Abstract
Background
Urea cycle disorders encompass several enzyme deficiencies that can result in cerebral damage, with a wide clinical spectrum from asymptomatic to severe.
Objective
The goal of this study was to correlate brain MRI abnormalities in urea cycle disorders with clinical neurological sequelae to evaluate whether MRI abnormalities can assist in guiding difficult treatment decisions.
Materials and methods
We performed a retrospective chart review of patients with urea cycle disorders and symptomatic hyperammonemia. Brain MRI images were reviewed for abnormalities that correlated with severity of clinical neurological sequelae.
Results
Our case series comprises six urea cycle disorder patients, five with ornithine transcarbamylase deficiency and one with citrullinemia type 1. The observed trend in distribution of brain MRI abnormalities as the severity of neurological sequelae increased was the peri-insular region first, extending into the frontal, parietal, temporal and, finally, the occipital lobes. There was thalamic restricted diffusion in three children with prolonged hyperammonemia. Prior to death, this site is typically reported to be spared in urea cycle disorders.
Conclusion
The pattern and extent of brain MRI abnormalities correlate with clinical neurological outcome in our case series. This suggests that brain MRI abnormalities may assist in determining prognosis and helping clinicians with subsequent treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
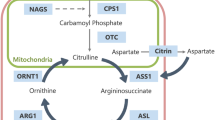
Urea cycle disorders include both enzyme and transporter deficiencies that result in impaired elimination of waste nitrogen and resultant hyperammonemia. The overall incidence of urea cycle defects is estimated to be at least 1 in 25,000 [1]. High plasma ammonia levels lead to high glutamine levels in plasma as well as in the central nervous system (CNS). Glutamine is one mediator of CNS toxicity associated with hyperammonemia, though the mechanism(s) is unclear [2]. Characteristic findings on MRI include prolonged T1 and T2 in the cerebral cortex, with a predilection for the peri-insular cortex and basal ganglia. The thalami are reported to be typically spared, differentiating this pattern from hypoxic-ischemic injury in neonates [3]. The perirolandic and occipital cortices in older patients are characteristically spared [4], although perirolandic involvement has been described in severe disease [4]. Additionally, when observed, restricted diffusion has been termed metabolic stroke, reflecting tissue injury during the hyperammonemic episode [4–7].
Urea cycle disorders have a wide phenotypical spectrum. In neonatal-onset disease, there is a virtually complete enzyme deficiency that may result in marked hyperammonemia, and severe neurological sequelae [8], whereas in childhood and adult-onset forms, there is partial enzyme deficiency with variable symptoms [9–11]. However, outcome prediction is not clear-cut [12] and there is no current direct correlation between genotype and phenotype [13]. Symptoms in females in X-linked OTC deficiency are dependent upon the X-inactivation pattern. Peak ammonia level at diagnosis has been reported to be predictive of neurological outcome [10], although specific values are not known and these were not predictive in another study [14]. Assessing the likely prognosis is crucial to clinicians when making difficult treatment decisions, such as whether to recommend continued support and treatment, including possible liver transplantation, for a neonate who has had severe hyperammonemia.
In 1984, correlation between IQ at 12 months and severity of brain CT findings, using a scoring system from 0 to 9, was found in 26 children with neonatal-onset disease [15]. The CT score described by these authors also correlated with the duration of hyperammonemic coma, a parameter used clinically for assessing prognosis in neonatal-onset disease. MRI findings and clinical manifestations have been correlated in a case series of seven individuals with late-onset OTC deficiency [16]. In this study, asymptomatic or mildly symptomatic individuals had normal brain MRIs. The goal of our study was to determine which brain MRI abnormalities correlated with more severe clinical neurological sequelae in patients with symptomatic hyperammonemia. We hypothesize that certain brain MRI patterns correlate with more severe neurological sequelae and, therefore, may be a useful prognostic tool to aid clinicians in making difficult treatment decisions.
Materials and methods
The electronic medical record at Children’s Hospital Colorado (Aurora, CO) was searched using several keywords (i.e. urea cycle defect, ornithine transcarbamylase, citrullinemia, carbamyl phosphate synthetase, hyperammonemia) to select patients with a urea cycle disorder and brain MRI exam(s) from 1999 to 2010. Cases were excluded if no brain MRI was obtained within 1 month of a hyperammonemic episode, due to the time sensitivity of diffusion-weighted imaging. An exempt status for this retrospective review was approved by the Institution Review Board.
Brain MRI exams were all performed at 1.5 T at Children’s Hospital Colorado with the following protocol: T1-weighted axial and sagittal images, T2-weighted axial images, T2 fluid attenuation inversion recovery (FLAIR) axial images, and diffusion-weighted images with apparent diffusion coefficient map. Postcontrast T1-weighted images were obtained in five of the six children
Each diagnosis was suggested by biochemical testing and confirmed by DNA sequencing. Children treated for critical hyperammonemic episodes received IV ammonia scavenging medications, IV arginine and hemodialysis (patients 1, 2, 3 and 5). Chronic management (patients 1, 4, 5 and 6) included oral ammonia scavenging medications, a protein-restricted diet and amino acid supplementation.
The clinical history of each child was reviewed with assessment of neurological sequelae, graded as mild, moderate or severe by physicians specializing in metabolic disease. Neurological sequelae were graded as mild if there was resolution of symptomatic hyperammonemia (altered mental status, seizures, emesis), without apparent permanent abnormalities on neurological exam or impaired cognition; moderate if there were persistent abnormalities on neurological exam or developmental delay, and severe if there was severe spastic quadriplegia or profound developmental delay.
Each brain MRI was reviewed by two radiologists. The type, distribution and extent of brain MRI abnormalities were then correlated with the clinical severity of neurological sequelae. The small size and heterogeneity of the patient population did not permit statistical analysis. The correlation between MRI findings and neurological sequelae was expected to be a trend correlation.
Results
This case series of six children includes four boys and two girls (see Table 2 for summary). Five had ornithine transcarbamylase deficiency—three boys with neonatal-onset disease and two girls with late-onset disease. One boy had neonatal onset citrullinemia type I (CIT 1) deficiency. Two children received a liver transplant, one with early-onset disease (patient 5) and one with late-onset disease (patient 6). In two children with early-onset disease, support was withdrawn because of poor neurological prognosis (patients 2 and 3). One boy with early-onset OTC deficiency was treated (patient 1) despite the poor prognosis, at parental request, and died of an apparent respiratory event at 15 months. Two children had one brain MRI each, two had two brain MRIs and two had three MRIs, for a total of 12. See Table 1 for clinical summaries and Table 2 for MRI findings.
Clinical severity of neurological sequelae ranged from moderate to severe. Three children had known or presumed severe neurological sequelae and three had moderate neurological sequelae. Two children were excluded from the series (one with mild and one with moderate neurological sequelae) because their MRIs were performed remote (months to years) from the hyperammonemic episode. The two children with severe neonatal-onset OTC or CIT 1 deficiency (patients 2 and 3), for whom support was withdrawn, were classified as severe, as this was the presumed clinical neurological outcome.
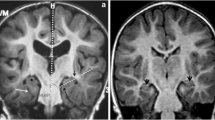
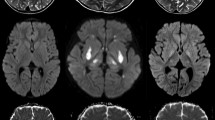
In five of six children, the peri-insular region demonstrated T2 and FLAIR hyperintense signal (Fig. 1), with the addition of restricted diffusion in all three cases with severe neurological sequelae and one with moderate neurological sequelae (patient 5, Fig. 2). In addition, all cases with severe neurological sequelae demonstrated restricted diffusion in areas beyond the peri-insular region and frontal lobes. The observed trend in distribution as the severity of neurological sequelae increased was the peri-insular region first, extending into the frontal, parietal, temporal and, finally, the occipital lobes (Figs. 3 and 4). There was restricted diffusion in the thalami in all three brain MRI exams prior to death or withdrawal of support (patients 1–3). There was restricted diffusion in the brainstem of all with severe neurological sequelae, with the exception of patient 1 (only T2 hyperintense signal), prior to death. Hyperintense signal on T2-weighted images without restricted diffusion occurred in the brainstem of one child with moderate neurological sequelae (patient 4). There was restricted diffusion in the basal ganglia in all three with severe neurological sequelae and in one with moderate neurological sequelae (patient 5).
Only one child had no signal abnormality on brain MRI; mild cerebral volume loss was the only abnormality (patient 6, who had moderate neurological sequelae: developmental delay). Four children had cerebral volume loss (patients 1, 4, 5 and 6), three of whom demonstrated progression over time (patients 1, 4 and 5). There was occipital cortical enhancement in a single case (patient 4, Fig. 5).
The presence or absence of restricted diffusion had a trend correlation with severity of elevated ammonia and glutamate levels (Table 1). All children who demonstrated restricted diffusion had ammonia levels at or above 2,196 μmol/L and glutamate levels at or above 2,883 μmol/L. Those without restricted diffusion had ammonia levels at or below 655 μmol/L and glutamate levels at or below 1,445 μmol/L.
Discussion
In individuals with a urea cycle disorder, different parts of the brain have different sensitivities to damage associated with hyperammonemic episodes, documented in previous studies [4] as well as in our series. The age at which hyperammonemia occurs, as well as the duration affects brain MRI findings and neurological sequelae. Mild hyperammonemia and/or hyperammonemia of limited duration causes less damage, affects only the most sensitive areas and results in limited or no long-term neurologicalal sequelae. More severe and prolonged episodes of hyperammonemia cause more widespread neurological damage, affect areas more resistant and have more severe neurological sequelae. We hypothesize that brain MRI abnormalities reflect this differential distribution of brain involvement, particularly in the acute setting (optimally within 3–5 days of hyperammonemia), and may help determine neurological outcome.
The most important discriminating factors in the severity of neurological sequelae were the presence or absence of restricted diffusion (dependent on timing of MRI with respect to hyperammonemia) and its distribution. In the neonatal-onset cases in which the ammonia levels were high and duration of hyperammonemia prolonged, restricted diffusion involved lobes beyond the peri-insular region and frontal lobes. The observed trend in distribution as the severity of neurological sequelae increased was the peri-insular region first, extending into the frontal, parietal, temporal and, finally, the occipital lobes. There was restricted diffusion in the thalami in all three fatal cases, more typical of anoxic injury. Severe neonatal citrullinemia with restricted diffusion in the basal ganglia and the thalami has been reported [17]. Findings similar to anoxic injury may be related to a common mechanism that anoxia and hyperammonemic states in urea cycle disorders share. Elevated glutamate/glutamine has been demonstrated by MR spectroscopy in both anoxic brain injury [18] and urea cycle disorders [19, 20]. Restricted diffusion in the thalami was absent in the children who survived, the more typical pattern described in urea cycle disorders [3]. Patient 2, a neonate, required extracorporeal membrane oxygenation (ECMO) and pressors for profound hypotension; therefore, hypoperfusion may have contributed to MRI abnormalities. In such cases, the consequences of a metabolic insult may not be distinguishable from hypoperfusion-related ischemia.
In our series, restricted diffusion only occurred during, or shortly after, a hyperammonemic episode. However, not all children imaged during a hyperammonemic episode had restricted diffusion, such as patients 4 and 6, both with late-onset OTC deficiency and lower peak ammonia values (Table 2). Their ammonia levels were much lower than those with early-onset disease. Another neonate we evaluated, not included in this series, had transient hyperammonemia, without restricted diffusion, despite an ammonia level of 4,720 μmol/L, higher than all patients in this series, but with less elevation of plasma glutamine (1,193 μmol/L). In all patients with restricted diffusion, the plasma glutamine was greater than 2,000 μmol/L. We hypothesize the brain MRI abnormalities observed in our series do not reflect hyperammonemic states in general, but rather resulting neurological injury and therefore outcome in urea cycle disorders, which are not due to elevated ammonia alone. Cerebral blood flow autoregulation fails in diseases with hyperammonemia, resulting in uncoupled metabolism and decreased cerebral blood flow [21]. Experiments in rats have suggested that cerebral edema and hypoxia may result from the accumulation of glutamine in the brain rather than from hyperammonemia alone [22, 23]. Furthermore, accumulation of ammonia and glutamine has been implicated in several neurotoxic effects, such as increased blood-brain barrier permeability, cell energy metabolism depletion, and altered neurotransmitter and amino acid levels. A recent review also discusses the detrimental effect of excess glutamine on astrocyte morphology and function [2]. In urea cycle disorders, in addition to elevated glutamine [20], there is elevated 5-hyroxyindoleacetic acid (5-HIAA) and quinolinic acid (an excitotoxin at the NMDA glutamate receptor), which may contribute to neurological sequelae.
The presence of restricted diffusion is time-sensitive to proximity of the hyperammonemic episode. Diffuse cerebral volume loss (including basal ganglia volume loss in patient 1) was the only evidence of remote cerebral insult, as demonstrated by five of the patients, four of whom had progressive volume loss. Some or all of the T2 hyperintense signal resolved on follow-up MRI in each neonatal-onset patient who had an early acute MRI and subsequent follow-up MRI(s) (patients 1 and 5). Resolution of signal abnormalities has been previously described in citrullinemia after medical treatment [24] and liver transplantation [25]. No brain signal abnormalities were apparent after liver transplantation in patient 5. Expected resolution of restricted diffusion without new restricted diffusion occurred after medical treatment on 1-month follow-up MRI in patients 1 and 5, though increased T2 signal abnormalities remained on the second MRI in patient 1.
Given the resolution of brain MRI abnormalities in our series, some of these metabolic insults may be reversible. In ischemic stroke, sites of restricted diffusion do not always result in irreversible infarction [26]; the same is likely true for restricted diffusion at sites of metabolic stroke. The observed distribution of brain MRI abnormalities reflects the severity of initial cerebral insult from a hyperammonemic episode(s). Patient 5 is a clear example of reversible brain MRI abnormalities following liver transplant with subsequent nearly normal brain MRI (other than volume loss).
A practical application of the proposed MRI-neurological sequelae correlation is provided by the contrast between patient 5 and patients 1, 2 and 3. All presented as newborn boys with severe neonatal ornithine transcarbamylase deficiency. Patient 2’s condition was recognized belatedly. Despite aggressive management with rapid correction of hyperammonemia, he showed extensive restricted diffusion throughout the brain 3 days after resolution of hyperammonemia. After normalization of his ammonia level, he developed seizures, hypertonia and had minimal spontaneous movement. Patients 1 and 3 had similar clinical courses. In sharp contrast, patient 5 developed similarly severe hyperammonemia, but of much shorter duration, identified 8 h after symptom onset. After equally aggressive treatment, his ammonia was corrected within 24 h. Brain MRI obtained 3 days after resolution of hyperammonemia showed limited restricted diffusion in the peri-insular regions and basal ganglia. This correlated with slow resumption of neurological function over several weeks. He had no further episodes of hyperammonemia and received a liver transplant at age 3 months. At age 12 months, he was progressing well developmentally with only mild motor delays. The difference in the brain MRI abnormalities in the neonatal period, very shortly after similar hyperammonemic episodes, but of different durations, reflected accurately the neurological injury incurred. Our patients who had less severe neurological sequelae had involvement limited to the peri-insular regions, with or without involvement of the frontal lobes and basal ganglia. Brain MRI could therefore help identify patients who are likely to have a more favorable neurological outcome and assist families and clinicians in difficult treatment decisions, including liver transplantation.
Brain MRI is one factor, in conjunction with clinical history and serial neurological examinations, in guiding care decisions. Early prognostic information is essential in guiding treatment decisions in children with severe neonatal hyperammonemia. The current study is limited in being retrospective with a small number of patients from a single center. A larger prospective multicenter study would be useful to further validate the trends observed in this case series. Also, further studies should address whether restricted diffusion (shortly after an acute hyperammonemic episode) is more extensive in children with an elevated glutamine level above 2,000 μmol/L, as suggested by our series (see Table 2).
An interesting MRI abnormality in patient 4 was bilateral occipital cortex enhancement (Fig. 5) associated with vision loss, which improved after treatment for OTC. Cortical vision loss has been described in a case report of OTC where brain MRI showed bilateral occipital cortex enhancement [27], similar to our case. In 1987, an adult with OTC with bilateral vision impairment was reported [28], but without MRI imaging. Pathophysiology of OTC involvement of the occipital cortex is unclear.
Brain MRI exams may not demonstrate abnormalities in patients with urea cycle disorders with few or absent symptoms. Diffusion tensor imaging (DTI) would be more sensitive in detecting microscopic white matter changes [17], and MR spectroscopy may demonstrate metabolite changes, such as elevate glutamate and decreased myoinositol [19, 20, 29]; neither was performed in our series.
Conclusion
Patterns of brain MRI abnormalities reflected the severity of neurological outcome in our case series of urea cycle disorders. The observed trend in distribution as the severity of neurological sequelae increased was the peri-insular region first, extending into the frontal, parietal, temporal and, finally, the occipital lobes. In three fatal cases, restricted diffusion occurred in the thalami, which has been reported to be typically spared in urea cycle disorders. Recognizing those abnormalities in the brain that correlate with severity of neurological outcome could be a useful tool in assessing likely prognosis and in assisting clinicians with treatment decisions, especially for individuals with early-onset disease.
Abbreviations
- OTC:
-
Ornithine transcarbamylase deficiency
- CIT 1:
-
Citrullinemia type 1
References
Summar ML, Dobbelaere D, Brusilow S et al (2008) Diagnosis, symptoms, frequency and mortality of 260 patients with urea cycle disorders from a 21-year, multicentre study of acute hyperammonaemic episodes. Acta Paediatrica 97:1420–1425
Brusilow SW, Koehler RC, Traystman RJ et al (2010) Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics 7:452–470
Barkovich AJ (2005) Pediatric neuroimaging. Lippincott Williams & Wilkins, Philadelphia
Takanashi J, Barkovich AJ, Cheng SF et al (2003) Brain MR imaging in acute hyperammonemic encephalopathy arising from late-onset ornithine transcarbamylase deficiency. AJNR 24:390–393
Sperl W, Felber S, Skladal D et al (1997) Metabolic stroke in carbamyl phosphate synthetase deficiency. Neuropediatrics 28:229–234
Mamourian AC, du Plessis A (1991) Urea cycle defect: a case with MR and CT findings resembling infarct. Pediatr Radiol 21:594–595
Bajaj SK, Kurlemann G, Schuierer G et al (1996) CT and MRI in a girl with late-onset ornithine transcarbamylase deficiency: case report. Neuroradiology 38:796–799
Maestri NE, Clissold D, Brusilow SW (1999) Neonatal onset ornithine transcarbamylase deficiency: a retrospective analysis. J Pediatr 134:268–272
McCullough BA, Yudkoff M, Batshaw ML et al (2000) Genotype spectrum of ornithine transcarbamylase deficiency: correlation with the clinical and biochemical phenotype. Am J Med Genet 93:313–319
Nicolaides P, Liebsch D, Dale N et al (2002) Neurological outcome of patients with ornithine carbamoyltransferase deficiency. Arch Dis Child 86:54–56
Smith W, Kishnani PS, Lee B et al (2005) Urea cycle disorders: clinical presentation outside the newborn period. Crit Care Clin 21(4 Suppl):S9–S17
Bachmann C (2003) Long-term outcome of patients with urea cycle disorders and the question of neonatal screening. Eur J Pediatr 162(Suppl):S29–S33
Picca S, Dionisi-Vici C, Abeni D et al (2001) Extracorporeal dialysis in neonatal hyperammonemia: modalities and prognostic indicators. Pediatr Nephrol 16:862–867
Arnoux JB, Dupic L, Barbier V et al (2010) Neurological outcome of pediatric patients with urea cycle disorders. J Inherit Metab Dis 33(Suppl 1):S121
Msall M, Batshaw ML, Suss R et al (1984) Neurologic outcome in children with inborn errors of urea synthesis. Outcome of urea-cycle enzymopathies. N Engl J Med 310:1500–1505
Kurihara AT, Tomita M, Kobayashi K (2003) Magnetic resonance imaging in late-onset ornithine transcarbamylase deficiency. Brain Dev 25:40–44
Majoie CBLM, Mourmans JM, Akkerman EM et al (2004) Neonatal citrullinemia: comparison of conventional MR, diffusion-weighted, and diffusion tensor findings. AJNR 25:32–35
Pu Y, Li Q-F, Zeng C-M et al (2000) Increased detectability of alpha brain glutamate/glutamine in neonatal hypoxic-ischemic encephalopathy. AJNR 21:203–212
Gropman AL, Seltzer RR, Yudkoff M et al (2008) 1H MRS allows brain phenotype differentiation in sisters with late onset ornithine transcarbamylase deficiency (OTCD) and discordant clinical presentations. Mol Genet Metab 94:52–60
Gropman A (2010) Brain imaging in urea cycle disorders. Mol Genet Metab 100(Suppl 1):S20–S30
Larsen FS (2002) Cerebral blood flow in hyperammonemia: heterogeneity and starling forces in capillaries. Metab Brain Dis 17:229–235
Takahashi H, Koehler RC, Brusilow SW et al (1991) Inhibition of brain glutamine accumulation prevents cerebral edema in hyperammonemic rats. Am J Physiol 261(3 Pt 2):H825–H829
Hirata T, Koehler RC, Brusilow SW et al (1995) Preservation of cerebral blood flow responses to hypoxia and arterial pressure alterations in hyperammonemic rats. J Cereb Blood Flow Metab 15:835–844
Chen Y-F, Huang Y-C, Liu H-M et al (2001) MRI in a case of adult-onset citrullinemia. Neuroradiology 43:845–847
Kawata A, Suda M, Tanabe H (1997) Adult-onset type II citrullinemia: clinical pictures before and after liver transplantation. Intern Med 36:408–412
Kranz PG, Eastwood JD (2009) Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR 30:1206–1212
Anderson JM, Brodsky MC (2010) Protracted cortical visual loss in a child with ornithine transcarbamylase deficiency. J Neuroopthalmol 30:99–101
Snebold NG, Rizzo JF, Lessell S et al (1987) Transient visual loss in ornithine transcarbamoylase deficiency. Am J Ophthalmol 104:407–412
Gropman AL, Fricke ST, Seltzer RR et al (2008) 1H MRS identifies symptomatic and asymptomatic subjects with partial ornithine transcarbamylase deficiency. Mol Genet Metab 95:21–30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bireley, W.R., Van Hove, J.L.K., Gallagher, R.C. et al. Urea cycle disorders: brain MRI and neurological outcome. Pediatr Radiol 42, 455–462 (2012). https://doi.org/10.1007/s00247-011-2253-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2253-6