Abstract
Background
Lymphobronchial tuberculosis (TB) is tuberculous lymphadenopathy involving the airways, which is particularly common in children.
Objective
To describe CT findings of lymphobronchial TB in children, the parenchymal complications and associated abnormalities.
Materials and methods
CT scans of children with lymphobronchial TB were reviewed retrospectively. Lymphadenopathy, bronchial narrowing, parenchymal complications and associations were documented.
Results
Infants comprised 51% of patients. The commonest site of lymphadenopathy was the subcarinal mediastinum (97% of patients). Bronchial compression was seen in all children (259 bronchi, of these 28% the bronchus intermedius) with severe or complete stenosis in 23% of affected bronchi. Parenchymal complications were present in 94% of patients, including consolidation (88%), breakdown (42%), air trapping (38%), expansile pneumonia (28%), collapse (17%) and bronchiectasis (9%), all predominantly on the right side (63%). Associated abnormalities included ovoid lesions, miliary nodules, pleural disease and intracavitary bodies.
Conclusion
Airway compression was more severe in infants and most commonly involved the bronchus intermedius. Numerous parenchymal complications were documented, all showing right-side predominance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of tuberculosis (TB) is increasing globally. South Africa has the third highest incidence in the world with respect to both TB and multidrug-resistent (MDR) TB [1]. Lymphadenopathy is considered the hallmark of the radiological diagnosis of TB in children [2]. When primary tuberculous infection within the lymph nodes involves the airways, the result is lymphobronchial TB, historically known as epituberculosis [3, 4]. This term encompasses a spectrum of airways involvement and associated complications [5]. The lymph nodes enlarge, obstructing the airway by external compression, intraluminal occlusion by inflammatory change in the bronchial wall or herniation of caseating lymph nodes [4, 6, 7]. This usually affects the right main bronchus or bronchus intermedius [8]. The pathological process starts with partial occlusion of the lumen, with a ball-valve effect that leads to air trapping in the affected segment or lobe, which may in turn consolidate. In consolidated segments with completely occluded bronchi, the lung may fill with fluid, resulting in so-called “drowned lung” [9]. Other radiologically demonstrable parenchymal complications of obstruction include collapse, expansile pneumonia, necrotising pneumonia and liquefaction with or without cavitation [10]. Erosion of tuberculous lymph nodes into the airways can cause transbronchial spread, resulting in distant alveolar or bronchopneumonic consolidation [11]. Lymphobronchial TB is a complication of TB seen almost exclusively in children, who are at risk because of the small calibre and compressibility of their airways. Radiological demonstration of lymphobronchial TB and the initial or advanced parenchymal complications may prompt surgical intervention to relieve the obstruction [12–14] and in some instances salvage of the lung.
Our purpose was to describe the features on CT of lymphobronchial TB in children, to document the parenchymal complications and to identify any associated abnormalities.
Materials and methods
A retrospective descriptive analysis of patient records, previously collated into an established database for use in a larger clinical project, was performed. The larger study group consisted of children who had had bronchoscopy, but not all these children had had CT. The number of children in the larger study was 212. The number of children with CT scans available for evaluation was 98. Ethics approval for this study was obtained from the Human Research Ethics Committee (Medical) of the University of the Witwatersrand, Johannesburg (clearance certificate M10441).
The database comprised children younger than 13 years with known TB, presenting to a paediatric pulmonologist with symptoms and signs of compression of large airways. These included persistent coughing and wheezing, unilateral hyperinflation, expansile pneumonia, pneumonia not responding to treatment, and lobar collapse. TB was confirmed either by culture or by demonstration of acid-fast bacilli in gastric or bronchial aspirates. The HIV status of all the patients was determined by PCR and was extracted from the available clinical data. The patients had been treated for 30 days with a quadruple anti-TB drug regimen, to which steroids were added. If, after 30 days of treatment, they had persistent clinical (e.g. coughing or wheezing) or radiologic (narrowed airway on radiographs, unilateral hyperinflation, persistent pneumonia, expansile pneumonia or lobar collapse) evidence of airways obstruction, fibreoptic tracheobronchoscopy and CT of the chest were performed to investigate the cause and level of obstruction. This was part of the routine clinical care.
CT was performed with a 4-slice multi-detector scanner (Aquilion, Toshiba, Nasu, Japan) using a routine chest CT protocol for children with 120-kVp tube voltage and 50-mA tube current. Intravenous iodinated contrast medium (low osmolar, non-ionic, 300 mg/ml iodine content) was used routinely at a dose of 2 ml/kg body weight, administered by hand injection. Standard image construction yielded soft-tissue images, standard lung images and high-resolution lung images. CTDI and DLP were not available.
All CT scans were reviewed by the same paediatric radiologist, who assessed:
-
Airways stenosis: presence, distribution and diameter at the level of maximum stenosis
-
Lymphadenopathy: presence and distribution of all lymphadenopathy. The size of the nodal group implicated in airway stenosis was measured
-
Parenchymal complications: consolidation, breakdown, air trapping, expansile pneumonia, collapse and bronchiectasis
-
Associated findings related to TB: ovoid focal bodies, miliary nodules, pleural disease and intracavitary bodies.
These radiologic findings were entered into the database as categorical and binary variables.
Our data collection regarding nodules included all varieties of nodules: larger nodules as well as ill-defined smaller nodules (tree-in-bud). We did not record tree-in-bud nodules as a separate entity. Nodules of any type at the periphery of a confluent area of consolidation were considered part of the airspace process and not as distinct nodules.
The measured value of the airway diameter at the level of maximum stenosis was compared to the normal value of the airway diameter for the child’s age group [15, 16] and was expressed as a percentage of attenuation. This percentage was then stratified into a category of severity of compression as follows: mild (0–33%), moderate (34–66%), severe (67–99%) and complete (100%) for each airway in each age group.
Normal values for the diameter of the bronchial tree distal to the main bronchi, i.e. left upper lobe bronchus, bronchus intermedius (third order bronchi), and right lower lobe bronchus (fourth order bronchus), were not available and were therefore estimated by the formula [17, 18]:
where d = diameter of bronchus, z = generation of bronchus, and d 0 = diameter of trachea
The resulting estimated values for each age category were rounded off to the nearest millimetre to facilitate comparison with the calliper measurements on CT images. The data was analysed using STATISTICA 9.1; (a statistical software package by StatSoft, Inc.) with specific reference to the number of compressions by site, age and severity, as well as the number and type of parenchymal changes present including laterality. Results were expressed as frequencies and percentages for categorical variables.
Results
The study included 98 children with confirmed lymphobronchial TB. There were 55 males and 43 females, with ages ranging from 2 to 144 months (mean age, 26.6 months; median age, 12 months). The largest age category was that of infants, 50 patients (51%). The HIV status was positive for 17 children (17.3%), negative for 80 children (81.6%) and unknown for 1 (1%) child.
Lymphadenopathy was seen in all 98 children. Multiple sites were usually involved. Only one child had single-site involvement. The distribution of lymphadenopathy is summarised in Table 1. The most common site of lymphadenopathy was the subcarinal region, followed by the right paratracheal and right hilar regions. Of 588 possible lymph node sites (6 sites × 98 individuals), lymphadenopathy was documented at 432 sites (73.5%). The mean number of affected sites was 4.4 per child.
Tracheobronchial compression
CT revealed a total of 259 sites (37.8%) of large-airways compressions out of a possible 686 sites (7 sites × 98 individuals). The number and severity of airways compression is summarised by age group in Table 2.
In the infant age group, the largest age group, we found 140/259 of all compressions (54.1%). The number of compressions per child was similar across age groups ranging from 43/19 (2.3 compressions per patient) for the 2–6 year age group to 140/15 (2.8 compressions per patient) in the 0–1 year age group. Severe and complete stenoses formed 30/259 (11.6%) and 30/259 (11.6%), respectively, of all compressions. The infant group demonstrated the highest proportion of severe and complete compressions, 41/60 (68.3%). (Total of 41 severe and complete compressions in the infant age group; total of 60 severe and complete compressions in all patients.) 41/50 (82%) of the infants had severe or complete compression at some level. (Total of 41 severe and complete compressions in the infant age group; total of 50 infants with severe and complete compressions.) Only 4/60 (6.7%) of severe and complete compressions were in children older than 6 years. (Total of 4 severe and complete compressions in children older than 6 years; total of 60 severe and complete compressions in all patients.)
Levels of compression (by seven predetermined levels) were documented in the database and are summarised, by severity, in Table 3.
The most common site of compression was the bronchus intermedius comprising 73/259 sites (28.2%), followed by the left main bronchus (63/259; 24.3%) and trachea (62/259; 24%). Most (34/259; 13.1%) of the severe/complete compressions, and 56.7% of all severe and complete compressions (18 + 16/30 + 30) involved the bronchus intermedius. Severe and complete compressions made up 34/73 bronchus intermedius compressions (46.6%). Tracheal compression was most commonly mild or moderate (60/62; 96.8%).
Endoluminal lesions were seen in 14 children, ten on the right side and four on the left. It was not possible to differentiate granulomatous tissue from debris.
A total of 262 instances of parenchymal complication were identified in 92/98 children (93.9%). Table 4 shows the instances of specific parenchymal complications. The most common complication was consolidation (44.7%), followed by breakdown (16.4%) and air trapping (15.6%). Overall, parenchymal complications were more frequent in the right lung, (164/262; 62.6%) than in the left (98/262; 37.4%). All categories of complications were more frequent in the right lung. The mean number of complications per child was 2.8 (92/262).
The number of patients in the database with specific parenchymal complications that can be ascribed to lymphobronchial TB, together with laterality of the lesions, are summarised in Table 5. The most common complication was consolidation (87.7%), followed by breakdown (41.8%) and air trapping (37.8%). The majority of children (66/98) had multiple complications, either uni- or bilaterally. A total of 81 associated findings were documented in 56 children. These included ovoid focal lesions (Ghon focus, a classic radiological sign of primary TB), pleural disease, miliary nodules and intracavitary bodies. These findings are summarised in Table 6. The most common associated finding was ovoid focal lesions (33/81 = 40.1%), followed by pleural disease, 27.2% (16/81 = 19.8% on right + 6/81 = 7.4% on left) and miliary nodules, 24.7% (20/81).
Six children with lymphobronchial TB had no parenchymal complications. The mean age of these patients was 19 months (median 8 months) compared to the overall group who had an mean age of 26 months (median 12 months). None in the subgroup of six children without parenchymal complications had complete obstruction of any airway. There was a higher proportion of tracheal compression (83%) in comparison to the overall group (63%), with a higher proportion of severe obstruction of the trachea (20% of this subgroup vs 3% of the overall group). There was a lower proportion of bronchial involvement in the subgroup (BI 67% vs 75%; LMB 50% vs 64%; RMB 17% vs 20%) with no severe compressions of any of the bronchi in children without parenchymal complications. Ovoid focal bodies noted in three children of the subgroup (50% vs 34% in the overall group) were indistinguishable from the parenchymal complications of lymphobronchial TB.
Discussion
The incidence of TB is increasing worldwide, and children are at increasing risk of being exposed to the disease [19]. The subsequent increase in the number of children with TB has led to an increase in the number of children with complications such as lymphobronchial TB. Primary TB results from the inhalation of droplets containing the causative organism Mycobacterium tuberculosis. This initiates a localised pneumonic process called the Ghon focus. From here, the bacilli spread to regional lymph nodes via lymphatics. This local infective focus with associated lymphangitis and lymphadenopathy is known as the primary complex or Ghon complex [2, 20]. This regional lymphadenopathy is considered the hallmark of the radiologic diagnosis of primary TB in children [2, 21].
The radiologic findings of lymphobronchial TB on chest radiographs are well-established, but the findings on CT are not well-documented, nor is the prevalence of parenchymal complications. Similarly, CT findings in patients with TB are well-documented, but there is a paucity of published data regarding CT of lymphobronchial TB. Known findings include enlarged lymph nodes, hyperinflation, consolidation, wet lung, necrosis, bulging fissures and eventual cavitation [6, 8].
Contrast-enhanced chest CT may identify lymph nodes that are not visible on radiographs [4, 21–24]. All the patients in our study had lymphadenopathy, and 99% had more than one site involved. The most common sites were the subcarinal, right hilar and paratracheal sites. This compares well with the known distribution of lymphadenopathy on radiographs [2, 20, 25].
The current study was compared to two previous studies that reported the prevalence and distribution of TB lymphadenopathy on CT. In the study by Andronikou et al. [21], the sample population included patients suspected but not proved to have TB, as was the case in the study by Mukund et al. [26] and our study. Both previous studies demonstrated airway disease in approximately one-third of patients whereas all patients in our study had airway involvement judged by our inclusion criteria. The median age of patients in the study by Mukund [26] was higher (11 years) than that found by Andronikou et al (21.5 months) [21] and the current study (12 months).
With regards to distribution of lymphadenopathy, our study demonstrates a higher prevalence overall and at specific sites when compared to both previous studies. In particular, we show a higher prevalence of hilar lymphadenopathy than the study by Mukund, and this can probably be explained by our preselection of childen with airway disease. This preselection in a predominantly infant population accounts for the higher prevalence of hilar lymphadenopathy, which is more likely to result in airway disease because bronchi in infants are more easily occluded due to their lack of cartilage and smaller diameter.
We show a higher prevalence of tracheal lymphadenopathy when compared to Andronikou et al. [21], who used a single-slice CT scanner. The use of MDCT in the our study presumably gave the reader (same reader as in the previous study) a higher confidence for distinguishing lymphadenopathy from the thymus and from poorly enhancing vasculature.
When tuberculous lymph nodes affect the airways, the resulting lesions define lymphobronchial TB. This may take the form of external airway compression, erosion, ulceration, infiltration, intraluminal caseating material or granulation tissue [6]. Not all children with TB develop lymphobronchial TB. The reported incidence of airway narrowing by tuberculous lymph nodes varies from 35% [21] to 40% [25]. The patient population in our study is a super-selected group of children presenting with symptoms of airway compression. It is not known what percentage they represent of all paediatric TB patients at our institution.
Younger children are more likely to develop TB, including lymphadenopathy, after infection with M. tuberculosis [2]. Younger children are also more likely to have tuberculous lymph nodes, probably because their immune systems are less mature [27], allowing organisms to proliferate within the reticulo-endothelial system. Younger children, by nature of their anatomical size, have narrower airways with less supportive cartilage, making them more compressible. This age-specific disease risk and the characteristic lymphadenopathy in primary TB offer plausible explanations why infants were more likely to develop lymphobronchial TB in our study. Although our patients ranged from 2 months to 12 years of age, the majority (51%) were younger than 1 year. This age group also demonstrated more severe compressions, with 82% of patients less than 1 year of age showing severe or complete compressions at least at one site.
The prevalence of HIV in our study population was 17%. This is in keeping with the prevalence of HIV co-infection with TB in children in other studies. Reported HIV prevalences in these studies vary from 11.2% in Ethiopia [28] and 18% in Zambia [29] in 2002, to 22.3% [30] and 21% [31] in more recent studies (2007, 2009) in South Africa. This similarity between the HIV prevalence of our study population with lymphobronchial TB and other study populations with unspecified TB implies that HIV infection is not a specific predisposing factor for the development of lymphobronchial TB.
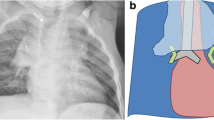
The children in our study showed a high percentage of lymphadenopathy at documented sites (73.5% of all possible sites showed nodal enlargement) but compression of the tracheobronchial tree was present at only 38% of sites. Although any of the airways may show compression by lymph nodes, our study showed that the bronchus intermedius is compressed most frequently (28% of all compressions). This is probably because it is a third-order bronchus and therefore narrower than the left and right main bronchi. It also is longer, more vertical and situated between two nodal groups that are often enlarged (subcarinal, 96.9% and right hilar, 85.7%). The predominant form of bronchus intermedius-compression was of a nutcracker-type, with combined medial and lateral compression (Fig. 1). Less typical, larger, more extensive lymphadenopathy compressed it circumferentially (Fig. 2). The bronchus intermedius was also the most severely compressed airway segment (severe and complete in 47% of cases of involvement). Goussard and Gie [6] also found the bronchus intermedius a common site for complete obstruction.
Contrast-enhanced axial chest CT of an 11-month-old boy with lymphobronchial TB demonstrates right hilar and subcarinal lymphadenopathy resulting in severe circumferential compression of the bronchus intermedius (white arrow) and moderate compression of the left main bronchus (black arrow) against the left pulmonary artery
The right and left main bronchi abut the pulmonary arteries, which may explain why they do not exhibit nutcracker-type compression by lymph nodes. When extensive lymphadenopathy encases the main bronchi, their larger calibre appears to initially protect them from significant compression. The longer and narrower left main bronchus accounted for 24% of total airway compressions, the right main bronchus for 8%.
The trachea accounted for 24% of nodal compressions; however, these were mostly (97%) mild or moderate, probably because the trachea is larger and more rigid than the bronchi and because it is not usually circumferentially involved and therefore responds by displacement rather than compression.
Nodal compression of airways leads to air trapping (Fig. 3), consolidation (Fig. 4), collapse, expansile pneumonia (Fig. 5), necrosis and breakdown (Fig. 6). The most common complication documented in our study was consolidation (88% of the children). Of note, however, is that it is not possible to differentiate consolidation secondary to lymphobronchial TB from that of primary TB, as the radiologic appearances are indistinguishable. Consequently, our results may overestimate the frequency of consolidation as a complication in lymphobronchial TB.
19-month-old boy with lymphobronchial TB. Constrast-enhanced axial chest CT demonstrates parenchymal abnormalities in the left lung as a result of left main bronchus compression (not shown). There is consolidation posteriorly (enhancing viable consolidated lung with air-bronchograms) and lung necrosis (low-density non-enhancing lung without air-bronchograms, black arrow) anteriorly. Multifocal ovoid soft-tissue density interstitial lesions (white arrows) are seen in the right lung
Constrast-enhanced axial chest CT of a 7-month-old girl with lymphobronchial TB demonstrates right lower lobe necrotic expansile penumonia (white arrow) secondary to compression of the bronchus intermedius (black arrow) by subcarinal tuberculous lymph nodes. Less severe parenchymal abnormalities are seen in the left lung
10-month-old boy with lymphobronchial TB. Contrast-enhanced axial chest CT demonstrates multiple cavities (black arrows) within the consolidated left lung. This was secondary to compression of the left main bronchus (not shown). There are subcarinal lymphadenopathy and multifocal soft-tissue nodules in the right lung (white arrows)
Evaluation of the subgroup of children that did not demonstrate any parenchymal complications of lymphobronchial TB (6% of all children) indicates that these children had a higher proportion of tracheal involvement rather than bronchial involvement and that there were no cases of severe or complete bronchial obstruction. We surmise that the lower proportion of severe and complete bronchial compression accounts for the absence of parenchymal complications of lymphobronchial TB. In addition we noted that there was a higher proportion of moderate and severe tracheal involvement in this subgroup without associated parenchymal complications.
The children in our study showed a predominance of right-side parenchymal complications, both overall and in each category of complication. This is to be expected, as lymphadenopathy was most commonly on the right and the most frequent and severe bronchial occlusions were also on the right. In addition, primary TB is reported to be more common on the right, which is thought to be related to the anatomy of the bronchial tree [32].
Other findings associated with TB, but not necessarily complications of lymphobronchial TB, were documented. These included pleural disease, ovoid focal bodies, miliary nodules and intracavitary bodies. All of these findings were also more common on the right.
TB is a progressively evolving, dynamic pathological process, and imaging findings depend on its stage. Our patients showed a variety of synchronous imaging abnormalities, e.g. features of primary TB (ovoid focal bodies) coexisting with abnormalities of advanced lymphobronchial TB (breakdown and cavitation; Fig. 6). Other children showed abnormalities of early lymphobronchial TB (air trapping) together with chronic lung disease in the form of bronchiectasis (Fig. 7). Interestingly, it has also been reported that treatment for TB can cause nodes to paradoxically enlarge [25], giving rise to more acute findings in patients with advanced (established) lymphobronchial TB.
Identifying the complications and associations of lymphobronchial TB has clinical relevance. These patients may need surgical intervention in the form of enucleation of an obstructing node to decompress a bronchus, or bronchoscopic removal of intraluminal obstructions [12–14]. Recovering patency of the airway and allowing resolution of the parenchymal disease associated with the obstruction to thus be achieved. In such cases, CT images serve as a road map for the surgical approach [33], and follow-up CT may confirm resolution [34]. Although CT may not replace bronchoscopy, it provides useful complementary information [35].
Our study was conducted purely from collected data without reviewing the CT scans. However, one author performed the original CT reporting from which the study data were compiled. The recorded data did not include sites of consolidation according to lobar distribution in detail, but recorded only the side of involvement. As a consequence, we could not draw direct conclusions from comparing of the site of the compression and the exact site of consolidation. CT is unable to differentiate consolidation of primary TB from consolidation as a complication of bronchial occlusion in lymphobronchial TB. The number of recorded instances of consolidation in our study may therefore include primary TB consolidations, falsely elevating the number of parenchymal complications of lymphobronchial TB in our study sample.
We identified a variety of future research questions including correlation of the degree of compression with nodal size. This study indicates that larger nodes are more likely to cause more severe airway compression. Determining whether there is a correlation between the consistency of lymph nodes identified at bronchoscopy, node density at CT and signal intensity at MRI may be interesting. So will further analysis to determine any relationship between lymph node consistency and likelihood of airway compression.
The hypothesis of pathogenesis developed from our study data may be confirmed by serial imaging of children with lymphobronchial TB, which may offer further insight into the disease process.
Conclusion
The most important CT finding in children with lymphobronchial TB is airway compression due to lymphadenopathy. Compression most frequently involves the right-side airways, most notably the bronchus intermedius. Compression is more frequent and more severe in infants. Parenchymal complications distal to an airway narrowing predominantly involve the right lung and include air trapping, consolidation/collapse, expansile pneumonia, breakdown and bronchiectasis. Associated findings in TB include miliary nodules, pleural disease, parenchymal ovoid focal bodies and intracavitary bodies, which are seen in a variety of combinations with the parenchymal complications.
CT of children with lymphobronchial TB is useful both for diagnosis and care planning. Recognition of lymphobronchial TB, identification of the location of airway compression, and identification of related parenchymal complications are relevant for paediatric radiologists, paediatric pulmonologists and thoracic surgeons and enable the planning of lung-sparing interventions. Development of protocols for scanning children with lymphobronchial TB, in collaboration with referring pulmonologists and thoracic surgeons, might be of multi-disciplinary benefit.
References
WHO (2010) Global tuberculosis control: a short update to the 2009 report. http://www.who.int/tb/publications/global_report/2009/update/en/index.html Accessed on 22/3/2010
Marais BJ, Gie RP, Schaaf HS et al (2004) The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 8:392–402
Beyers JA (1979) The radiological features of primary pulmonary tuberculosis. S Afr Med J 55:994–997
Daltro PA, Nunez-Santos E (2008) Pediatric tuberculosis. In: Lucaya J, Strife JL (eds) Pediatric chest imaging: chest imaging in infants and children. Springer, Berlin, pp 165–181
Marais BJ, Gie RP, Schaaf HS et al (2004) A proposed radiological classification of childhood intra-thoracic tuberculosis. Pediatr Radiol 34:886–894
Goussard P, Gie R (2007) Airway involvement in pulmonary tuberculosis. Paediatr Respir Rev 8:118–123
Gie RP, Goussard P, Kling S et al (2004) Unusual forms of intrathoracic tuberculosis in children and their management. Paediatr Respir Rev 5 Suppl A:S139–S141
Andronikou S, Vanhoenacker FM, De Backer AI (2009) Advances in imaging chest tuberculosis: blurring of differences between children and adults. Clin Chest Med 30:717–744
Venta LA, Shapir J (1985) Enhancement of pulmonary vasculature in pulmonary consolidation as seen by computed tomography. J Comput Tomogr 9:133–135
Andronikou S, Wieslthaler N (2009) Imaging for tuberculosis in children. In: Schaaf HS, Zumla A (eds) Tuberculosis: a comprehensive clinical reference. Saunders Elsevier, Philadelphia, pp 261–295
Griffith-Richards SB, Goussard P, Andronikou S et al (2007) Cavitating pulmonary tuberculosis in children: correlating radiology with pathogenesis. Pediatr Radiol 37:798–804
Papagiannopoulos KA, Linegar AG, Harris DG et al (1999) Surgical management of airway obstruction in primary tuberculosis in children. Ann Thorac Surg 68:1182–1186
Hewitson JP, Von Oppell UO (1997) Role of thoracic surgery for childhood tuberculosis. World J Surg 21:468–474
Freixinet J, Varela A, Lopez RL et al (1995) Surgical treatment of childhood mediastinal tuberculous lymphadenitis. Ann Thorac Surg 59:644–646
Hammer GB, Fitzmaurice BG, Brodsky JB (1999) Methods for single-lung ventilation in pediatric patients. Anesth Analg 89:1426–1429
Respiration and circulation (biological handbooks) (1971) Federation of American Societies for Experimental Biology. Bethesda, MD, pp 105–108
Weibel ER (1963) Morphometry of the human lung. Springer, Berlin
The lung: scientific foundations (1997) (2nd edn) Lippincott-Raven, Philadelphia, pp 1064–1066
Middelkoop K, Bekker LG, Morrow C et al (2009) Childhood tuberculosis infection and disease: a spatial and temporal transmission analysis in a South African township. S Afr Med J 99:738–743
Parisi MT, Jensen MC, Wood BP (1994) Pictorial review of the usual and unusual roentgen manifestations of childhood tuberculosis. Clin Imaging 18:149–154
Andronikou S, Joseph E, Lucas S et al (2004) CT scanning for the detection of tuberculous mediastinal and hilar lymphadenopathy in children. Pediatr Radiol 34:232–236
de Charnace G, Delacourt C (2001) Diagnostic techniques in paediatric tuberculosis. Paediatr Respir Rev 2:120–126
Delacourt C, Mani TM, Bonnerot V et al (1993) Computed tomography with normal chest radiograph in tuberculous infection. Arch Dis Child 69:430–432
Venkateswaran RV, Barron DJ, Brawn WJ et al (2005) A forgotten old disease: mediastinal tuberculous lymphadenitis in children. Eur J Cardiothorac Surg 27:401–404
Andreu J, Caceres J, Pallisa E et al (2004) Radiological manifestations of pulmonary tuberculosis. Eur J Radiol 51:139–149
Mukund A, Khurana R, Bhalla AS et al (2011) CT patterns of nodal disease in pediatric chest tuberculosis. World J Radiol 3:17–23
Newton SM, Brent AJ, Anderson S et al (2008) Paediatric tuberculosis. Lancet Infect Dis 8:498–510
Palme IB, Gudetta B, Bruchfeld J et al (2002) Impact of human immunodeficiency virus 1 infection on clinical presentation, treatment outcome and survival in a cohort of Ethiopian children with tuberculosis. Pediatr Infect Dis J 21:1053–1061
Chintu C, Mudenda V, Lucas S et al (2002) Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 360:985–990
Schaaf HS, Marais BJ, Whitelaw A et al (2007) Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Infect Dis 7:140
Hesseling AC, Cotton MF, Jennings T et al (2009) High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis 48:108–114
Leung AN, Muller NL, Pineda PR et al (1992) Primary tuberculosis in childhood: radiographic manifestations. Radiology 182:87–91
Papaioannou G, Young C, Owens CM (2007) Multidetector row CT for imaging the paediatric tracheobronchial tree. Pediatr Radiol 37:515–529
Maydell A, Goussard P, Andronikou S et al (2010) Radiological changes post-lymph node enucleation for airway obstruction in children with pulmonary tuberculosis. Eur J Cardiothorac Surg 38:478–483
Lawler LP, Corl FM, Haponik EF et al (2002) Multidetector row computed tomography and 3-dimensional volume rendering for adult airway imaging. Curr Probl Diagn Radiol 31:115–133
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lucas, S., Andronikou, S., Goussard, P. et al. CT features of lymphobronchial tuberculosis in children, including complications and associated abnormalities. Pediatr Radiol 42, 923–931 (2012). https://doi.org/10.1007/s00247-012-2399-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2399-x