Abstract
Magnetic resonance enterography (MRE) now plays a central role in diagnosing pediatric inflammatory bowel disease (IBD), and its role in other intestinal pathologies such as scleroderma is gradually expanding. MRE helps distinguish between Crohn disease and ulcerative colitis, defining extent and severity. Standard MRE protocols can be optimized in children and adolescents to be diagnostic and well tolerated, both of which are important with increasing use of serial MRE in pediatric IBD for monitoring treatment response and evaluating complications. MRI is especially suited to this role given its lack of ionizing radiation. MRE compliance can be improved through patient education. Differing from adult MRE, pediatric MRE protocols use weight-based formulas to calculate oral and intravenous contrast media and antispasmodic agent doses, using either hyoscine-N-butylbromide or glucagon. Nausea is more commonly experienced with glucagon; however vomiting occurs in <10% of children with either agent. Standard and advanced sequences applied in adults are also used in children and adolescents. These include static and cinematic balanced steady-state free precession sequences, single-shot T2-weighted sequences, diffusion-weighted imaging and pre- and post-contrast 3-D T1-weighted gradient echo sequences. Magnetization transfer imaging and quantitative assessment of bowel to distinguish inflammation and fibrosis are not yet standard in pediatric MRE, but show promise.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bowel evaluation with MRI has been evolving since the 1980s. This was initially propelled by the advent of rapid sequences such as echoplanar imaging to demonstrate bowel motility [1], then by recognition that use of oral and intravenous gadolinium-based contrast agents (GBCAs) improved bowel visualization [2, 3]. In 2016, these elements remain the basis for performing magnetic resonance enterography (MRE) in children and adolescents, and this modality is now central to assessing the small bowel in pediatric inflammatory bowel disease (IBD) [4].
Anti-peristaltic agents further minimize motion artifact, complementing the rapid sequences [5]. Modifications in the type and volume of oral contrast agent and the timing of imaging after intravenous GBCA delivery have resulted in better depiction of the bowel for qualitative, and increasingly quantitative, assessment of disease activity [6–9]. More refined cinematic (cine) techniques than those initially trialed by Stehling et al. [1] allow better demonstration of bowel motility and strictures. Diffusion-weighted imaging (DWI) and magnetization transfer imaging (MTI) are leading the way in tissue characterization, together with quantification of delayed enhancement, although work remains in reliably demonstrating fibrosis [6, 10, 11].
This review details a standard MRE protocol and sequences used for children and adolescents at a large tertiary pediatric center. It also addresses key aspects of achieving studies that are high in diagnostic quality and well tolerated in a pediatric population, both of which are particularly important with serial MRE being more routinely used. Repeat imaging is often performed to assess disease progression, to monitor treatment response or to evaluate acute flare-ups. Lack of ionizing radiation in MRE is an advantage, whether it is performed once or more than once in children and adolescents, who are at increased cancer risk with irradiation [12]. As such, MRE in imaging pediatric IBD has largely replaced barium follow-through or enteroclysis. MRE provides a viable alternative to CT enterography, with which it compares similarly or better, not entirely replacing it. US examination continues to have an ongoing and complementary role [13–17].
We discuss the guidelines for performing MRE in pediatric IBD that have been established at our institution. Image interpretation and value adding of MRE in pediatric IBD management are addressed separately in this supplement.
Pre-MRE
Indication
The major indication for MRE in children and adolescents is evaluating the small intestine in confirmed or suspected IBD [18–20]. As MRE becomes more widely adopted, other uses are being found. These include bowel assessment for infiltrative disorders (e.g., scleroderma), polyposis syndromes, abdominal pain +/- weight loss where US is normal, vascular malformations, occult gastrointestinal bleeding or vasculitis, transplanted small bowel, malrotation or masses [18–23]. As a consequence technical modifications are likely to evolve, but for now standard MRE is optimized to assess the distal small bowel, the region most commonly affected in pediatric Crohn disease [24].
Risk factors
The MRE referral should always be reviewed prospectively to ensure appropriateness of the investigation and to identify risk factors. Occasionally a prior investigation is warranted, such as abdominal US for nonspecific abdominal pain. Screening for MRI contraindications (e.g., implants, allergies — either dietary or rarely prior reactions to intravenous GBCAs potentially requiring premedication to minimize anaphylaxis, renal failure or the need for sedation/general anesthesia) is critical to ensure safety and allow proper planning.
Scheduling
Timing of MRE depends on the clinical indication. In pediatric IBD this might be required urgently to evaluate an acute flare-up, checking for an abscess or fistula, or at longer intervals to assess treatment response, balancing a potential management change and service capacity. Correctly identifying which category the patient falls into helps prioritize MRE patients. At our institution clinical guidelines for scheduling MRE for children with IBD have been established in collaboration with our gastroenterology colleagues (Table 1).
We aim to perform MRE within 4–6 weeks in children with suspected or newly diagnosed pediatric IBD, ideally prior to initiation of medical therapy to best estimate disease severity and extent. This is in conjunction with the child’s global assessment, including esophagogastroduodenoscopy and ileocolonoscopy, which are seldom done urgently [4]. As such, initial MRE can often be performed as an outpatient examination unless there is concern regarding a complication such as an abscess or bowel stricture that might warrant more rapid evaluation and intervention.
Serial MRE to monitor treatment response varies, ranging from 12 months to 18 months (our institutional guideline), although in cases where rapidly progressive disease is suspected, MRE might be repeated within 3 months.
Abdominal US is the first choice for imaging an acute flare-up, ideally performed within 24 h of presentation. This is to confirm bowel involvement, define disease activity and extent, and look for complications such as strictures, abscesses and fistulae. Some limitations include limited visualization of the lower sigmoid colon and rectum [16, 17]. Where US is non-diagnostic or inconsistent with the patient’s clinical status, urgent MRE is performed. CT enterography is a viable alternative, particularly if access to MRI is limited or the child is unable to tolerate the MRI environment. Occasionally conventional abdominal CT is performed if the child is too unwell to tolerate oral contrast administration. Depending on availability and quality of US imaging, both MRE and CT enterography can have a primary role in assessing an acute flare.
Education
Informing the child and parent/caregiver about what MRE entails is very helpful in alleviating patient anxiety and improving tolerance and compliance, resulting in a high-quality diagnostic MRE. Patient education can range from simple instructions about practicing breath-holds and lying still, to interactive games or Apps, to more MRE-specific tools such as brochures and videos [25]. Use of a mock MRI or simulator can limit the need for general anesthesia in younger patients [26]. Clinician education about the merits and challenges of performing MRE in their patients is also vital, and clinico-radiologic meetings provide a useful forum for such discussions.
Sedation/general anesthesia
Because oral contrast agent is used, MRE is not performed under sedation. Occasionally non-sedative doses of anti-anxiolytic agents can be beneficial. In a recent audit at our institution, children as young as 5 years old successfully underwent MRE without general anesthesia, although from a cohort of 757 children undergoing MRE without general anesthesia between 2011 and 2012, only 5% (39) were younger than 10 years (mean age 8.5 years). This parallels the experience of Courtier et al. [27] in reliably performing MRE in children ages 4–7 years without general anesthesia, although with some sequence modification.
However, a small subset of patients might require general anesthesia because of age or developmental need [22, 23, 28]. Mollard et al. [28] recently published their experience in performing 110 diagnostic-quality MREs under general anesthesia in children younger than 10 years. Following endotracheal intubation and nasogastric or orogastric tube insertion, oral contrast agent was instilled as two 5-ml/kg boluses, initially and at 30 min, imaging at ~45 min and withholding glucagon, with terminal ileal filling in 31%. Nausea and vomiting were infrequent; however given the aspiration risk, aspirating the stomach prior to extubation is mandatory. Because of bladder distension, insertion of an indwelling bladder catheter is advisable.
Preparation
No special diet is required prior to MRE. Fasting is 6 h at our institution, ranging 4–6 h at different centers, with adequate pre-procedure fasting thought to promote gastric emptying and minimize filling defects in bowel that can be mistaken for masses or polyps [18, 19, 22, 29].
Magnetic resonance enterography
Protocol
Oral contrast agent
Suitability of an oral contrast agent depends on whether it is acceptable, distensible and biphasic to improve patient tolerance and diagnostic capability.
Acceptable
Most children and adolescents manage to ingest contrast orally without too much difficulty, and this is better tolerated than MR enteroclysis [30]. Compliance can be optimized by giving the patient input into choice of flavoring, keeping drinks chilled and giving lots of positive encouragement. Good distension of the bowel is aided by drinking boluses quickly rather than as slow continuous sips. A few children require nasogastric tubes (only 2 of 50 children in one study [31]). Water chasers or lying on the right side immediately pre-MRE can help with gastric emptying, and anecdotally, staying active between boluses is helpful. Ko et al. [31] evaluated 3% sorbitol tolerance in 50 consecutive children undergoing MRE. Using a Likert-like scale of 1–10, they found that most had no difficulty with the flavor, texture and speed of ingestion (Fig. 1).
Chart shows patient grades regarding acceptance of 3% sorbitol oral contrast agent [31]
Distensible
Hyperosmolar solutions provide better distensibility than water, which is too rapidly absorbed. These solutions are typically administered in divided aliquots more than 60 min pre-MRE for optimal intestinal distension [22, 23, 30, 32–34). These include low-density barium sulphate solutions (VoLumen®; Bracco Diagnostics, Princeton, NJ), polyethylene glycol, methylcellulose, locust bean gum, sorbitol and mannitol — alone or in combination (Breeza®; Beekley Corp., Bristol, CT) [5, 22, 23, 30]. Choice of agent often depends on local access and institutional preference. Volumes are weighted-based in children and adolescents, usually less than 1,500 ml, most ranging between 900 and 1,350 ml [5, 22, 23, 30, 32–35]. Smaller volume regimens have been proposed but not widely applied [36].
At our institution we use 3% sorbitol prepared by our pharmacy (Appendix 1).
This is ingested according to a weighted-based formula in 3 divided doses at 60 min, 30 min and immediately pre-scan. For children <50 kg, these are 10 ml/kg, 5 ml/kg and 5 ml/kg, respectively, with an optional extra 5 ml/kg; and for patients ≥50 kg, equal doses of 450 ml are given at each time-point, up to a maximum of 1,350 ml.
Biphasic
Biphasic agents allow distinction of bowel wall from lumen depending on the sequence and wall signal characteristics. T2-positive agents have luminal hyperintensity on T2-weighted sequences, improving mucosal detail, and hypointensity on T1-weighted sequences, which is most beneficial for mural assessment post contrast administration. Commonly used agents are the hyperosmolar agents described in the prior “Distensible” section. T2-negative agents show low signal on T1-weighted and T2-weighted sequences and are often less readily available, less palatable and more expensive [30, 32, 33].
Anti-peristaltic agents
Glucagon, widely used in the United States, and hyoscine-N-butylbromide (BuscopanTM; Boehringer Ingelheim Pharma, Ingelheim, Germany) in Canada, Australia and Europe, have an anti-peristaltic effect to improve bowel visualization, although recent data suggest this is less critical in disease detection than initially considered [5, 37–39]. Both agents have a rapid onset of gastrointestinal effect of <1 min and duration 6.8+/-5.3 min for hyoscine-N-butylbromide and 18.3+/-7 min for glucagon [40–43]. Dose regimen and mode of administration vary among centers, from 1 to 3 doses, single or split, and given subcutaneously, intramuscularly or more commonly intravenously (IV), as a slow injection or infusion [22, 37–43].
At our institution, hyoscine-N-butylbromide is the agent of choice, being well-tolerated and less expensive than glucagon, administered intravenously as two doses each of 0.3 mg/kg to a maximum of 20 mg per dose. The first dose is injected slowly immediately after surveillance and cinematic images, the second pre-IV contrast agent.
Ko et al. [31] prospectively evaluated the incidence of adverse effects relating to hyoscine-N-butylbromide in 50 consecutive children undergoing MRE, at the time and immediately after MRE (Fig. 2). Approximately 20% of children experienced mild blurred vision, tachycardia and a dry mouth, and less than 10% mild vomiting. A few had moderate tachycardia and one child had more severe vomiting and blurred vision. All symptoms were transient, resolving within 1 h without treatment.
Graph depicts patients’ adverse reactions from intravenous hyoscine-N-butylbromide [31]
Glucagon is given if hyoscine-N-butylbromide is contraindicated at our institution, also intravenously as two doses each of 0.25 mg in children <20 kg or 0.5 mg in children ≥20 kg by slow injection. We have found rebound hypoglycemia can result from glucagon’s hyperglycemic effect, and children benefit from being given fruit juice post MRE. Nausea and vomiting are the most commonly encountered adverse effects, and Dillman et al. [38] supported this finding in a study of 50 pediatric patients undergoing MRE, where 48% of patients described nausea but only 8% had frank emesis.
Intravenous contrast agent
Dynamic contrast-enhanced imaging improves detection of diseased bowel and helps to define its extent and severity [5, 30, 37, 44]. Recently Rimola et al. [6] showed that delayed imaging reliably demonstrates marked fibrosis (submucosal or transmural), with or without superimposed inflammation. When comparing contrast-enhanced MRE with DWI, Seo et al. [43] found disease detection in the terminal ileum comparable between modalities, but contrast-enhanced imaging was better for penetrating disease. It is likely that advanced sequences such as DWI, with its response to therapy not yet fully elucidated, will not only be viable alternatives but will ultimately replace contrast-enhanced sequences. This is particularly so given concerns regarding gadolinium retention with repeat imaging, but for now its use remains standard practice in MRE [43–46].
We use a gadolinium-based contrast agent administered intravenously by power injector at a rate of 2 ml/s with a saline flush, using a weight-based dose of 0.1 mmol/kg to a maximum of 5 mmol (e.g., 1 mmol/ml gadobutrol [Gadovist®; Bayer Schering Pharma, Berlin, Germany] to a maximum dose of 5 ml).
MRE allows imaging at multiple time-points. This is of particular value post contrast administration. At our institution, we commence imaging in the coronal plane during the enteric phase at 45 s post-injection, followed by axial imaging in the portal venous phase at approximately 70 s. Because immediate post-contrast dynamic imaging correlates variably with different grades of active inflammation and fibrosis [44, 45] this is not routinely used at our center. However because recent evidence comparing enhancement at 7 min with 70 s correlates well with marked fibrosis [6], we are incorporating this additional time-point into our standard protocol.
Technical parameters
MRE is successfully performed at 1.5 T and 3 T. At our institution we use MRI scanners of both field strengths interchangeably, although 1.5 T is used more frequently. Minor modifications are required at 3 T to address sequence-related artifact, with chemical shift, banding artifact and susceptibility artifact increased, and bowel gas more problematic with steady-state free precession sequences [23, 32]. Issues relating to inhomogeneous signal and fat suppression can still be encountered at 3 T. This is partially mitigated by multi-transmit technology, with use of parallel imaging improving spatial resolution and reducing susceptibility artifact. Although increased signal-to-noise ratio at 3 T provides some improvement in contrast resolution, the gain in spatial resolution is more pronounced [23, 32, 47].
We prefer scanning children supine, having found that this is better tolerated in young children with fluid-filled bowel distending their abdomen; additionally, there is insufficient benefit from slightly reduced peristalsis, bowel loop separation and decreased anteroposterior diameter to warrant prone imaging. MR-compatible audiovisual distraction devices to play music and movies during MRE are truly beneficial in improving procedure tolerance, and this is balanced against sufficient cooperation to follow breath-hold instructions.
Before getting underway, we ask children to empty their bladder pre-scan to minimize compression of bowel loops from an over-distended bladder, and this also reduces patient discomfort (Fig. 3). It is also helpful to reassure children that passage of loose stool at this time is normal.
Benefits of pre-scan voiding. Surveillance scans at 1.5 T using coronal single-shot T2-weighted turbo spin echo in an 11-year-old girl show Crohn disease of the distal ileum (arrows). a The distended bladder superiorly displaces the thick-walled distal ileum. b Post void, the affected bowel loop is better visualized
Adequate coverage with phased-array torso/body coils consists of imaging from the diaphragm to the perineum, including all of the small bowel and potential extra-intestinal sites of IBD involvement such as the liver and perianal region. Extending the field of view to just beyond these regions can reduce impact of wrap and end-of-stack artifact. Number of stacks in axial imaging is dictated by patient size, and the terminal ileum and cecal pole should be included wholely in one or the other stack if two stacks are needed. Centering the mid-slice in coronal cine imaging on the ileocecal region optimizes visualization of the terminal ileum.
Standard sequences
Standard sequences used in MRE in adults are also used in children and adolescents. Surveillance and cine imaging stacks have thicker slices and wider interslice gaps compared with the remaining sequences, with slice thicknesses of 3–5 mm with no gap. For examples of multivendor sequence parameters at a single tertiary pediatric center, please refer to Table 2.
A surveillance scan using rapid-acquisition static coronal balanced steady-state free precession (SSFP) is acquired with free breathing to ensure adequate distension of the terminal ileum to optimize timing of anti-peristaltic agent administration. If the terminal ileum is not fluid-filled, occasionally this sequence is repeated at intervals of 5 min, sometimes longer, until oral contrast agent is shown to reach the terminal ileum (Fig. 4).
Terminal ileum assessment. Surveillance scans at 1.5 T using coronal balanced steady-state free precession (SSFP) show moderate small bowel distension with oral contrast agent reaching the ileocecal region. a Terminal ileal distension is limited in this 17-year-old girl with Crohn disease because of a terminal ileal stricture (arrow) with surrounding fibrofatty proliferation. b In contrast, there is good distension of the normal terminal ileum in this 16-year-old girl with suspected Crohn disease (arrow)
Cine balanced SSFP sequences, with 40 acquisitions in 12 s at each of 5–7 slices, are acquired with breath-holds to assess bowel motility and strictures, and on occasion these provide better jejunal interrogation if it is suboptimally distended on later static sequences. Both static and cine SSFP images provide good extra-intestinal definition, less so for bowel wall delineation because of black edge artifact and increased susceptibility artifact from bowel gas, especially at 3 T. (Supplementary Online Material)
After the first antispasmodic injection, respiratory triggering or navigation is used for fat-suppressed single-shot T2-weighted turbo spin-echo acquisitions in axial and coronal planes to assess mural signal, mucosal detail and mesenteric edema, with less peri-intestinal definition (Figs. 5 and 6).
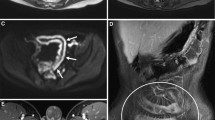
Bowel wall assessment. Half-Fourier single-shot T2-weighted turbo spin-echo sequence at 1.5 T in a 15-year-old girl with longstanding Crohn disease. a Axial fat-suppressed imaging shows small bowel wall thickening with diffuse low signal and luminal narrowing consistent with fibrosis (long arrows) and adjacent deep ulcers reflecting co-existent active inflammation (short arrows). b Non-fat-suppressed coronal image shows pseudosacculation (arrowheads) together with thick low-signal bowel wall (arrows), again suggesting fibrosis
Bowel wall assessment. Additional images acquired at 1.5 T with half-Fourier single-shot T2-weighted turbo spin-echo sequence with fat suppression in a 12-year-old girl. a, b Coronal images show more active inflammation with duodenal (a) and ileal (b) bowel wall thickening, mural T2 hyperintensity (straight arrows) with mesenteric edema and fibrofatty proliferation (curved arrows)
Axial balanced-SSFP post anti-spasmodic can be acquired either free breathing, which is slightly faster, or with respiratory triggering or breath-holds, typically smoother. This sequence permits additional peri-intestinal assessment, including fibrofatty proliferation and vascular engorgement (comb sign), vessel patency, and lymph node number and size (Fig. 7).
Pre-contrast ultrafast 3-D T1-W gradient echo fat-suppressed images are acquired with breath-holds in the coronal plane to delineate high-signal luminal content (e.g., stool and baseline signal of bowel, jejunum slightly brighter than ileum) prior to the second dose of antispasmodic (Fig. 8).
Imaging pre- and post-enhancement in a 12-year-old boy with Crohn disease. a Pre- and (b) post-intravenous gadolinium-based contrast coronal 3-D T1-weighted gradient echo images with fat suppression acquired at 1.5 T. There is marked transmural enhancement (b) of thick-walled jejunum, with a deep ulcer (arrows) better seen post-contrast agent. Mesenteric changes (arrowheads) include fibrofatty proliferation, mesenteric enhancement (b) and prominent vasa recta (comb sign)
Post IV GBCA, ultrafast 3-D T1-W gradient echo fat-suppressed images are acquired with breath-holds at 45 s in the coronal plane and 70 s in the axial plane (Fig. 8). Enhancement improves detection and characterization of diseased bowel segments and visualization of penetrating disease (e.g., sinuses, fistulae or abscesses; Fig. 9). Rarely, sagittal planes are acquired for problem-solving (Fig. 10).
Sagittal imaging. a Sagittal single-shot T2-weighted turbo spin-echo and (b) T1-W fast spin-echo fat-suppressed post-contrast sequences at 3 T in a 15-year-old girl with an abdominal abscess. Images show dark signal from gas (straight arrows) and anterior abdominal wall abscess (arrowheads) secondary to fistularizing ileal Crohn disease. Note the ileal sinus communicating with the abscess (curved arrows)
Advanced sequences
Diffusion-weighted imaging (DWI) is performed routinely prior to the second dose of antispasmodic and GBCA, with free-breathing fat-suppressed echoplanar imaging acquired in the axial plane. Usually multidirectional, 3–8 b values of 0–800 mm2/s are acquired, typically 3–5 b values for qualitative analysis (Fig. 11) (Table 2). The b value (trace) images are compared with the apparent diffusion coefficient (ADC) maps to demonstrate restricted diffusion, which is seen as high signal on trace images and low signal on ADC maps. This correlates with actively inflamed bowel and lymph nodes, with the appearance of bowel fibrosis on DWI not yet defined. Restricted diffusion is also seen in extra-intestinal complications such as abscesses [43, 48]. Increasingly, quantitative assessment of ADC maps is being performed to better define active inflammation and possibly fibrosis and relative contributions of fast and slow diffusion to ADC values, using up to eight b values for more accurate ADC calculations [9, 48–51]. As discussed, DWI might ultimately replace contrast-enhanced MRE sequences.
Diffusion-weighted imaging (DWI). Axial DWI acquired at 3 T in a 14-year-old boy with Crohn disease shows restricted diffusion in the proximal ileum (arrows) and sigmoid colon (arrowheads). a Note increased signal intensity on the trace image (b = 600 mm/s2). b There is corresponding decreased signal intensity on the apparent diffusion coefficient map
Magnetization transfer imaging (MTI) also offers the potential to quantitatively measure fibrosis, although indirectly, by calculating relative signal decrease with magnetization transfer applied in tissue where macromolecules (collagen) are present. Although clinical data are limited [10], work on Crohn disease animal models shows that MTI can detect intestinal fibrosis [11, 51].
Increasingly, quantification on standard and advanced sequences offers the potential for accurate disease characterization. Delayed post-contrast comparative bowel wall enhancement [6] and MTI and T2-weighted signal quantification [11] in a research setting correlate with fibrosis and inflammation. Translating these to a clinical environment would require robust, reproducible MRE studies for longitudinal quantitative assessment.
Conclusion
Optimal MRE in children and adolescents can be readily achieved by using a standardized protocol, applying simple steps to improve patient tolerance and compliance, both of which are especially important when performing MRE serially. Together, standard and advanced sequences provide diagnostic information for qualitative and increasingly quantitative assessment of pediatric IBD.
References
Stehling MK, Evans DF, Lamont G et al (1989) Gastrointestinal tract: dynamic MR studies with echo-planar imaging. Radiology 171:41–46
Semelka RC, Shoenut JP, Silverman R et al (1991) Bowel disease: prospective comparison of CT and 1.5-T pre- and postcontrast MR imaging with T1-weighted fat-suppressed and breath-hold FLASH sequences. J Magn Reson Imaging 1:625–632
Hahn PF, Saini S, Cohen MS et al (1992) An aqueous gastrointestinal contrast agent for use in echo-planar MR imaging. Magn Reson Med 25:380–383
Levine A, Koletzko S, Turner D et al (2014) ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 58:795–806
Low RN, Francis IR (1997) MR imaging of the gastrointestinal tract with i.v., gadolinium and diluted barium oral contrast media compared with unenhanced MR imaging and CT. AJR Am J Roentgenol 169:1051–1059
Rimola J, Planell N, Rodríguez S et al (2015) Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol 110:432–440
Menys A, Atkinson D, Odille F et al (2012) Quantified terminal ileal motility during MR enterography as a potential biomarker of Crohn’s disease activity: a preliminary study. Eur Radiol 22:2494–2501
Tielbeek JA, Ziech ML, Li Z et al (2014) Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 24:619–629
Kovanlikaya A, Beneck D, Rose M et al (2015) Quantitative apparent diffusion coefficient (ADC) values as an imaging biomarker for fibrosis in pediatric Crohn’s disease: preliminary experience. Abdom Imaging 40:1068–1074
Pazahr S, Blume I, Frei P et al (2013) Magnetization transfer for the assessment of bowel fibrosis in patients with Crohn’s disease: initial experience. Magn Reson Mater Phys 26:291–301
Dillman JR, Swanson SD, Johnson LA et al (2015) Comparison of noncontrast MRI magnetization transfer and T2-weighted signal intensity ratios for detection of bowel wall fibrosis in a Crohn’s disease animal model. J Magn Reson Imaging 42:801–810
Mathews JD, Forsythe AV, Brady Z et al (2013) Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346:f2360
de Bie CI, Buderus S, Sandhu BK et al (2012) Diagnostic workup of paediatric patients with inflammatory bowel disease in Europe: results of a 5-year audit of the EUROKIDS registry. J Pediatr Gastroenterol Nutr 54:374–380
Horsthuis K, Bipat S, Bennink RJ et al (2008) Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 247:64–79
Qiu Y, Mao R, Chen BL et al (2014) Systematic review with meta-analysis: magnetic resonance enterography vs. computed tomography enterography for evaluating disease activity in small bowel Crohn’s disease. Aliment Pharmacol Ther 40:134–146
Dillman JR, Smith EA, Sanchez RJ et al (2015) Pediatric small bowel Crohn disease: correlation of US and MR enterography. Radiographics 35:835–848
Ahmad TM, Greer M-L, Walters TD et al (2016) Bowel sonography and MR enterography in children. AJR Am J Roentgenol 206:173–181
Mann GS, Greer MC, Nadel HR (2015) Paediatric Crohn’s disease. In: Rajesh A, Sinha R (eds) Crohn’s disease: current concepts. Springer, Cham, pp 130–135
Absah I, Bruining DH, Matsumoto JM et al (2012) MR enterography in pediatric inflammatory bowel disease: retrospective assessment of patient tolerance, image quality, and initial performance estimates. AJR Am J Roentgenol 199:W367–W375
Torkzad MR, Masselli G, Halligan S et al (2015) Indications and selection of MR enterography vs. MR enteroclysis with emphasis on patients who need small bowel MRI and general anaesthesia: results of a survey. Insights Imaging 6:339–346
Amzallag-Bellenger E, Oudjit A, Ruiz A et al (2012) Effectiveness of MR enterography for the assessment of small-bowel diseases beyond Crohn disease. Radiographics 32:1423–1444
Anupindi SA, Podberesky DJ, Towbin AJ et al (2015) Pediatric inflammatory bowel disease: imaging issues with targeted solutions. Abdom Imaging 40:975–992
Mollard BJ, Smith EA, Dillman JR (2015) Pediatric MR enterography: technique and approach to interpretation — how we do it. Radiology 274:29–43
de Bie CI, Paerregaard A, Kolacek S et al (2013) Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-year analyses of the EUROKIDS registry. Inflamm Bowel Dis 19:378–385
Greer MC, Krajewski D, Brown N et al (2014) MRE tour at SickKids. https://www.youtube.com/watch?v=9CHml_CrAEQ. Accessed 19 Feb 2016
Carter AJ, Greer ML, Gray SE et al (2010) Mock MRI: reducing the need for anaesthesia in children. Pediatr Radiol 40:1368–1374
Courtier J, Cardenas A, Tan C et al (2015) Nonanesthesia magnetic resonance enterography in young children: feasibility, technique, and performance. J Pediatr Gastroenterol Nutr 60:754–761
Mollard BJ, Smith EA, Lai ME et al (2016) MR enterography under the age of 10 years: a single institutional experience. Pediatr Radiol 46:43–49
Rajesh A, Sinha R (2015) CT and magnetic resonance enterography in Crohn’s disease. In: Rajesh A, Sinha R (eds) Crohn’s disease: current concepts. Springer, Cham, pp 75–80
Grand DJ, Guglielmo FF, Al-Hawary MM (2015) MR enterography in Crohn’s disease: current consensus on optimal imaging technique and future advances from the SAR Crohn’s disease-focused panel. Abdom Imaging 40:953–964
Ko HS, Greer MC, Aziza A et al (2011) 3% sorbitol as oral contrast and IV hyoscine butylbromide are well tolerated in children undergoing MR enterography. Pediatr Radiol 41:S311–S428
Masselli G, Gualdi G (2012) MR imaging of the small bowel. Radiology 264:333–348
Riordan RD, Khonsari M, Jeffries J et al (2004) Pineapple juice as a negative oral contrast agent in magnetic resonance cholangiopancreatography: a preliminary evaluation. Br J Radiol 77:991–999
Kuehle CA, Ajaj W, Ladd SC et al (2006) Hydro-MRI of the small bowel: effect of contrast volume, timing of contrast administration, and data acquisition on bowel distention. AJR Am J Roentgenol 187:W375–W385
Kinner S, Kuehle CA, Herbig S et al (2008) MRI of the small bowel: can sufficient bowel distension be achieved with small volumes of oral contrast? Eur Radiol 18:2542–2548
Young BM, Fletcher JG, Booya F et al (2008) Head-to-head comparison of oral contrast agents for cross-sectional enterography: small bowel distention, timing, and side effects. J Comput Assist Tomogr 32:32–38
Siddiki H, Fidler J (2009) MR imaging of the small bowel in Crohn’s disease. Eur J Radiol 69:409–417
Dillman JR, Smith EA, Khalatbari S et al (2013) I.V. glucagon use in pediatric MR enterography: effect on image quality, length of examination, and patient tolerance. AJR Am J Roentgenol 201:185–189
Grand DJ, Beland MD, Machan JT et al (2012) Detection of Crohn’s disease: comparison of CT and MR enterography without anti-peristaltic agents performed on the same day. Eur J Radiol 81:1735–1741
(2003) Glucagon for injection (rDNA origin). U.S. Food and Drug Administration website. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20928slr010_glucagon_lbl.pdf. Accessed 19 Feb 2016
Tytgat GN (2008) Hyoscine butylbromide — a review on its parenteral use in acute abdominal spasm and as an aid in abdominal diagnostic and therapeutic procedures. Curr Med Res Opin 24:3159–3173
Froehlich JM, Daenzer M, von Weymarn C et al (2009) Aperistaltic effect of hyoscine N-butylbromide versus glucagon on the small bowel assessed by magnetic resonance imaging. Eur Radiol 19:1387–1393
Seo N, Park SH, Kim KJ et al (2015) MR enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology 278:762–772
Taylor SA, Punwani S, Rodriguez-Justo M et al (2009) Mural Crohn disease: correlation of dynamic contrast-enhanced MR imaging findings with angiogenesis and inflammation at histologic examination — pilot study. Radiology 251:369–379
Oto A, Kayhan A, Williams JT et al (2011) Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 33:615–624
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Patak MA, von Weymarn C, Froehlich JM (2007) Small bowel MR imaging: 1.5T versus 3T. Magn Reson Imaging Clin N Am 15:383–393
Morani AC, Smith EA, Ganeshan D et al (2015) Diffusion-weighted MRI in pediatric inflammatory bowel disease. AJR Am J Roentgenol 204:1269–1277
Freiman M, Perez-Rossello JM, Callahan MJ et al (2013) Characterization of fast and slow diffusion from diffusion-weighted MRI of pediatric Crohn’s disease. J Magn Reson Imaging 37:156–163
Rosenbaum DG, Rose ML, Solomon AB et al (2015) Longitudinal diffusion-weighted imaging changes in children with small bowel Crohn’s disease: preliminary experience. Abdom Imaging 40:1075–1080
Adler J, Swanson SD, Schmiedlin-Ren P et al (2011) Magnetization transfer helps detect intestinal fibrosis in an animal model of Crohn’s disease. Radiology 259:127–135
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author has no financial interests or investigational uses to disclose. Off-label use of hyoscine-N-butylbromide is discussed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
3% Sorbitol oral contrast preparation. Abbreviation: W/V - weight per volume. (GIF 26 kb)
Supplementary online material
(movie clip) Coronal cine steady-state free precession image acquired at 1.5 T demonstrates a distal ileal stricture in a 17-year-old girl with Crohn disease. Each image slice is acquired as 40 images over 12 seconds, with 5–7 slices at a slice thickness of 10–15 mm (MOV 1404 kb)
Rights and permissions
About this article
Cite this article
Greer, ML.C. How we do it: MR enterography. Pediatr Radiol 46, 818–828 (2016). https://doi.org/10.1007/s00247-016-3596-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3596-9