk of haemorrhage and dissemination in the peritoneal cavity.
CT had been the examination of choice to assess tumour characteristics for several reasons. Given the short acquisition time, it rapidly offers all the information needed (location, boundary, relation with surrounding vessels, assessment of tumour rupture/bleeding, peritoneal free fluid, metastasis), which is a true advantage considering the patience of children confronted with the discomfort or pain caused by the mass and also the ease of access to the machine in emergency. However, in the literature [11, 14, 15] MRI is considered the examination of choice when it comes to assessing the surgical resectability of the mass as well as the boundaries between the tumour and surrounding biliary and vascular structures.
None of our patients presented with metastasis at the time of diagnosis, although there are literature reports of secondary lesions in the lungs [4, 9], thymus [2], pleura and peritoneum [11].
In most of the cases studied here, the diagnosis was made histopathologically from material provided by US-guided biopsy as in the series of Ismail et al. [3], where five of seven results were obtained by using US guidance. While occasional non-diagnostic US-guided biopsies can occur [9], this did not happen in our series.
Pathology reveals a neoplasm of a mesenchymal origin with sarcomatous features consisting of variably shaped cells (spindle, oval and stellate) associated with a myxoid stroma [5, 16]. A definitive pathological diagnosis of undifferentiated embryonal sarcoma is based on immunohistochemical marking that is positive for CD56, CD68, vimentine and desmine; it is negative for myogenine, which differentiates undifferentiated embryonal sarcoma from rhabdomyosarcoma [5].
When it comes to the accuracy in establishing the right diagnosis, errors can occur. In our series, imaging interpretation was responsible for two diagnosis errors, haematoma in one case and cystic lymphangioma in another; anatomopathology was responsible for the wrong initial diagnosis in two other cases, hepatoblastoma and cystic mesenchymal hamartoma. In case 5, a child who was initially diagnosed with a haematoma, the post-traumatic context of the discovery of the mass influenced the radiologist’s report. In case 9, the lesion appeared predominantly exophytic to the liver, with multiple cystic areas with fluid/fluid levels separated by septa on CT, leading to the false initial diagnosis of cystic lymphangioma (Figs. 1 and 8). Ismail et al. [3] reported the case of a child with undifferentiated embryonal sarcoma diagnosed initially with hepatoblastoma who therefore was administrated an inadequate chemotherapy and, because the tumour did not respond in the awaited manner, he had to undergo liver transplant. In the series of Kim et al. [8], one out of the six children with undifferentiated embryonal sarcoma was initially diagnosed with cystic mesenchymal hamartoma. A wrong initial histological diagnosis occurred in one-third of the series published by Wildhaber et al. [6], who therefore recommended the collection of enough biopsy material either by needle or open biopsy, the demand of a second opinion if any doubt exists, and the complete resection of solid–cystic masses in order to get an accurate final diagnosis and a proper treatment.
The association between cystic mesenchymal hamartoma and undifferentiated embryonal sarcoma is known and debated in the literature. In a systematic review of cystic mesenchymal hamartoma, Stringer and Alizai [17] consider that there is clinical and histological evidence that undifferentiated embryonal sarcoma can develop within a pre-existing cystic mesenchymal hamartoma because both entities share common features on gross pathology (cystic and solid components), histology (mesenchymal elements with benign bile duct epithelial structures) and immunohistochemistry (positive staining for vimentin, desmin, α-1 antitrypsin, actin, cytokeratins). In addition, Lauwers et al. [18] found a cytogenetic aberration of the region of chromosome 19q13 in an undifferentiated embryonal sarcoma within a cystic mesenchymal hamartoma, the same aberration that was also found by O’Sullivan et al. [19]. Shehata et al. [20] suggest a continuum between undifferentiated embryonal sarcoma and cystic mesenchymal hamartoma.
The main differential diagnostic for undifferentiated embryonal sarcoma is cystic mesenchymal hamartoma of the liver, with which it may be associated as mentioned. Depending on the gross pathological appearance, cystic mesenchymal hamartoma can vary from a predominantly cystic mass containing septa to a predominantly solid mass containing a few small cysts [21]. Wildhaber et al.’s [6] study concerning the diagnosis issues of cystic mesenchymal hamartoma and undifferentiated embryonal sarcoma also demonstrated that both tumours can be completely cystic, solid or mixed cystic–solid. If the tumour is entirely cystic or solid, making the difference between the two in imaging is challenging. Because bleeding into a cyst is uncommon in cystic mesenchymal hamartoma [17], if blood features are found within, it should raise the suspicion of undifferentiated embryonal sarcoma. As described in our series, the presence of serpiginous vessels within the mass (a feature not found within cystic mesenchymal hamartoma) is in favor of undifferentiated embryonal sarcoma.
Given the common imaging features between the two, the age of the child should give a good argument because cystic mesenchymal hamartoma is usually diagnosed by the age of 2 and undifferentiated embryonal sarcoma is seldom seen in children younger than 5 years [11]. However, this age criteria is not absolute [6]. The youngest case of undifferentiated embryonal sarcoma described in the literature was 4 months [3] and the oldest child with cystic mesenchymal hamartoma was 8 years at diagnosis [6]. Our series confirms the fact that age is not a reliable element when it comes to distinguishing these two entities.
Undifferentiated embryonal sarcoma should also be differentiated from other hepatic mesenchymal malignancies such as rhabdomyosarcoma. Rhabdomyosarcoma originates more often in major bile ducts and is responsible for biliary obstruction and dilatation with jaundice; if it arises from a distant biliary duct, it might be undistinguishable from other primary hepatic malignancies such as undifferentiated embryonal sarcoma.
Hepatoblastoma and hepatocellular carcinoma rarely have a cystic appearance, but in most cases they have an elevated level of alpha fetoprotein, a feature not usually found in undifferentiated embryonal sarcoma [11]. Also, hepatoblastoma usually occurs in the first 3 years of age and can be multifocal. Cystic liver metastatic lesions have also been reported in children [11]. Finally the differential diagnosis of a hydatid cyst should be taken in consideration in endemic areas [11].
As mentioned in the literature [5, 9], neoadjuvant chemotherapy has an important role in reducing the initial tumour volume. This finding was confirmed by our study, in which 10 children benefited from it, showing an evident decrease in the initial size of the mass despite the cystic and haemorrhagic appearance of the lesion.
Conclusion
The diagnosis of undifferentiated embryonal sarcoma should be suggested when dealing with a non-secreting, unifocal liver tumour with cyst-like components and frequent serpiginous intra-tumoural vessels, even moreso if there are signs of bleeding or rupture. The case of the mixed tumour associating undifferentiated embryonal sarcoma components with cystic mesenchymal hamartoma elements discovered at the age of 9 months emphasises the possible association of the two entities regardless of the child’s age.
References
Stocker JT, Ishak KG (1978) Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer 42:336–348
Chocarro G, Amesty MV, Hernández F et al (2013) Embryonal sarcoma of the liver. Pediatr Surg Int 29:1261–1266
Ismail H, Dembowska-Bagińska B, Broniszczak D et al (2013) Treatment of undifferentiated embryonal sarcoma of the liver in children — single center experience. J Pediatr Surg 48:2202–2206
Upadhyaya M, McKiernan P, Hobin D et al (2010) Primary hepatic sarcomas in children — a single-center experience over 19 years. J Pediatr Surg 45:2124–2128
Walther A, Geller J, Coots A et al (2014) Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transpl 20:191–199
Wildhaber BE, Montaruli E, Guérin F et al (2014) Mesenchymal hamartoma or embryonal sarcoma of the liver in childhood: a difficult diagnosis before complete surgical excision. J Pediatr Surg 49:1372–1377
Merli L, Mussini C, Gabor F et al (2015) Pitfalls in the surgical management of undifferentiated sarcoma of the liver and benefits of preoperative chemotherapy. Eur J Pediatr 25:132–137
Roebuck DJ, Aronson D, Clapuyt P et al (2007) 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol 37:123–132
Kim D-Y, Kim K-H, Jung S-E et al (2002) Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg 37:1419–1423
Wu JT, Book L, Sudar K (1981) Serum alpha fetoprotein (AFP) levels in normal infants. Pediatr Res 15:50–52
Chung EM, Lattin GE, Cube R et al (2011) From the archives of the 457 AFIP: pediatric liver masses: radiologic-pathologic correlation. Part 458 2. Malignant tumors. Radiographics 31:483–507
Buetow PC, Buck JL, Pantongrag-Brown L et al (1997) Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology 203:779–783
Ros PR, Olmsted WW, Dachman AH et al (1986) Undifferentiated (embryonal) sarcoma of the liver: radiologic-pathologic correlation. Radiology 161:141–145
Qiu L-L, Yu R-S, Chen Y (2011) Sarcomas of abdominal organs: computed tomography and magnetic resonance imaging findings. Semin Ultrasound CT MR 32:405–421
Iqbal K, Xian ZM, Yuan C (2008) Undifferentiated liver sarcoma — rare entity: a case report and review of the literature. J Med Case Reports 2:20
Wei ZG, Tang LF, Chen ZM et al (2008) Childhood undifferentiated embryonal liver sarcoma: clinical features and immunohistochemistry analysis. J Pediatr Surg 43:1912–1919
Stringer MD, Alizai NK (2005) Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg 40:1681–1690
Lauwers GY, Grant LD, Donnelly WH et al (1997) Hepatic undifferentiated (embryonal) sarcoma arising in a mesenchymal hamartoma. Am J Surg Pathol 21:1248–1254
O’Sullivan MJ, Swanson PE, Knoll J et al (2001) Undifferentiated embryonal sarcoma with unusual features arising within mesenchymal hamartoma of the liver: report of a case and review of the literature. Pediatr Dev Pathol 4:482–489
Shehata BM, Gupta NA, Katzenstein HM et al (2011) Undifferentiated embryonal sarcoma of the liver is associated with mesenchymal hamartoma and multiple chromosomal abnormalities: a review of eleven cases. Pediatr Dev Pathol 14:111–116
Chung EM, Cube R, Lewis RB et al (2010) From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics 30:801–826
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Gabor, F., Franchi-Abella, S., Merli, L. et al. Imaging features of undifferentiated embryonal sarcoma of the liver: a series of 15 children. Pediatr Radiol 46, 1694–1704 (2016). https://doi.org/10.1007/s00247-016-3670-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3670-3