Abstract
Background
Subglottic haemangioma causes progressive and life-threatening stridor, typically manifesting at age 2–3 months. Standard diagnosis is by laryngoscopy. Larynx sonography is rarely used but allows assessment of the presence and extension of a mass that impinges on the subglottic airway. The additional use of colour Doppler enables demonstration of the vascular nature of such masses.
Objective
To compare US and endoscopic findings in infants with subglottic haemangioma and to evaluate accuracy of US and colour Doppler imaging in this diagnosis.
Materials and methods
We report eight infants with subglottic haemangioma seen in our institution over the last decade. They presented with laryngeal stridor and were all investigated with both US and endoscopy. Six infants underwent colour Doppler sonography.
Results
US and endoscopic findings showed excellent anatomical correlation in lateral subglottic haemangioma. Colour Doppler imaging was deemed helpful in four infants.
Conclusion
Larynx sonography with complementary colour Doppler imaging was non-invasive and helpful in the diagnosis of subglottic haemangioma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infantile haemangiomas are benign endothelial cell neoplasms that are not present at birth but generally appear after a few weeks. They grow rapidly throughout the first year of life, then slowly involute. Haemangiomas are easily diagnosed at US as well-defined mostly hypoechoic soft-tissue masses with prominent vessels. Fluctuations of the sonographic pattern are related to phases of evolution.
Ten to twelve percent of children develop haemangiomas and 70–90% of these are evident by 1 month of age [1]. By the age of 7 years, 70% of all haemangiomas have disappeared [2].
Locations may vary, but the head and neck are involved in about 60%. A subglottic location is rather infrequent and was found in only 7.2% of 687 congenital malformations of the larynx [3, 4]. Fifty percent of all infants with subglottic haemangiomas also have cutaneous haemangiomas and associated central nervous system lesions [2, 5]. Associated dysmorphic syndrome-like anomalies have been described [6]. There is a female preponderance. Most subglottic haemangiomas are capillary [7].
Haemangiomas in the subglottic region require urgent diagnosis and intervention as persistent growth can lead to life-threatening airways compression. Thus, any upper airways obstruction in infants under 6 months of age requires instant investigation [2]. Early clinical diagnosis is difficult since respiratory symptoms like stridor may be mistaken for laryngomalacia or protracted spasmodic croup, and improvement with steroid therapy may be misleading. Later in childhood, laryngeal granular cell tumour can mimic the clinical and imaging features of subglottic haemangioma [8].
Laryngoscopy is a proven diagnostic tool for identifying airways narrowing, including at subglottic level [9]. However, laryngoscopy is not universally available, does not provide information about tumour aetiology, requires sedation and does not convey robust information about infiltration. Therefore, an imaging technique able to produce a cross-sectional view of the region of interest is desirable. CT and MRI are possible candidates but have several disadvantages: Small lesions are demanding to image appropriately and sedation or general anaesthesia is often required.
US provides cross-sectional views of the subglottic area and adjacent structures without need for sedation or a costly procedure and may therefore be used preferentially in this group of patients. This paper investigates high-resolution US in infants with inspiratory stridor and upper airway narrowing caused by subglottic haemangioma.
Materials and methods
Patients
Children admitted to our hospital between 1997 and 2009 with signs of subglottic stenosis and clinical suspicion of subglottic haemangioma were included. Subglottic haemangioma was clinically suspected when stridor was apparent some time after birth with increasing severity. Clinical investigation was performed according to good clinical practice. This retrospective study was approved by the local institutional review board.
Laryngeal sonography
US images were obtained, using an ATL Apogee 800 Plus, a Toshiba Xario XG or Aplio XG diagnostic US system. A 5–11 MHz linear transducer provided high-resolution images of the larynx in an axial plane. Thyroid and cricoid cartilages, the thyroid gland and the carotid artery were the anatomical landmarks of the region. The focal zone was placed at ½-¾ of the depth of the field of view. Colour Doppler imaging used a pulse repetition frequency suitable for a velocity scale of ±10–30 cm/s. The examination was carried out in the spontaneous sleeping infant, if possible in the supine position with the neck in mild hyperextension using a cushion. Orientation followed the following signs: the epiglottis, an elastic stratified cartilage, has a hyperechoic appearance, while the hyaline cartilages (thyroid, cricoid and arytenoid) are poorly echogenic with an echogenic rim. Vocal cords are hypoechoic because of their muscular nature. Plicae ventriculares (false vocal cords) contain fat and are therefore hyperechoic. In an axial plane, the arciform subglottic air column between the lobes of the thyroid gland hides all structures behind it because of acoustic shadowing (Fig. 1). Lack of posterior acoustic shadowing behind the subglottic air is a sign of a space-occupying lesion within the larynx. Delineation of the lesion, evaluation of its echogenicity and/or blood flow, visualisation of a possible haemangioma extension outside of the confines of the cricoid and tracheal walls were also evaluated. In addition to intralaryngeal morphology, US provides information about the dynamics of the vocal cord during respiration (Fig. 1).
Transaxial US of the larynx. a Normal findings at subglottic level include the arciform shape of the subglottic air (arrow) with acoustic shadowing between the lobes of thyroid gland. b At glottic level, there is a punctate hyperechoic focus representing glottic air (black arrow). The vocal cords show a triangular hyperechoic configuration. In this neonate, stridor was due to paralysis of the right vocal cord with visible abduction movement of the left vocal cord (white arrow) only during inspiration
Laryngoscopy
Laryngoscopy was performed using an Olympus XT-160 (Olympus Optical, Hamburg, Germany) digital bronchoscope or an Olympus BF-3C20 (Olympus Optical, Hamburg, Germany) fiberoptic bronchoscope. All children were sedated with propofol at a dose of 2–4 mg/kg for the procedure. The endoscope was inserted in the more easily accessible nostril and advanced to the rhinopharynx.
Results
The clinical characteristics are summarised in Table 1. Eight infants (seven females) with a mean age of 2.9 months (range, 8 weeks to 4 months) were included. Two (25%) had skin haemangiomas. Histological proof of capillary haemangioma was obtained in six (patients 1–6).
Laryngeal sonography was performed immediately before laryngoscopy in seven infants. Due to organisational reasons, patient 5 had laryngoscopy before laryngeal US.
Laryngoscopy
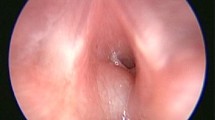
Subglottic haemangiomas in this cohort were regularly associated with airway narrowing of at least 70%. The mucosa covering the subglottic haemangioma appeared pink (normal) in five (63%), a purple or bluish-livedoid colour in two (25%) and superficial angiectasia in one (13%). Five were left-sided only, one right anterolateral only, one posterior and one bilateral (Figs. 2, 3, 4).
Correspondence between endoscopy (left column) and transaxial subglottic US in three infants with left-sided subglottic haemangioma. a Patient 2 in Table 1. Endoscopy shows subglottic stenosis due to a left-sided tumour that is covered with normal-coloured mucosa. On US, there is a left-sided lesion with poorly or mixed echogenicity (arrowheads, 4 mm in diameter) with compression of the subglottic airway, which takes a crescent-shaped configuration (arrow). b Patient 5 in Table 1. Endoscopy shows a left-sided purple subglottic tumour. On US, there is a round, mixed-echogenicity lesion (arrowheads) with narrowing of the subglottic airway from the left to a small orifice (arrows) and rich vascularisation seen with colour Doppler. c Patient 6 in Table 1. Endoscopy shows a left-sided ventral and dorsal bilobed subglottic mass with superficial angiectasis. On US, there is a mass with an anterior and posterior hypoechoic lobe (arrowheads) displacing the subglottic air (arrow) left to right
Comparison of endoscopy (left column) and transaxial US in three infants with subglottic haemangioma. a Patient 1 in Table 1. Endoscopy shows severe subglottic stenosis due to a right-sided mass. The mucosa is normal. On US, there is a hypoechoic round mass (arrowheads) to the right of a markedly reduced subglottic airway. b Patient 3 in Table 1. Endoscopy shows the subglottic lumen narrowed to 10% due to a dorsal tumour covered with bluish mucosa. On US, there is a widened subglottic airway that hides the deeper tumour. c Patient 7 in Table 1. Endoscopy shows symmetrical subglottic narrowing to 10%. On US there, are two hypoechoic round lesions to the right (white arrowheads) and left (black arrowheads) of the narrow punctate and midline subglottic air (arrow)
Endoscopy (left column) and transaxial US in patient 8 (Table 1). a Endoscopy at primary diagnosis shows a left-sided pink subglottic tumour (arrow). On US, there is a round mixed-echogenicity lesion (arrowhead) with narrowing of the subglottic airway to a small orifice. Colour Doppler shows high vascularity. b Following 2 months of propranolol, endoscopy and US are normal. c Relapse 4 months following end of treatment with propranolol. Left-sided haemangioma seen both on endoscopy (arrow) and US (arrowhead)
Sonography
In general, the haemangiomas were hypoechoic and round. The normal arciform subglottic air column changed into a punctate or crescent-shaped configuration due to compression. This was particularly well seen in several left-sided haemangiomas (Figs. 2, 4). Except for the midline haemangioma, which was adherent to the posterior laryngeal wall, the sonographic diagnosis of a subglottic haemangioma was readily suggested in all infants by visualising both the lesion and the modified shape of the subglottic air. The infant with a midline lesion sonographically had an enlarged airway, stretched horizontally, as if it was compressed from behind, but the haemangioma itself was not clearly seen (Fig. 3). One bilobed horseshoe haemangioma (Fig. 2) and one bilateral haemangioma (Fig. 3) were better demonstrated by US than by endoscopy. Colour Doppler imaging used in six infants showed hyper-vascularisation in four, in whom it was contributory in the diagnosis of subglottic haemangioma (Figs. 2, 4). Motion artefacts made Doppler non-diagnostic in two. There was no extra-laryngeal extension seen in any of the lesions.
Therapy and control of treatment efficacy (Table 2)
Five infants initially received steroids, generally before a further procedure like surgery, laser therapy or prolonged intubation. In one child steroids were given after an initial laser therapy. Without any surgical and/or laser therapy, steroids induced remission only in two children: the first one (patient 7) had also been intubated for a prolonged period of time; an additional benefit from compression of the haemangioma by the endotracheal tube may therefore be presumed; in the second one (patient 8) steroids were associated to propranolol and prolonged intubation. Propranolol has to be considered here as the major responsible of the favourable issue. In one child, tapering of the steroid dose was twice followed by relapse, so surgical treatment was initiated. In one child, initial steroid therapy was without any effect (Table 2).
Surgical treatment was required in four. Open surgical techniques were used twice and performed without tracheostomy. They consisted of cricoid ring enlargement with costal cartilage grafting and posterior cricotomy and excision of the haemangioma. The child with a midline haemangioma had unsuccessful laser therapy before open surgery.
A watch-and-wait approach with tracheostomy without surgical excision was performed in two. The subglottic airway narrowing of these infants was endoscopically found to be the most severe of the series with a subtotal stenosis of 95%. Decannulation was possible at the age of 14 and 20 months, respectively.
CO2 laser vaporisation was used in four children. In two of these, laser coagulation had to be completed by a further surgical procedure and it was followed once by steroids and prolonged intubation. In one, several sessions of laser vaporisation were necessary for resolving the respiratory symptoms.
Oral treatment with propranolol was initiated in the last child in the series (Fig. 4). This girl presented at 3 months of age with stridor that had increased in severity over 4 weeks. On admission, the child was severely dyspneic, and could only breathe in an opisthotonoid posture. US revealed a left-sided hypoechoic round lesion in the subglottic region. At laryngoscopy, a smooth, pink submucosal lesion was seen to obstruct more than 80% of the airway cross-section. Endotracheal intubation was performed due to severe respiratory distress. Propranolol was then given at a dose of 1 mg/kg body weight in three daily doses for 3 days, subsequently at a dose of 2 mg/kg. Extubation was possible 12 days after initiation of therapy. Laryngoscopy 2 days after extubation showed reduction in size of the haemangioma and less than 20% airway obstruction. The child was discharged at day 16 without stridor. Propranolol treatment was continued at 2 mg/kg for 5 months with no adverse effects. At the age of 1 year (4 months after the end of therapy), the child re-presented with stridor, and recurrence of the haemangioma was diagnosed by US and by endoscopy. A second course of propranolol was given for 4 months. The symptoms resolved a few days into treatment.
Discussion
We have reported eight children with infantile subglottic haemangioma. The condition was diagnosed by high-resolution US and confirmed endoscopically. The results suggested that US combined with colour Doppler imaging is accurate for diagnosing subglottic haemangioma.
When faced with stridor in neonates and infants, the whole spectrum of laryngotracheal and vascular malformations need to be considered [10].
Laryngomalacia, a functional and self-limiting abnormality, remains to be the most frequent diagnosis in infants with stridor that is evident shortly after birth, increases with crying and agitation and improves in the prone position or with neck extension.
A less frequent but more severe cause of stridor in this age group is subglottic haemangioma, which is characterised by a later onset of stridor. There is a female and left-sided preponderance, as also seen in our small series [7].
Endoscopy remains the diagnostic reference standard for subglottic haemangioma, but it is not completely accurate. The correct diagnosis may be missed if endoscopy is performed while the child is on steroid therapy or when there is an endotracheal tube in situ. In such cases, a ruptured laryngeal cyst may then be suspected, as in one of our patients. The overlying mucosa is not necessarily abnormal (purple discolouration, angiectasia), as shown in a majority in our series. An underlying haemangioma may therefore be missed endoscopically [11]. Inflammatory change of the subglottic mucosa as in spasmodic croup or severe gastro-oesophageal reflux may mimic the endoscopic appearance of haemangioma. It follows that complementary or alternative diagnostic techniques may provide benefits compared with laryngoscopy alone. Although not widely used, US seems a good candidate for imaging the infantile larynx, since the cartilaginous skeleton of the larynx permits good sonographic views [11, 12].
In our series, most subglottic haemangiomas were seen as hypoechogenic soft-tissue masses. They were typically located posteriorly and laterally and compressed the airway lumen, and were therefore seen between the subglottic air column and the posterior cricoid ring [9, 10]. When colour Doppler sonography showed conspicuous blood flow within the lesion, the diagnosis of a subglottic haemangioma was considered very likely. However, in practice, Doppler examination is not always easy in a restless infant with respiratory distress. Typical US findings suggest subglottic haemangioma as the most likely differential diagnosis. In the context of laryngeal stridor in young infants, acquired subglottic ductal retention cyst in ex-premature infants with a history of neonatal intubation may be considered, but a sonographic semiology of this entity has not yet been described [13]. It is nevertheless likely that the cystic nature of these would be obvious sonographically. Unilateral and/or bilateral vocal cord palsy may be considered if there is asymmetrical and/or absent abduction of the vocal cords during inspiration (Fig. 1).
Visualisation of subglottic haemangiomas could also be achieved directly or by virtual laryngotracheal endoscopy using fast high-resolution CT [14–17]. However, deglutition and breathing movements can make interpretation difficult. MRI of the larynx gives good tissue contrast and offers the possibility of multiplanar imaging. The great advantage of MRI is in evaluating large cervicothoracic enhancing haemangiomas with diffuse tracheal lumen compromise. Detection of the full extent of haemangiomas is generally better with MRI than with CT because of the high signal intensity of haemangiomas relative to muscle on T2-weighted images. However, in infancy, the requirement of sedation or anaesthesia may be a drawback for MRI. Furthermore, with standard MRI coils, the spatial resolution may be poor because of the small size of the infantile larynx. There are only a few reports of MRI diagnosis of isolated subglottic haemangiomas in infants [5, 18].
After intubation or tracheostomy, an informative laryngeal US examination is impossible. The lesions we followed with US typically showed a decrease in echogenicity, and not the typical increase seen in spontaneously involuting haemangiomas in other locations (Fig. 4).
Laser vaporisation followed by steroids and proplonged intubation has been the treatment of choice for subglottic haemangioma. The therapeutic strategy changed after the 2008 discovery of the very favourable effects of propranolol on cutaneous haemangiomas [19]. A similar favourable therapeutic response to propranolol in subglottic haemangioma has been described in several case reports [20–24].
In conclusion, US showed an excellent diagnostic and topographical correlation with endoscopic findings in subglottic stenosis in a series of eight infants. The correspondence was particularly good in lateral lesions. High vascularity could be demonstrated in four out of six infants where colour Doppler imaging was performed. The addition of colour Doppler is, therefore, thought to provide complementary information of high diagnostic value. Doppler examination may, however, be difficult in young infants with respiratory distress.
References
Silverman RA (1991) Hemangiomas and vascular malformations. Pediatr Clin North Am 38:811–834
Denoyelle F (1996) Angiome sous-glottique et trachéal du nourrisson. In: Garabédian EN, Bobin S, Monteil JP, Triglia JM (eds) ORL de l’enfant: pathologie de l’oreille. Flammarion, Paris, pp 205–208
Altman KW, Wetmore RF, Marsh RR (1999) Congenital airway abnormalities in patients requiring hospitalization. Arch Otolaryngol Head Neck Surg 125:525–528
Narcy P, Bobin S, Contencin P et al (1984) Laryngeal anomalies in newborn infants. Apropos of 687 cases. Ann Otolaryngol Chir Cervicofac 101:363–373
Fan HC, Hung CH, Juan CJ et al (2000) Subglottic hemangioma associated with cutaneous and cerebellar hemangiomas detected by MRI: report of one case. Acta Paediatr Taiwan 41:214–217
Buzenet C, Hamel-Teillac D, Acar P et al (2000) Facial hemangioma associated with arterial anomalies, coarctation of the aorta, and eye abnormalities: PHACES syndrome. Ann Dermatol Venereol 127:292–295
Choa DI, Smith MC, Evans JN et al (1986) Subglottic haemangioma in children. J Laryngol Otol 100:447–454
Royal SA (2000) Pediatric laryngeal granular cell tumor. Pediatr Radiol 30:869–870
Riding K (1992) Subglottic hemangioma: a practical approach. J Otolaryngol 21:419–421
Mancuso RF (1996) Stridor in neonates. Pediatr Clin North Am 43:1339–1356
Garel C (2002) Ultrasonography of the larynx. In: King SJ, Boothroyd AE (eds) Pediatric ENT radiology. Springer, Berlin, pp 345–350
Garel C, Hassan M, Legrand I et al (1991) Laryngeal ultrasonography in infants and children: pathological findings. Pediatr Radiol 21:164–167
Agada FO, Bell J, Knight L (2006) Subglottic cysts in children: a 10-year review. Int J Pediatr Otorhinolaryngol 70:1485–1488
Chetty A, Mischler E, Gregg D (1997) Diagnosis of subglottic hemangioma by chest CT. Pediatr Pulmonol 23:464–467
Liu P, Daneman A (1984) Computed tomography of intrinsic laryngeal and tracheal abnormalities in children. J Comput Assist Tomogr 8:662–669
Triglia JM, Nazarian B, Sudre-Levillain I et al (2002) Virtual laryngotracheal endoscopy based on geometric surface modeling using spiral computed tomography data. Ann Otol Rhinol Laryngol 111:36–43
Frey EE, Smith WL, Grandgeorge S et al (1987) Chronic airway obstruction in children: evaluation with cine-CT. AJR 148:347–352
Nozawa K, Aihara T, Takano H (1995) MR imaging of a subglottic hemangioma. Pediatr Radiol 25:235–236
Leaute-Labreze C, Dumas de la Roque E, Hubiche T et al (2008) Propranolol for severe hemangiomas of infancy. N Engl J Med 358:2649–2651
Jephson CG, Manunza F, Syed S et al (2009) Successful treatment of isolated subglottic haemangioma with propranolol alone. Int J Pediatr Otorhinolaryngol 73:1821–1823
Denoyelle F, Leboulanger N, Enjolras O et al (2009) Role of Propranolol in the therapeutic strategy of infantile laryngotracheal hemangioma. Int J Pediatr Otorhinolaryngol 73:1168–1172
Buckmiller L, Dyamenahalli U, Richter GT (2009) Propranolol for airway hemangiomas: case report of novel treatment. Laryngoscope 119:2051–2054
Leboulanger N, Fayoux P, Teissier N et al (2010) Propranolol in the therapeutic strategy of infantile laryngotracheal hemangioma: a preliminary retrospective study of French experience. Int J Pediatr Otorhinolaryngol 74:1254–1257
Peridis S, Pilgrim G, Athanasopoulos I et al (2011) A meta-analysis on the effectiveness of propranolol for the treatment of infantile airway hemangiomas. Int J Pediatr Otorhinolaryngol 75:455–460
Acknowledgements
The authors would like to thank Mrs. Greifenberg and Mr. Müller (photo laboratory, St. Josef-Hospital, Bochum) for their great help in processing of the images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossler, L., Rothoeft, T., Teig, N. et al. Ultrasound and colour Doppler in infantile subglottic haemangioma. Pediatr Radiol 41, 1421–1428 (2011). https://doi.org/10.1007/s00247-011-2213-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2213-1