Abstract
Background
Optic pathway glioma (OPG) is a characteristic hallmark of neurofibromatosis type I (NF-I).
Objective
To evaluate the feasibility of magnetic resonance diffusion tensor imaging (MRDTI) at 3T to detect abnormalities of the optic nerves and optic radiations in children with NF-I.
Materials and methods
3-T MRDTI was prospectively performed in 9 children with NF-I (7 boys, 2 girls, average age 7.8 years, range 3–17 years) and 44 controls (25 boys, 19 girls, average age 8.1 years, range 3–17 years). Fractional anisotropy (FA) and mean diffusivity were determined by region-of-interest analysis for the optic nerves and radiations. Statistical analysis compared controls to NF-I patients.
Results
Two NF-I patients had bilateral optic nerve gliomas, three had chiasmatic gliomas and four had unidentified neurofibromatosis objects (UNOs) along the optic nerve pathways. All NF-I patients had statistically significant decreases in FA and elevations in mean diffusivity in the optic nerves and radiations compared to age-matched controls.
Conclusion
MRDTI can evaluate the optic pathways in children with NF-I. Statistically significant abnormalities were detected in the diffusion tensor metrics of the optic nerves and radiations in children with NF-I compared to age-matched controls.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis type I (NF-I) is a common inherited disorder with an approximate incidence of 1:4,000. The development of optic pathway gliomas is a known complication with reported incidence ranging from 1.5% to 24% [1, 2]. Although optic pathway gliomas are designated by the World Health Organization (WHO) as grade I tumors with similar histopathology to juvenile pilocytic astrocytomas, these typically non-aggressive tumors can cause significant visual impairment despite their benign classification [3–5]. Optic pathway gliomas typically have a self-limiting growth pattern and are thought to have a more indolent course in children with NF-I [4, 6–10].
The National Institutes of Health NF-I Optic Nerve Task Force recommends ophthalmological examination of children newly diagnosed with NF-I and without known optic pathway glioma [11]. This task force recommends yearly ophthalmological exams until age 6 followed by longer intervals after age 6; neuroimaging is only recommended when clinically indicated such that routine screening is not advocated [11]. However, optic pathway gliomas might be present prior to onset of clinical symptoms, and vision loss can be difficult to accurately detect in young children [2, 12]. Thus, the role of neuroimaging in children with NF-I is sometimes considered controversial despite these guidelines.
There have been reports of clinical deterioration in children with NF-I diagnosed with optic pathway gliomas at much later stages than previously described, which is concerning because these occurred after long periods of presumed stability [6, 13]. In one study, progression occurred 8 years after initial diagnosis [14]. In another study, during 8 years of follow-up, 50% of patients had clinical or radiological progression of their optic pathway gliomas [6]. Reports of spontaneous regression of optic pathway gliomas as documented by serial MR exams with no improvement in visual function at time of regression have been reported [15–17].
The inability to differentiate or predict progressive from non-progressive optic pathway tumors in children with NF-I remains problematic even with increasingly sophisticated MR imaging [21]. Prediction of tumor progression or morbidity is not possible because studies that have attempted to track the natural history of optic pathway gliomas have been limited by small patient samples, short follow-up longitudinally, and failure to differentiate NF-I with optic pathway gliomas from NF-I without tumor [6]. Tumor location has been suggested as an outcome predictor given that chiasmal lesions tend to act more aggressively [18–20] and often have bilateral visual impairment [6].
MRDTI has been used to assess quantitative differences in mean diffusivity in normal-appearing and abnormal-appearing white matter in children with NF-I compared to age-matched controls, which has suggested microstructural abnormality in the white matter [22]. Recently the feasibility of optic nerve DTI has been demonstrated in patients with septo-optic dysplasia [24]. Given that the optic nerve pathways are essentially a pure white matter fiber tract, we hypothesize that MRDTI can be used to quantitatively and reliably assess the FA and mean diffusivity of the optic nerve and optic radiations in children with NF-I with and without optic pathway gliomas, and this might provide a useful in vivo quantitative bioimaging marker for the optic pathway in children with NF-I.
Materials and methods
This study was compliant with the Health Insurance Portability and Accountability Act (HIPPA) and approved by the Committee on Human Research at our institution. MRDTI was prospectively performed at 3T in nine children with NF-I (7 boys, 2 girls, average age 7.8 years, range 3–17 years). Three children had chiasmatic glioma with unilateral extension into the optic nerve, two children had unilateral optic nerve gliomas with chiasmal involvement, and four had normal-appearing optic nerves but unidentified neurofibromatosis objects (UNOs) along portions of the optic radiations bilaterally. Furthermore, there were 44 retrospectively identified controls (25 boys, 19 girls, average age 8.1 years, range 3–17 years), none of whom had signs, symptoms or findings of optic nerve or pathway pathology. All controls had a brain MR for complaint of headache or migraine and had normal brain MR scans. In all cases, the controls had no significant medical history, including no history of prematurity, seizure, developmental delay, metabolic disorder or traumatic brain injury. All of these cases are part of a normative data bank, HIPPA-compliant and approved by the Committee on Human Research at our institution, which can be reviewed retrospectively as all pediatric brain MR protocols are performed at 3T and include MRDTI.
All MR images were acquired with a 3-T Achieva system (Philips Healthcare, Best, Netherlands) using an 8-channel SENSE head coil. Per hospital protocol, children younger than 7 years were scanned under anesthesia. The diffusion tensor imaging (DTI) was acquired on each child using an axial fat-suppressed single-shot echo planar imaging sequence with field of view (FOV) 220–260 mm, TR/TE 7,620 msec/57 msec, flip angle 90°, acquired in plane voxel resolution of 1.9 × 2 × 2 mm, slice thickness 2 mm, slice gap 0 mm, six directions with two b values of 0 and 1,000 s/mm2, averages = 1, SENSE factor 3, EPI factor 39, BW/pixel 30.2 Hz, halfscan factor of 0.678, and number of slices and scan duration varying with patient size. Six-direction DTI was used as a practical choice in the clinical setting for which the NF-I patients are scanned as the scan duration is less than 3 min. In a paper by Yamamoto et al. [24], no statistically significant differences in mean diffusivity were detected using 6-, 12-, 40-, or 81-directional gradients for DTI. In addition, for anatomical MR imaging, 3D T1-weighted turbo field echo (TFE) imaging was obtained with imaging parameters of TR/TE 8.2 msec/3.8 msec, FOV 200 mm, flip angle 8°, in plane voxel resolution of 0.98 × 1.09 × 1.00 mm, slice thickness 1 mm with no gap, and scan time duration of 6 min 20 s. Axial and coronal T2-weighted anatomical images were obtained with TR 2,640 msec/TE 80 msec, flip angle of 90°, FOV 200 mm, slice thickness 4 mm, slice gap 0.44 mm, effective reconstruction voxel size of 0.57 × 0.72 × 1.0 mm, and 30 slices with scan time duration of 2 min 39 s.
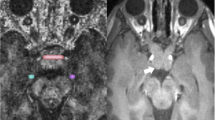
Post-processing data analysis and fiber tracking was performed on a Philips Extended Workspace (EWS, Philips, Best, Netherlands) image processing workstation beginning with a 2D registration of the diffusion-weighted (DW) images with the non-DW images. Pixel-based FA and mean diffusivity values were calculated. Single voxels within the mid to posterior third of the intraorbital optic nerve were identified on T2-weighted images with FA color map overlay, and contiguous points within ipsilateral nerves were merged into one region of interest (ROI) from which FA and mean diffusivity values were generated (Fig. 1). ROIs were placed in the center of the optic nerves to avoid the effects of movement, vascular pulsation and cerebrospinal fluid pulsation closer to the retina. ROIs were placed along optic radiations to determine FA and mean diffusivity (Fig. 2). Location of ROIs was confirmed by tractography of the optic radiations. Deterministic streamline fiber tracking, using the fiber assignment by continuous tracking (FACT) method, was employed using methods adopted by the authors of this study and part of previous research studies of the optic radiations at 3T [24]. This method consists of using either the T1-weighted TFE or T2-weighted images so that ROIs are placed near the lateral geniculate and another ROI is placed in subcortical white matter of calcarine cortex in an area where optic radiations course. Tracts were drawn by the software program and coursed through all ROIs and met tractography software default of minimum FA value of 0.15, minimum fiber length of 10 mm, and maximum angle changes of 27°. Once tractography confirmed optic radiations (Fig. 2), manual ROIs were placed along the course of optic radiations as shown in Fig. 3. The software package provided FA and ADC (mean diffusivity) values for the ROIs drawn of the optic radiations.
The reproducibility of ROI placement was excellent, with interobserver correlation coefficients greater than 0.90 for both optic nerve and optic radiations bilaterally. To determine intraobserver reliability of region-of-interest placement, the same observer repeated the measurements in a subset of 20 cases (controls and NF-I patients) with single-measure intraclass correlation coefficient (ICC) calculated. One observer was a 4th-year medical student trained by the other observer, a neuroradiologist with certificate of added qualification (CAQ) and special interest in pediatric neuroradiology; both observers did manual region-of-interest measurements for the study. ICCs were greater than 0.90.
Statistical analysis
Statistical analysis was performed using the PASW 17.0 (SPSS, Chicago, IL, USA) by a qualified instructor in methodology and statistics. Although the control group was small, the authors assume that members of the control group are drawn from a normal distribution and that the patients with NF-I and optic pathway pathology are representative of NF-I patients with optic pathway pathology given that the Levene test for variance yielded results that were not statistically significant. Independent sample t-tests are appropriate for the data given the presence of only two groups of data: patients with NF-I and the control group. The independent sample t-test ought to be a conservative measure of statistical significance given the propensity for all small datasets to yield inflated standards of error in parametric testing and thereby creating a greater difficulty in achieving low P-values. Statistical significance set at P < 0.05. To explore the mean group differences, values were average between right and left optic nerve as well as right and left optic radiations.
Results
The fractional anisotropy and the mean diffusivity measurements of the right and left optic nerves and the FA and mean diffusivity values for the right and left optic radiations are given in Tables 1 and 2 for the children with NF-I and controls. The average values with statistical significance are summarized in Table 3. Fractional anisotropy was 0.409 +/- 0.052 in the right optic nerve and 0.389 +/- 0.066 in the left optic nerve in children with NF-I, and these values in the controls were 0.545 +/- 0.064 in the right optic nerve and 0.553 +/- 0.057 in the left optic nerve. Children with NF-I had lower FA values in the optic nerves bilaterally that met statistical significance at P < 0.05. The mean diffusivity of the right optic nerve was 1.31 +/- 0.079 and 1.27 +/- 0.078 in the left optic nerve in children with NF-I, which was higher compared to age-matched controls who had values of mean diffusivity in the right optic nerve of 1.080 +/- 0.091 and in left optic nerve of 1.047 +/- 0.097, which was statistically significant at P < 0.05.
The fractional anisotropy in right optic radiations was 0.573 +/- 0.054 and in left optic radiations was 0.570 +/- 0.057 in children with NF-I, which was lower compared to controls, who had values in right optic radiations of 0.620 +/- 0.054 and in left optic radiations of 0.628 +/- 0.061, which was statistically significant at P < 0.05. The mean diffusivity in right optic radiations was 0.944 +/- 0.067 and in left optic radiations was 0.948 +/- 0.077 in children with NF-I, which was higher than the mean diffusivity in optic radiations of controls, which measured 0.888 +/- 0.078 on the right and 0.887 +/- 0.083 on the left, which was statistically significant at P < 0.05.
Discussion
The results of our study demonstrate the feasibility of MRDTI in the evaluation of the optic nerve and optic pathways in children with NF-I. The average FA of the optic nerves and optic radiations was significantly lower than that of age-matched controls in all cases. Values were lowest in the regions where optic nerve glioma and chiasmatic glioma were present. The average mean diffusivity was significantly elevated in the optic radiations and optic nerves of NF-I patients at all levels measured and in all cases.
Information about the microstructural integrity of white matter fiber tracts can be inferred from the fractional anisotropy derived from diffusion tensor MR imaging. Synaptic density, axonal packing, relative membrane permeability to water, internal axonal structure, myelination itself, and tissue water content can affect the fractional anisotropy of white matter fiber tracts [25]. In our study, the elevation in the mean diffusivity in the optic nerves and optic radiations in children with NF-I could represent increased tissue water content, either intracellular or extracellular, or might reflect decreased or abnormal axonal packing or synaptic density, which has been theorized in other studies of adults with NF-I [26–30]. In our study, the marked decreases in FA in optic nerves and optic radiations could represent abnormal myelination even in regions lacking abnormal T2 prolongation or other microstructural dysgenesis or alterations to myelin, which has been theorized in adults with NF-1 [30]. To our knowledge, this is a unique study specifically examining the optic nerves and optic radiations in children with NF-I at 3T. In a prior study at 1.5T FA values in children with NF-I were not significantly lower in areas of subcortical white matter that were assessed with MRDTI, but that study did not specifically examine the optic radiations [22]. In another study that just assessed ADC values of the optic pathway at 1.5T, there were elevations in mean diffusivity of the optic nerve but not in optic radiations [31]. Our findings of both reduced anisotropy of optic radiations and elevations in mean diffusivity might partly relate to choosing a more coherent white matter fiber tract (optic nerve) and higher magnetic field strength (3T), as well as selecting patients with NF-I who either had chiasmatic or optic nerve gliomas or multifocal UNOs that, in part, affected the optic radiations.
However, in our study, four children with NF-I, who did not have OPG, had UNOs along portions of the optic radiations, but many of the visual pathways appeared normal on MR (Fig. 4). In these cases there were elevations in mean diffusivity and reductions in fractional anisotropy at all levels that were studied. The exact nature and relevance of UNOs remains unclear [30]. Based on a histopathological study by DiPaolo et al. [34], there is a commonly accepted theory that spongiotic and vacuolating alterations in myelin structure are responsible for UNOs that are commonly seen in NF-I [30]. A recent study has shown that alterations in diffusivity in patients with NF-I at 1.5T, suggesting intramyelinic abnormality related to increased water content as an etiology for the abnormalities detected on diffusion tensor imaging [22, 33]. Our observation of abnormal FA and mean diffusivity (ADC values) in the setting of normal-appearing optic nerve or optic radiations has been reported in a study that noted diffuse alterations in both mean diffusivity and FA in all normal-appearing areas of subcortical white matter on brain MR that were analyzed in adult patients with NF-I [23, 32], suggesting that MRDTI has the ability to detect abnormalities not detected on routine MR imaging.
In our study, we have shown the feasibility of MRDTI to assess quantitative differences in the optic nerves and pathways in children with NF-I compared to age-matched controls. Further longitudinal research is needed to determine whether abnormalities detected on MRDTI correlate quantitatively with long-term clinical outcome or can predict tumor progression tumor or UNO development.
There are limitations to our study. Our sample size of nine patients with NF-I is small, but this is balanced by the large number of normative measurements of the optic nerves and optic radiations whose values, particularly in the older children, are consistent with other reports [32, 35, 36]. Furthermore, post hoc power analyses showed that the power was greater than 0.80 for all comparisons between the children with NF-I and controls. There is lack of histopathological confirmation for the abnormalities observed. The deterministic method of fiber assignment with continuous tracking (FACT) was used. CSF pulsation, patient movement and distortion artifacts can impede the utility of the FACT method [15, 24]. Furthermore, the FACT method is user-dependent, which can affect reproducibility as it requires user-defined ROIs based on prior anatomical knowledge to restrict the fiber tracts [15], which we used for the FA and ADC measurements of the optic radiations. However, in this study, we had interobserver reproducibility greater than 0.9.
Another potential weakness is insufficient ophthalmological acuity data at the time of MR imaging, as all the children were essentially screened at the time of diagnosis. A prospective study in which children are screened according to task force guidelines based on ophthalmological exam versus those screened with both eye exam and MR imaging would be needed to establish evidence-based guidelines for early detection and management of optic pathway gliomas in children with NF-I [21].
Conclusion
This study demonstrates the feasibility of performing MRDTI to assess the visual pathway in children at 3T from intraorbital optic nerve to optic radiations. Significant decreases in FA and elevations in mean diffusivity are present in the optic nerves and optic radiations of children with NF-I who have optic pathway pathology, which suggests that MRDTI offers increased sensitivity over conventional imaging and might serve as a quantitative bioimaging marker of white matter disease of the optic pathways in children with NF-I.
References
Stern J, Jakobiec FA, Housepian EM (1980) The architecture of optic nerve gliomas with and without neurofibromatosis. Arch Ophthalmol 98:505–511
Lewis RA, Gerson LP, Axelson KA et al (1984) Neurofibromatosis. II. Incidence of optic nerve gliomata. Ophthalmology 91:929–935
Gayre GS, Scott IU, Feuer W et al (2001) Long-term visual outcome in patients with anterior visual pathway gliomas. J Neuroophthalmol 21:1–7
Rush JA, Younge BR, Campbell RJ et al (1982) Optic glioma: long-term follow-up of 85 histopathologically verified cases. Ophthalmology 89(11):1213–1219
Lund AM, Skovby F (1991) Optic gliomas in children with neurofibromatosis type I. Eur J Pediatr 150:835–838
Thiagalingam S, Flaherty M, Billson F et al (2004) Neurofibromatosis type I and optic pathway gliomas: follow-up of 54 patients. Ophthalmology 111:568–577
Listernick R, Charrow J, Greenwald M et al (1994) Natural history of optic pathway tumors in children with neurofibromatosis type I: a longitudinal study. J Pediatr 125:63–66
Hoyt WF, Baghdassarian SA (1969) Optic glioma of childhood: natural history and rationale for conservative management. Br J Ophthalmol 53:793–798
Listernick R, Darling C, Greenwald M et al (1995) Optic pathway tumors in children: the effect of neurofibromatosis type I on clinical manifestations and natural history. J Pediatr 127:718–722
Wright JE, McDonald WI, Call NB (1980) Management of optic nerve gliomas. Br J Ophthalmol 64:545–552
Listernick R, Louis DN, Packer RJ et al (1997) Optic pathway gliomas in children with neurofibromatosis I: consensus statement from the NF-I Optic Nerve Pathway Glioma Task Force. Ann Neural 41:143–149
Listernick R, Charrow J, Guttmann DH (1999) Intracranial gliomas in neurofibromatosis type I. Am J Med Genet 89(1):38–44
Grill J, Lather V, Rodriguez D et al (2000) When do children with optic pathway tumors need treatment? An ontological perspective in 106 patients treated in a single centre. Eur J Pediatr 159:692–696
Deliganis AV, Geyer JR, Berger MS (1996) Prognostic significance of type I neurofibromatosis (von Recklinghausen disease) in childhood optic glioma. Neurosurgery 38:1114–1118
Gottschalk S, Tavakolian R, Buske A et al (1999) Spontaneous remission of chiasmatic/hypothalamic masses in neurofibromatosis type I: a report of two cases. Neuroradiology 41:199–201
Schmandt SM, Packer RJ, Vezina LG (2000) Spontaneous regression of low-grade astrocytomas in childhood. Pediatr Neurosurg 32:132–136
Perilongo G, Moras P, Carollo C et al (1999) Spontaneous partial regression of a low-grade glioma in children with neurofibromatosis-I: a real possibility. J Child Neurol 14:352–356
Blazo MA, Lewis RA, Chintagumpala MM (2004) Outcomes of systematic screening for optic pathway tumors in children with neurofibromatosis type I. Am J Med Genet A 127A(3):224–229
Stern J, DiGiacinto GV, Housepian EM (1979) Neurofibromatosis and optic glioma: clinical and morphological correlations. Neurosurgery 4:524–528
Borit A, Richardson EP Jr (1982) The biological and clinical behavior of pilocytic astrocytomas of the optic pathways. Brain 105:161–187
Balcer LJ, Liu GT, Heller G et al (2001) Visual loss in children with neurofibromatosis type I and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol 131(4):442–445
van Engelen SJPM, Krab LC, Moll HA et al (2008) Quantitative differentiation between healthy and disordered brain matter in patients with neurofibromatosis type I using diffusion tensor imaging. AJNR 29:816–822
Salmela MB, Cauley KA, Nickerson JP et al (2010) Magnetic resonance diffusion tensor imaging (MRDTI) and tractography in children with septo-optic dysplasia. Pediatr Radiol 40(5):708–713
Yamamoto A, Miki Y, Urayama S (2007) Diffusion tensor fiber tractography of the optic radiation: analysis with 6-, 12-, 40-, and 81-directional motion-probing gradients. A preliminary study. AJNR 28:92–96
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design, and data analysis-a technical review. NMR Biomed 15:457–467
Mori H, Abe O, Okubo T et al (2001) Diffusion property in a hamartomatous lesion of neurofibromatosis type I. J Comput Assist Tomogr 25:537–539
Eastwood JD, Fiorella DJ, MacFall JF et al (2001) Increased brain apparent diffusion coefficient in children with neurofibromatosis type I. Radiology 219:354–358
Alkan A, Sigirci A, Kutlu R et al (2005) Neurofibromatosis type I: diffusion weighted imaging findings of brain. Eur J Radiol 56:229–234
Tognini G, Ferrozzi F, Garlaschi G et al (2005) Brain apparent diffusion coefficient evaluation in pediatric patients with neurofibromatosis type I. J Comput Assist Tomogr 29:298–304
Zamboni SL, Loenneker T, Boltshauser E et al (2007) Contribution of diffusion tensor MR imaging in detecting cerebral microstructural changes in adults with neurofibromatosis type I. AJNR 28:773–776
Nickerson JP, Salmela MB, Koski CJ et al (2010) Diffusion tensor imaging of the pediatric optic nerve: intrinsic and extrinsic pathology compared to normal controls. J Magn Reson Imaging 32:76–81
Sener RN (2002) Diffusion MR in neurofibromatosis type I: ADC evaluations of the optic pathways and a comparison with normal individuals. Comput Med Imaging Graph 26(2):59–64
Ono J, Harada K, Mano T et al (1997) Differentiation of dys- and demyelination using diffusional anisotropy. Pediatr Radiol 16:63–66
DiPaolo DP, Zimmerman RA, Rorke LB et al (1995) Neurofibromatosis type I: pathologic substrate of high signal intensity foci in the brain. Radiology 195:721–724
Trip SA, Wheeler-Kingshott C, Jones SJ et al (2006) Optic nerve diffusion tensor imaging in optic neuritis. Neuorimage 30:498–505
Xu J, Sun SW, Naismith RT et al (2008) Assessing optic nerve pathology with diffusion MRI: from mouse to human. NMR Biomed 21:928–940
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filippi, C.G., Bos, A., Nickerson, J.P. et al. Magnetic resonance diffusion tensor imaging (MRDTI) of the optic nerve and optic radiations at 3T in children with neurofibromatosis type I (NF-1). Pediatr Radiol 42, 168–174 (2012). https://doi.org/10.1007/s00247-011-2216-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2216-y