Abstract
Background
Optic nerve tortuosity is often reported in children with neurofibromatosis type 1 (NF1).
Objective
To employ quantitative and subjective criteria to assess optic nerve tortuosity in individuals with NF1.
Materials and methods
A retrospective study over a period of 8 years was performed on children with NF1, with and without optic pathway glioma, compared with children without NF1. A tortuosity index was computed for the optic nerve in each child using a high-resolution 3-D T1-weighted magnetization-prepared rapid gradient-echo sequence, which was averaged and compared across groups.
Results
The tortuosity index for subjects with NF1, regardless of an optic pathway glioma, was greater than those without NF1. There was no difference in the tortuosity index between NF1 subjects with optic pathway glioma and NF1 subjects without optic pathway glioma. There was also no correlation between subjective measures of tortuosity and the quantitative scoring (tortuosity index) or between the degree of tortuosity and subject age or gender.
Conclusion
Individuals with NF1 have increased optic nerve tortuosity relative to unaffected individuals. Quantitative tortuosity index is a superior measure to subjective assessment in the evaluation of optic nerve tortuosity in children with NF1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis type 1 (NF1) is a common inherited cancer predisposition syndrome in which affected individuals are prone to the development of brain tumors [1]. In children, the most frequently identified brain tumor is the optic pathway glioma [2, 3]. This World Health Organization (WHO) grade I tumor can arise anywhere along the extent of the optic pathway, from the retro-orbital optic nerve to the post-chiasmatic optic tracts [4]. Optic pathway gliomas are usually infiltrative tumors with low proliferative indices, which lack a defined cystic component, as seen in their sporadic counterparts located elsewhere within the neuroaxis [5].
Previous clinical series have shown that 15-20% of children with NF1 have radiographic evidence of an optic pathway glioma [2, 6, 7]; however, most children with these tumors will not experience further visual decline and many will be asymptomatic [6, 8, 9]. In addition, a few reports have revealed additional optic nerve abnormalities, including optic nerve tortuosity and thickening [2, 10–12]. While these findings were incidentally identified, their clinical significance in the context of NF1 has not been explored. Moreover, accurate objective measures of tortuosity have not been applied to these abnormalities. This study sought to define a tortuosity index that quantifies the subjective interpretation of optic nerve tortuosity with higher precision and reproducibility. The purpose of this study was to employ a standardized quantitative measurement of optic nerve tortuosity to critically evaluate the significance of this frequently reported incidental finding in children with NF1.
Materials and methods
Subjects
This study was performed in accordance with an approved Human Studies Protocol at the Washington University School of Medicine. The retrospective study covered a period of 8 years from 2005 to 2012, and included children with NF1 who underwent magnetic resonance imaging (MRI) (n = 29) as well as children without NF1 who underwent MRI for the evaluation of headaches (n = 18). The children were categorized into three groups: controls (n = 18), NF1 subjects without optic pathway glioma (n = 18) and NF1 subjects with optic pathway glioma (n = 11) (Fig. 1). The identification of optic pathway glioma was previously determined based on the interpreting radiologist’s opinion, typically due to thickening of the optic nerve/chiasm and/or associated gadolinium enhancement on MRI as an indication of glioma (Fig. 1). Similarly, although tortuosity has not been clearly defined, radiologists have previously noted several tortuous nerves in certain children with NF1, based on subjective assessments.
Patients older than 18 years were excluded from this study. All children with NF1 had a diagnosis established using NIH Consensus Development Conference Diagnostic criteria [13].
Quantitative tortuosity scoring system
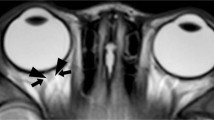
Clinical MRI scans were performed in children with NF1 as part of a standard of care protocol. MRI scans were performed in the control group for the clinical indication of headaches. Analysis was performed on a T1-weighted 3-D magnetization-prepared rapid gradient-echo (MPRAGE) sequence that was acquired with a sagittal slice thickness of 1 mm and an in-plane resolution of 1 mm × 1 mm that is variably interpolated to 0.5 mm × 0.5 mm. The full extent of both optic nerves extending from the optic chiasm to the globe was identified in the coronal plane and the coordinates of the center of the nerve were recorded approximately every 1 mm along the course of the nerve (using every slice in the 1-mm scans, and every other slice in the 0.5-mm scans) (Fig. 2). Two lengths were computed. A straight line length was computed as the Euclidean distance between the first and last point along the nerve. An estimation of the tortuous length of the nerve was calculated by summing the short segment lengths computed as the Euclidean distance between pairs of points on adjacent coronal slices. Finally, the tortuosity index was computed as the ratio between these lengths minus one, modeled on a similar measure of arterial tortuosity [14]. Hence, the formula for the tortuosity index was:
Schematic diagram illustrates the method used to measure the tortuosity index. The length of the nerve is calculated by adding the short line segment lengths (in black) estimated from the location of the nerve (black dots) in each of the coronal MRI views (vertical dashed gray lines) and numbered from 1 to n. This length is divided by the straight line length (dashed red line) between the two edge points, 1 and n, and then 1 is subtracted from this ratio to obtain the tortuosity index
with i indexing the slice number between 1 and n, and with x, y and z representing the coordinates of the optic nerve in each slice.
After the scans were scored, the subject set was randomized, their scans recoded with new scan IDs and a second quantitative analysis was performed to assess test-retest reliability of the objective scoring system. The retest was conducted with the same observer and the same equipment 1 month after the original measurements were completed.
To objectively estimate the prevalence of optic nerve tortuosity in our NF1 population, we used the average tortuosity index in the normal controls plus two standard deviations as an estimate of the upper limit of normal tortuosity index value.
Subjective tortuosity scoring system
Subjective analysis of the optic nerves was limited to children without optic pathway glioma. Using T1-weighted 3-D MPRAGE sequences, transaxial and coronal images of the optic nerves were cropped so that the optic nerves were visualized from the orbital apex to the globe. These steps prevented the reviewer from discerning the NF1 diagnostic status of the subject. The subjective assessment of optic nerve tortuosity was performed by a pediatric neuroradiologist with 13 years of experience (R.C.M.). Scans were scored on a three-point scale: normal (0), intermediate tortuosity (1) and marked tortuosity (2) (Fig. 3). After the scans were scored, the images set was randomized and recoded with new scan IDs and a second subjective analysis was performed to assess test-retest reliability of the subjective interpretation. The retest analysis was completed with the same radiologist (RCM) and the same equipment 1 month after the original assessment.
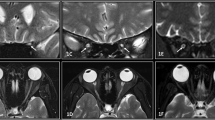
Subject axial MRI scans. Subjective optic nerve tortuosity scores were determined by a radiologist (R.C.M.) on a three-point scale (from 0 to 2). a Optic nerve with a score of 0 (normal) in a 4-year-old girl. b Optic nerve with score of 1 (intermediate tortuosity) in a 2-year-old boy with NF1. c Optic nerve with a score of 2 (marked tortuosity) in a 5-year-old boy with NF1
Data analysis
The distributions of tortuosity scores (by right-side and left-side separately) and demographics across the three subject groups (control, NF1 without optic pathway glioma and NF1 with optic pathway glioma) were summarized using means and standard deviations or counts and frequencies as appropriate. The differences of tortuosity scores between groups were compared using two-way analysis of variance (ANOVA) for repeated measurement data (quantitative tortuosity scores) or generalized estimating equation (subjective tortuosity scores), to account for potential correlations between scores measured from the same child. These models were also used to compare the between-group differences while adjusting for age and gender. The overall discriminating ability of tortuosity scores on each side was described using the concordance statistics (C-index). The C-index is analogous to the area under the receiver operating characteristic (ROC) curve and measures the ability of the model to classify two randomly selected subjects into correct disease groups. Finally, Spearman’s rank correlation coefficients (rho) were used to measure the reliability of test-retest for both quantitative and subjective scoring systems. All the analyses were two-sided and significance was set at a P-value of 0.05. Statistical analyses were performed using SAS (SAS Institute, Cary, NC).
Results
None of the control subjects harbored an optic pathway glioma, but several had central nervous system (CNS) pathologies that are not known to affect the optic nerves. While most of the MRI scans of the control subjects were unremarkable, a few of the children had findings such as an incidentally identified Chiari I malformation.
At the time of examination, the mean age of the control group was 10.22 years (SD = 4.58), while the mean ages of the NF1 groups with optic pathway glioma and without were 7.91 (SD = 4.35) and 9.33 (SD = 5.37), respectively. With respect to gender distribution, the control group was composed of 33.3% males, the NF1 group without optic pathway glioma had 50.0% males, and the NF1 group with optic pathway glioma had 54.5% males. No significant differences were found among the three child-patient groups with regard to these characteristics (Table 1).
Based on the quantitative measurements of the tortuosity index, the mean optic nerve tortuosity indices in the control group for the right and left nerves were 14.9% (SD = 2.1) and 15.0% (SD = 2.2), respectively. Within the NF1 subjects, those without optic pathway gliomas had mean tortuosity indices of 26.9% (SD = 9.0) and 24.3% (SD = 4.3) for right and left optic nerves, respectively. In addition, NF1 patients with optic pathway gliomas had mean tortuosity indices of 27.0% (SD = 6.9) and 24.5% (SD = 6.2) for right and left optic nerves, respectively (Table 2). Post hoc analysis demonstrated that the controls had significantly lower tortuosity than NF1 subjects with optic pathway glioma (P < 0.001) and NF1 subjects without optic pathway glioma (P < 0.001), but the difference between the two NF1 groups was not significant (P = 0.979) (Fig. 4). Multivariate analysis showed that age (P = 0.173) and gender (P = 0.100) were not significantly associated with the quantitative tortuosity score, and the conclusion regarding between-group differences remained the same after adjusting for age and gender. The same conclusions were replicated in the analyses using retest scores (Table 2).
Optic nerve tortuosity indices measured by the quantitative scoring system, indicating higher tortuosity indices for NF1 subjects than for controls (P < 0.001). a Optic nerves of all the children in the study. Optic nerves previously noted as “tortuous” by radiologists are represented by an asterisk. b Right optic nerves only. c Left optic nerves only
Using the average tortuosity index in the controls plus two standard deviations as an upper limit for normal, we estimated the prevalence of optic nerve tortuosity as 84% (49 out of 58 optic nerves) in the NF1 subjects analyzed. There was no difference between the optic pathway glioma and non-optic pathway glioma groups.
Comparing the distribution of subjective tortuosity scores across the controls and the NF1 subjects without optic pathway glioma (Table 3), the controls had significantly lower tortuosity than NF1 subjects without optic pathway gliomas (P = 0.002). Multivariate analysis showed that age (P = 0.934) and gender (P = 0.234) were not significantly associated with the subjective tortuosity score, and the same conclusion was reached after adjusting for age and gender. Additionally, the same results were replicated upon analysis of the retest scores.
To compare the two scoring systems, the overall discriminating ability was measured by the C-index, which has a value ranged from 0.5 to 1, with 1 indicating a perfect differentiation. The results showed that the quantitative scoring system could reliably distinguish between the controls and NF1 subjects without optic pathway glioma (with C-indices of 0.960 and 0.988 for the right and left optic nerves, respectively). Meanwhile, the subjective scoring system also performed well in the differentiation of controls versus NF1 subjects without optic pathway glioma but with relatively low precision (C-indices of 0.796 and 0.696 for the right and left optic nerves, respectively). Similar results were obtained in the retest measures of the quantitative scores (with C-indices of 0.929 and 0.923) and subjective scores (with C-indices of 0.758 and 0.731). As evidenced by the calculated C-indices, the quantitative scoring system has a higher discriminative ability than the subjective scoring system, and thus presents a more precise method of defining tortuosity.
Finally, the test-retest analyses showed that the quantitative scores could be reproduced with relatively high precision in both the right optic nerves (Spearman’s rho = 0.810, P < 0.001) and the left optic nerves (Spearman’s rho = 0.785, P < 0.001) (Fig. 5). The subjective scoring system also demonstrated a significant, but slightly weaker, correlation between the test and retest scores in both the right optic nerves (Spearman’s rho = 0.774, P < 0.001) and the left optic nerves (Spearman’s rho = 0.748, P < 0.001). Only a weak correlation in the right optic nerves (Spearman’s rho = 0.432, P = 0.008) and insignificant correlation in the left optic nerves (Spearman’s rho = 0.250, P = 0.141) were found upon comparison of the quantitative scores with the subjective scores (Fig. 6). In this regard, the quantitative method is more reproducible relative to the subjective method.
Discussion
Improvements in neuroimaging have resulted in more frequent detection of brain abnormalities in children with NF1, including T2 hyperintensities [15–17], heterotopias [18] and changes in corpus callosum thickness [19–21]. Within the optic pathway, four previous reports have described increased optic nerve tortuosity. In the computerized tomography era, 8 of 65 children with NF1 (12.3%) had tortuous or widened optic nerves (mean age, 3.3 years) as determined by an experienced neuroradiologist [2]. Similarly, using MRI, one study reported 8 of 26 subjects (30.7%) with NF1 exhibiting tortuous optic nerves [12], while another described only 2 of 50 subjects (4%) with the same finding [11]. In all cases, vision was normal. In contrast, our estimation of the prevalence of optic nerve tortuosity was much higher (84%), which likely reflects the use of a quantitative approach coupled with advances in MRI techniques.
In an effort to provide more objective methods for defining optic nerve tortuosity, Armstrong and colleagues [10] employed six radiographic features to independently assess MRI scans on individuals with NF1 whose optic nerves were suspected to be tortuous. The two most reliable features were (1) lack of congruity in greater than one coronal section and (2) dilation of the subarachnoid space surrounding the anterior portion of the optic nerve. The latter feature was highly specific and sensitive, but exhibited 64% concordance among three experienced neuroradiologists. The criterion used in this method is still relatively subjective, and thus limited in its utility for distinguishing normal from tortuous optic nerves. The method used in our study is both quantitative and reproducible, making it more easily translatable to future clinical studies.
For this reason, we applied a more objective measure of tortuosity, based on a quantitative method used to determine the relationship between vertebral artery tortuosity and clinical outcomes in children with connective tissue disorders [14]. We adopted this quantitative method of measuring vertebral artery tortuosity in order to measure tortuosity of the optic nerve. By employing this method, the average tortuosity for children with NF1 was higher than that in a similar population of children who underwent neuroimaging for headaches. Importantly, the increase in tortuosity was higher in children with NF1 regardless of the presence of optic gliomas. Furthermore, the tortuosity index did not depend on age or gender. In this respect, the optic nerves in individuals with NF1 appear morphologically abnormal, irrespective of neoplasm or clinical symptoms.
Interestingly, the quantitative and subjective scoring systems did not closely correlate with each other, despite establishing similar trends. The quantitative method demonstrated an improved ability to differentiate between the control and the NF1 patients, as well as a greater sensitivity to differences in optic nerve tortuosity. In addition, our conclusions were reproducible upon test-retest evaluation using the quantitative method, supporting the use of this scoring system as a more effective way of evaluating optic nerve tortuosity. It should be noted that one of the limitations of this study is the small number of children examined and the lack of longitudinal data. For this reason, future studies will be required to apply this measurement method to prospectively assess the value of optic nerve tortuosity as a possible distinguishing feature of the optic nerves of children with NF1.
The purpose of this study was not to employ tortuosity to diagnose children with NF1, but rather to define the clinical significance of this frequently reported incidental finding in the setting of NF1. We do not advocate sedated neuroimaging studies for asymptomatic children with only one feature of NF1 [7], but rather recommend that neurological and ophthalmological assessments guide the need for MRI in children with this brain tumor predisposition syndrome. The tortuosity index provides an objective and reproducible measurement that would be superior to the subjective assessment of tortuosity for future correlative studies aimed at examining the correlations of optic nerve tortuosity to visual function or optic pathway glioma development in children with NF1.
Conclusion
Children with NF1 as a group have increased optic nerve tortuosity relative to unaffected children, independent of the presence of an optic pathway glioma. Tortuosity index is a more precise measure of tortuosity than subjective interpretation and would be a more effective approach for further investigation of the correlations of optic nerve tortuosity with visual function and with optic pathway glioma development.
References
Friedman JM, Gutmann DH, MacCollin M et al (1999) Neurofibromatosis: phenotype, natural history and pathogenesis, 3rd edn. John Hopkins University Press, Baltimore
Listernick R, Charrow J, Greenwald MJ et al (1989) Optic gliomas in children with neurofibromatosis type 1. J Pediatr 114:788–792
Listernick R, Louis DN, Packer RJ et al (1997) Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic pathway glioma Task Force. Ann Neurol 41:143–149
Guillamo JS, Créange A, Kalifa C et al (2003) Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain 126:152–160
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Listernick R, Charrow J, Greenwald MJ et al (1994) Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr 125:63–66
King AA, Listernick R, Charrow J et al (2003) Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet 122A:95–99
Listernick R, Darling C, Greenwald M et al (1995) Optic pathway tumors in children: the effect of neurofibromatosis type 1 on clinical manifestations and natural history. J Pediatr 127:718–722
Balcer LJ, Liu GT, Heller G et al (2001) Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol 131:442–445
Armstrong GT, Localio AR, Feygin T et al (2007) Defining optic nerve tortuosity. AJNR Am J Neuroradiol 28:666–671
Van Es S, North KN, McHugh K et al (1996) MRI findings in children with neurofibromatosis 1: a prospective study. Pediatr Radiol 26:478–487
DiMario FJ Jr, Ramsby G, Greenstein R et al (1993) Neurofibromatosis type 1: magnetic resonance imaging findings. J Child Neurol 8:32–39
Stumpf DA, Alksne JF, Annegers JF et al (1988) Neurofibromatosis—conference statement. National institutes of health consensus development conference. Arch Neurol 45:575–578
Morris SA, Orbach DB, Geva T et al (2011) Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation 124:388–396
DiPaolo DP, Zimmerman RA, Rorke LB et al (1995) Neurofibromatosis type 1: pathologic substrate of high-signal-intensity foci in the brain. Radiology 195:721–724
Hyman SL, Gill DS, Shores EA et al (2003) Natural history of cognitive deficits and their relationship to MRI T2-hyperintensities in NF1. Neurology 60:1139–1145
Hyman SL, Gill DS, Shores EA et al (2007) T2 hyperintensities in children with neurofibromatosis type 1 and their relationship to cognitive functioning. J Neurol Neurosurg Psychiatry 78:1088–1091
Kato M, Mizuguchi M, Hattori S et al (1998) Loss of neurofibromin in the leptomeningeal astroglial heterotopia of NF-1. Pediatr Neurol 18:227–230
Kayl AE, Moore BD 3rd, Slopis JM et al (2000) Quantitative morphology of the corpus callosum in children with neurofibromatosis and attention-deficit hyperactivity disorder. J Child Neurol 15:90–96
Moore BD 3rd, Slopis JM, Jackson EF et al (2000) Brain volume in children with neurofibromatosis type 1: relation to neuropsychological status. Neurology 54:914–920
Pride N, Payne JM, Webster R et al (2010) Corpus callosum morphology and its relationship to cognitive function in neurofibromatosis type 1. J Child Neurol 25:834–841
Acknowledgments
We thank Anne C. Albers, PNP, and Mrs. Taylor Ferguson for assistance. The authors also wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, J., Shimony, J., Gao, F. et al. Optic nerve tortuosity in children with neurofibromatosis type 1. Pediatr Radiol 43, 1336–1343 (2013). https://doi.org/10.1007/s00247-013-2694-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-013-2694-1