Abstract
Congenital skeletal abnormalities compose a heterogeneous and complex group of conditions that affect bone growth and development and result in various anomalies in shape and size of the skeleton. Prenatal sonographic diagnosis of these anomalies is challenging because of the relative rarity of each skeletal dysplasia, the multitude of differential diagnoses encountered when the bony abnormalities are identified, lack of precise molecular diagnosis and the fact that many of these disorders have overlapping features and marked phenotypic variability. The following review is a preliminary summary of our experience at the Children’s Hospital of Philadelphia (CHOP) using low-dose fetal CT in the evaluation of severe fetal osseous abnormalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital skeletal abnormalities compose a heterogeneous and complex group of conditions that affect bone growth and development and result in various anomalies in shape and size of the skeleton. They comprise the osteochondrodysplasias or skeletal dysplasias (SD), the dysostoses and the reduction deformities. Skeletal dysplasias refer to a generalized developmental disorder of the chondro-osseous tissue. The dysostoses refer to a single or localized group of abnormal bones [1]. Reductions are secondary malformations of bones [2] caused by a disruption sequence, that is exposure to a toxic substance or infectious agent resulting in transient disruption of normal skeletal development and secondary malformations. The distinction between the first two groups has become blurred, as mutations in individual genes can produce diffuse abnormalities in the bone and cartilage or preferentially affect certain skeletal segments [3].
As reviewed by Krakow et al. [1], the fetal skeleton follows a programmed pattern of osseous development: the clavicles and mandibles ossify at about 8 weeks; appendicular skeleton, ileum and scapula, 12 weeks; metacarpals and metatarsals, 12–16 weeks; epiphyseal centers, about 20 weeks. The end point of many genetic mutations responsible for osseous congenital anomalies is an abnormal patterning of the skeleton, joint morphogenesis, abnormal linear growth and compromised integrity of the articular surface [4] so that the normal pattern of skeletal growth is altered.
To date, approximately 350 distinct disorders have been described. These may be inherited as autosomal-dominant, autosomal-recessive and X-linked disorders, or secondary to teratogen exposure or spontaneous mutations. Approximately 160 of these disorders have a causative genetic mutation that makes them amenable to prenatal genetic testing.
The prenatal evaluation of skeletal dysplasias includes a detailed second-trimester US exam and an extensive genetic family work-up. Sonographic evaluation requires a meticulous evaluation of the fetal skeleton. Extensive guidelines have been published for the sonographic evaluation of the fetal skeleton elsewhere [1, 5]. Briefly, shortening of the extremity long bones, bowed or fractured bones, demineralization and a small thorax are indications of an SD. Fetuses with long-bone measurements of more than 3 standard deviations below the mean are strongly suspected of having a skeletal dysplasia. The main goal of prenatal US is the identification of potential lethality, as manifested by death in utero, at or shortly after birth. Sonographic indications of lethality are a chest to abdominal circumference ratio of < 0.6 or femur length to abdominal circumference ratio of 0.16.
Yet prenatal sonographic diagnosis is challenging because of the relative rarity of each skeletal dysplasia, the multitude of differential diagnoses encountered when the bony abnormalities are identified, lack of precise molecular diagnosis and the fact that many of these disorders have overlapping features and marked phenotypic variability. In addition, some of these disorders are not apparent during the second trimester US and only become evident in the late prenatal period or early postnatal time, further complicating timely diagnosis. Last, it should be mentioned that although US constitutes the main imaging modality in the diagnostic armamentarium of skeletal dysplasias, its use is somewhat limited, even in the best of hands. Numerous studies indicate that the sensitivity of US in diagnosing skeletal dysplasias is anywhere from 40% to 60% [6, 7], thus the need to investigate new imaging tools to better evaluate these complex entities.
Case reports have been published about the utility of fetal MR in evaluating these osseous abnormalities [8–10]. MR affords a large field of view and accentuated soft-tissue contrast and, unlike US, can delineate fetal cartilage abnormalities in detail [9]. Yet in our hands, the use of fetal MR has not proved fruitful when evaluating these fetal skeletal abnormalities.
Recently, certain groups in Europe have started to use fetal CT as means of further evaluation of the fetal skeleton [11–13]. The use of this modality, however, has not propagated to the United States, largely because of an understandable fear of irradiating the developing fetus.
The following review is a summary of our experience at the Children’s Hospital of Philadelphia (CHOP) with low-dose fetal CT in the evaluation of severe fetal osseous abnormalities. We would like to stress that this imaging modality, as detailed below, is only advised for a small subset of fetuses with severe skeletal abnormalities, and that the decision to image should be carefully taken by a multidisciplinary team of radiologists, obstetricians and geneticists.
Low-dose fetal CT: who to image
The fetal CT program at CHOP was initiated because of the growing frustration in diagnosing skeletal dysplasias and the limited capabilities in counseling patients. At CHOP, the decision to image the fetus by CT is reached by a multidisciplinary team involving sonographers, radiologists, geneticists and obstetricians, when the US exam demonstrates severe osseous abnormalities and the diagnosis is indeterminate. It is important to emphasize this point, fetal CTs at CHOP are only done to evaluate the fetus with severe bony abnormalities, most of which are lethal. These CTs are only done in the second and third trimesters of gestation, when organogenesis has already taken place. In our relatively short experience, 18 weeks is the earliest gestational age at which we have done a fetal CT. Earlier scanning is not advised for a number of reasons, the strongest of which is to delay the radiation dose as much as possible from organogenesis. Although we have not scanned fetuses earlier than this, we can extrapolate that the osseous findings would be more difficult and subtle to observe in the younger fetus.
We have recently limited the use of this technology to mothers with large body habitus; in the larger patient, the radiation dose has to be increased in order to traverse the maternal abdomen, thus raising the radiation dose to the fetus.
Consent and radiation dose
The consent form that the mother signs prior to the examination discusses the benefits and risks of low-dose fetal CT imaging. The main benefit is the excellent visualization of the fetal bones, far superior to US, and the main risk is the radiation dose to the mother, and more important, the fetus.
As pediatric radiologists, we abide by and conform to the ALARA principle, which mandates keeping radiation dose low, or as low as reasonably achievable, a concept strongly endorsed by the Society for Pediatric Radiology. This is a particularly pressing matter in the pediatric population because fetuses and children are particularly sensitive to radiation and their cumulative radiation exposure risk. Yet, when performed for appropriate indications, when utilizing proper technical parameters, and when realizing that fetuses and children are far more sensitive to radiation than adults [14], the benefits of low-dose fetal CT might far exceed the small individual risk.
Regarding the radiation dose effect to the conceptus, there is a robust body of knowledge on the effects of radiation derived mostly from the sequelae of the atomic bombs at the end of World War II, but also derived from children whose mothers received medical irradiation (diagnostic and/or therapeutic) during pregnancy. The American College of Radiologists, in its guidelines for imaging of the pregnant patient, summarizes the suspected in utero deterministic radiation effects of radiation a dose of < 50 mSv as “none.” McCollough et al. [15] postulate that the absolute risk of fetal effects, including fetal cancer induction, is small at a conceptus dose of 100 mSv and negligible at doses of less than 50 mSv. Assuming a normal incidence of 0.07% incidence of childhood cancer and a natural incidence of malformation risk in the order of 4% in the normal population [16], the risk to the conceptus given a fetal dose of about 50 mSv results in a 96% probability of birthing a child with no malformation, or a 99.9% chance that the child will not develop cancer, or a 95.9% chance that the child will have no malformation and no cancer.
To date we have done approximately 22 low-dose fetal CTs. Calculation of the effective radiation dose for each fetus is estimated using ImPACT CT Patient Dosimetry Calculator (CT Scanner Evaluation Center, London, UK). The estimation is based on the scan techniques recorded by the CT scanner, including kVp, average mAs per rotation, collimation, scan pitch, and anatomical region covered by the fetal CT scan.
Of the 22 studies done to date, the mean radiation dose is 4.8 mSv; the median is 4.5 mSv. To put these numbers into context when obtaining consent from the patient, she is counseled that 5 mSv is the overall radiation dose that a gravid radiation worker is allowed to receive during the entire course of the pregnancy, and that 3.6 mSv is the radiation dose per year one may receive from background [17].
Fetal CT: how to
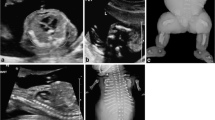
Once the consent is obtained, the patient is conducted to the CT suite, where she is placed in a supine position on the CT table. Propping up the legs with pillows under the knees and using head rests may help make the patient more comfortable during the examination. The top and bottom of the uterus are sonographically marked with radiopaque markers. A topogram of the uterus is then obtained imaging exclusively the abdominal contents included between the two markers. The topogram confirms fetal position if the bones are not demineralized. The unenhanced CT study is then done imaging exclusively the fetus in a 64-detector Siemens SOMATOM Sensation CT scanner (Siemens Healthcare, Erlangen, Germany). Our protocol utilizes 100 kVp, modulated mAs, pitch 1.2 mm, slice thickness 1.5 mm, on a 1.2-mm detector and rotation time 0.5 s. The actual image acquisition takes a few seconds. Three-dimensional volume-rendered images of the fetus are generated from the original 2D data in a TeraRecon Aquarius workstation (TeraRecon, Foster City, CA, USA) following digital segmentation and removal of the maternal pelvic bones, allowing complete visualization of the fetal skeleton, which can then be analyzed for overall proportions and morphological deformities (Fig. 1). Note that the study is done at very low radiation doses such that the fetal bones are seen with exquisite detail while the fetal organs are not well depicted.
Two-dimensional and 3D processing of CT images. a Axial image through the maternal pelvis in bone windows with partial visualization of the fetal skeleton. It is difficult to visually reconstruct the fetus on 2D axial images alone. b Three-dimensional reconstructions of the maternal abdomen show the fetus located within the lower maternal abdomen. Selective sectioning of the maternal organs allows exclusive visualization of the fetal skeleton in the 3D console, which can then be rotated in space for better evaluation of the fetus. Radiation dose in this case: 4.4 mSv. c, d A different case demonstrates alternative manners of rendering 3D visualization of the fetus. Radiation dose: 4.9 mSv
Fetal CT: image analysis
With postprocessing, 3D images of the fetal skeleton can be rotated in space to optimize visualization of the fetal bones. With these low radiation doses, the fetal hands and feet might not be well visualized in the early second trimester, particularly if bone mineralization is decreased. Yet the fetal hands and feet are well seen by US, obviating the need to increase the radiation dose by CT. In the late second trimester and third trimester, the fetal hands and feet are well seen with CT. Epiphysis can be seen at about 30 weeks’ gestation. Skull, clavicles, scapula, ribs, vertebral bodies and long bones should be visible in a normal fetus in the second and third trimesters, and their absence implies pathology.
Once the CT images are available in the 2D and 3D console, a systematic evaluation of the fetal bones is undertaken, as discussed in myriad prior articles [1, 2, 4, 18]. Briefly, the main features to evaluate by CT include bone mineralization, presence of fractures or bowing, relative length of long bones, shape of skull, shape of ribs and vertebral bodies, presence of segmentation anomalies or abnormal curvature of the spine and morphology of the pelvis.
There is a learning curve for appreciating bone mineralization by CT at the different gestational ages, as there are no standards published for fetal CT. For reference in the normal fetus, we found helpful the fetal radiology atlas of Schumacher et al. [19], which shows postmortem radiographs of normal fetus up to a gestational age (GA) of 23 weeks. For relative length of the long bones, we defer to US measurements, since they have been well established in the literature.
It is essential to emphasize that the imaging interpretation and overall prenatal diagnosis of skeletal dysplasia requires a multidisciplinary approach that includes geneticists, molecular biologists, obstetricians, radiologists and a host of surgical specialists for management of the possible postnatal complications. In our imaging practice, every study is reviewed by a team of geneticists, obstetricians and radiologists, who then formulate a team-approach diagnosis.
Examples
We include several cases seen in our practice.
-
Case 1
Figure 2 is a 21-week fetus with a preliminary sonographic diagnosis of campomelic dysplasia. US described normal mineralization, no fractures, bowed long bones and 11 bilateral ribs. CT images showed bone demineralization, particularly of the skull. There was diffuse long-bone shortening. A coarse, crumpled appearance of the bones and an asymmetrical bowed appearance of the femora were findings most consistent with healing fractures. A diagnosis of osteogenesis imperfecta was provided. Molecular diagnosis confirmed a diagnosis of osteogenesis imperfecta type III caused by a mutation GGA > GAA base pair substitution in exon 21 of the collagen gene COL 1A2. The radiation dose in this case was 4.4 mSv. Postnatal images confirmed the presence of multiple fractures and diffuse mineralization.
Fig. 2 Case 1. A 21-week fetus with sonographic diagnosis of campomelic dysplasia. a, b Low-dose CT of the fetal body (a) with detailed sectioning of the pelvis and bilateral lower extremities (b) shows diffusely decreased bone demineralization. There is marked asymmetrical bowing of the long bones, particularly the bilateral femora, with a crumpled, coarse appearance of the bones, more so the metaphysis, an appearance attributed to fractures. c, d Immediately postnatal images demonstrate multiple healing fractures of the ribs, left clavicle, right humerus and bilateral femora. A diagnosis of osteogenesis imperfecta type III was confirmed by molecular analysis. The fetal radiation dose in this case was 4.4 mSv (note that the radiation dose of the postnatal skeletal survey, as performed at our institution, is about 1 mSv)
-
Case 2
Figure 3 is a 21-week fetus with an abnormal US suggestive of thanatophoric dysplasia. Fetal CT showed mild bone demineralization, platyspondyly, marked foreshortening of the fetal ribs and long bones, characteristic bowing of the femora, and somewhat flattened acetabuli. A diagnosis of thanatophoric dysplasia was molecularly confirmed (FGFR 3 mutation in nucleotide 742 [C > T mutation resulting in R248C change]).
Fig. 3 Case 2. A 21-week fetus with sonographic diagnosis of skeletal dysplasia. Fetal CT shows diffuse foreshortening of the ribs, platyspondyly and a characteristic symmetrical bowing of the bilateral femora resembling a classic telephone receiver. A diagnosis of thanatophoric dysplasia was confirmed by molecular testing. The radiation dose to the fetus in this case was 3.9 mSv
-
Case 3
Of course, as with US, many fetal cases of skeletal dysplasia remain difficult to diagnose, as demonstrated by this recent case, which is pending molecular diagnosis (Fig. 4). This 34-week fetus was a product of consanguineous parents. The prior four pregnancies were unremarkable; the mother was 42 years of age. By US the long bones were determined to be approximately 16 weeks behind. CT showed overall normal mineralization. The rib cage was markedly small with anterior splaying of the foreshortened ribs. There was platyspondyly, not seen by US. Marked foreshortening of the long bones with bowing was seen, without discrete fractures. A trident pelvis was evident. The metaphysis of the long bones appeared bulbous. Hands and feet were normal in size and morphology without polydactyly identified. A differential diagnosis of thanatophoric dysplasia, asphyxiating thoracic dystrophy, and short rib polydactyly syndrome (even in the absence of polydactyly) was reached, in consultation with the International Registry of Skeletal Dysplasias, based at Cedars-Sinai Medical Center (Los Angeles, CA), and the International Skeletal Dysplasia Society, based in Switzerland. Molecular results are pending.
Fig. 4 Case 3. A 34-week fetus of consanguineous parents. Fetal CT demonstrates normal bone mineralization, markedly short ribs and long bones, and symmetrical bowing of the bilateral femora. There were no fractures. The vertebral bodies are remarkable for demonstrating platyspondyly. There is a characteristic trident appearance of the fetal pelvis. Hands and feet are normal in size and morphology, without evidence of platyspondyly. The final diagnosis in this case is pending. The radiation dose to the fetus was 4.9 mSv
-
Case 4
Finally, radiologists should be aware of a great mimicker of skeletal dysplasia, the fetus with intrauterine growth retardation or IUGR [20]. Figure 5 shows the 21-week fetus of a 37-year-old woman. By US the fetus had asymmetrical, short long bones, being 3–4 weeks behind on the left and 2–3 weeks behind on the right. The bones demonstrated normal mineralization with no evidence of bowing or fractures. A small thorax with short ribs was also described. By US the findings were suggestive of an SD, and a CT was obtained for further characterization. The CT examination showed no indication of skeletal dysplasia. There was asymmetrical prominence of the fetal head with respect to the overall small body, but an otherwise normal appearance of the fetal bones including bone mineralization, vertebral bodies, pelvis and long bones. A diagnosis of intrauterine growth retardation was provided, with sparing of the head. The case was sent in consultation to the International Skeletal Dysplasia Registry at Cedars-Sinai Medical Center, which agreed with this preliminary diagnosis. The patient delivered a stillborn child at 35 weeks. Autopsy confirmed a diagnosis of IUGR. Please, note that a fetal CT should NOT be done if IUGR is suspected. Fetal CT is only to be done in a small subset of fetuses. The goal of fetal CT is to help diagnose severe, life-threatening abnormalities of the fetal bone. If the diagnosis of IUGR is suspected, evaluation with US and MRI should be performed, never fetal CT.
Fig. 5 Case 4. A 21-week fetus who by US showed shortening of the long fetal bones and was suspected to have a skeletal dysplasia. By CT there was normal bone mineralization. An incongruent normal head size and overall small body, with otherwise normal appearance of the bones led to a CT diagnosis of IUGR, confirmed on autopsy. The radiation dose to this fetus was 4.5 mSv
Conclusion
Skeletal dysplasias are difficult entities to diagnose prenatally. Low-dose fetal CT imaging has emerged from the frustration and limited resources available to diagnose such conditions. In this review we report our preliminary experience with low-dose fetal CT in examining severe osseous abnormalities. Low-dose fetal CT affords exquisite imaging and detail of the fetal skeleton. Because of the obvious risk of radiation to the growing fetus, this type of study is only recommended in cases of severe, likely lethal abnormalities, and much care and thought is required before deciding who is a candidate for this examination. As always in medicine, the diagnosis of these difficult entities requires a multidisciplinary approach including radiologists, obstetricians and geneticists.
By providing exquisite visualization of the fetal bones, low-dose fetal CT might afford improved parental counseling, predelivery planning and postnatal management in fetuses carrying a preemptive diagnosis of skeletal dysplasia.
References
Krakow D, Lachman RS, Rimoin DL (2009) Guidelines for the prenatal diagnosis of fetal skeletal dysplasias. Genet Med 11:127–133
Schramm T, Gloning KP, Minderer S et al (2009) Prenatal sonographic diagnosis of skeletal dysplasias. Ultrasound Obstet Gynecol 34:160–170
Rimoin DL, Cohn D, Krakow D et al (2007) The skeletal dysplasias: clinical-molecular correlations. Ann N Y Acad Sci 1117:302–309
Krakow D, Alanay Y, Rimoin LP et al (2008) Evaluation of prenatal-onset osteochondrodysplasias by ultrasonography: a retrospective and prospective analysis. Am J Med Genet A 146A:1917–1924
Bowerman RA (1995) Anomalies of the fetal skeleton: sonographic findings. AJR 164:973–979
Doray B, Favre R, Viville B et al (2000) Prenatal sonographic diagnosis of skeletal dysplasias. A report of 47 cases. Ann Genet 43:163–169
Parilla BV, Leeth EA, Kambich MP et al (2003) Antenatal detection of skeletal dysplasias. J Ultrasound Med 22:255–258, quiz 259–261
Suzumura H, Kohno T, Nishimura G et al (2002) Prenatal diagnosis of hypochondrogenesis using fetal MRI: a case report. Pediatr Radiol 32:373–375
Yazici Z, Kline-Fath BM, Laor T et al (2010) Fetal MR imaging of Kniest dysplasia. Pediatr Radiol 40:348–352
Miller E, Blaser S, Miller S et al (2008) Fetal MR imaging of atelosteogenesis type II (AO-II). Pediatr Radiol 38:1345–1349
Brunelle F, Sonigo P, Simon I (2003) Fetal CT. Childs Nerv Syst 19:415–417
Ruano R, Molho M, Roume J et al (2004) Prenatal diagnosis of fetal skeletal dysplasias by combining two-dimensional and three-dimensional ultrasound and intrauterine three-dimensional helical computer tomography. Ultrasound Obstet Gynecol 24:134–140
Cassart M, Massez A, Cos T et al (2007) Contribution of three-dimensional computed tomography in the assessment of fetal skeletal dysplasia. Ultrasound Obstet Gynecol 29:537–543
Slovis TL (2002) The ALARA concept in pediatric CT: myth or reality? Radiology 223:5–6
McCollough CH, Schueler BA, Atwell TD et al (2007) Radiation exposure and pregnancy: when should we be concerned? Radiographics 27:909–917, discussion 917–918
Wagner LK, Hayman LA (1982) Pregnancy and women radiologists. Radiology 145:559–562
Huda W, Ovid Technologies Inc. Review of radiologic physics. Lippincott Williams & Wilkins, Baltimore, MD, pp xv, 255
Goncalves LF, Espinoza J, Mazor M et al (2004) Newer imaging modalities in the prenatal diagnosis of skeletal dysplasias. Ultrasound Obstet Gynecol 24:115–120
Schumacher R, Spranger JW, Seaver LH (2004) Fetal radiology: a diagnostic atlas. Springer, Berlin, New York
Partrick ME, Baker ER, Crow HC (1999) Abnormal skull shape in intrauterine growth retardation: report of two cases. J Ultrasound Med 18:161–163
Disclaimer
The supplement this article is part of is not sponsored by the industry. Dr. Victoria, Dr. Epelman, Dr. Bebbington, Dr. Johnson, Dr. Kramer, Dr. Wilson, and Dr. Jaramillo have no financial interest, investigational or off-label uses to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Victoria, T., Epelman, M., Bebbington, M. et al. Low-dose fetal CT for evaluation of severe congenital skeletal anomalies: preliminary experience. Pediatr Radiol 42 (Suppl 1), 142–149 (2012). https://doi.org/10.1007/s00247-011-2175-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2175-3