Abstract
Background
Congenital cytomegalovirus (CMV) infection can lead to severe neurological sequelae, but a defined brain magnetic resonance (MR) pattern and MR predictors of clinical outcome are still lacking.
Materials and methods
Clinical and MR findings of 14 children with symptomatic congenital CMV infection were retrospectively reviewed.
Results
Microcephaly, cerebral palsy and epilepsy were found in eight, six and seven patients, respectively (all concomitant in 6); 12 children developed sensory-neural hearing loss (SNHL). At first MRI (mean age 21 months, range 5–54 months), white matter (WM) involvement was not assessable in two children due to incomplete myelination. WM abnormalities were common (11/12 patients); deep WM was predominantly involved in 5/11; the largest WM lesion was in the parietal lobe in 6/11. Anterior temporal lobe abnormalities were found in 13/14. Six children underwent MRI examination after 2 years of life; in this subgroup, WM abnormalities were extensive and confluent (4/6), bilateral and multifocal (1/6) or absent (1/6). Four children showed a progression of myelination. Ventriculomegaly (9/14), migration disorders (6/14 polymicrogyria and 1/14 pachygyria-lissencephaly) and hippocampal dysplasia (6/14) correlated with severe neurological sequelae (p < 0.05, Fisher exact test), while the presence of WM abnormalities (11/12), periventricular cysts (6/14) and cerebellar hypoplasia (4/14) did not predict the outcome.
Conclusions
The spectrum of brain MR abnormalities in symptomatic congenital CMV infection is extremely wide. WM involvement is variable, difficult to evaluate at a very young age and unrelated to clinical outcome, while cortical malformations, ventriculomegaly and hippocampal dysplasia seem to be strong predictors of poor outcome except for SNHL.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cytomegalovirus (CMV) is the most common cause of congenital viral infection in the Western world affecting 0.5–2.4% of newborns [1–3]; however, only 10–15% have signs of congenital infection. Neurological signs include cognitive and motor impairments, sensorineural hearing loss (SNHL) and chorioretinitis [1, 4–9]. In more than two-thirds of these children, neuroimaging abnormalities are present and comprise cerebral (especially periventricular) calcifications, ventriculomegaly, periventricular white matter lesions, migration abnormalities, cortical atrophy, periventricular cysts or cerebellar hypoplasia [10]. A normal brain CT is predictive of good clinical outcome, while microcephaly is the most specific predictor for severe neurological sequelae [11]. Brain MRI is more sensitive than brain CT in detecting white matter (WM) abnormalities, cortical malformations (agyria, lissencephaly, pachygyria, polymicrogyria), periventricular cysts and hippocampal dysplasia [12, 13]; however, the prognostic role of these findings is not yet defined.
Specific patterns of WM involvement have been previously identified on a small cohort of congenital CMV patients [14] and used as MR criteria for the identification of this infection among patients affected by leukoencephalopathy of unknown origin. In particular, in this study all patients with proven congenital CMV infection presented multifocal lesions predominantly involving deep parietal white matter, with or without gyral abnormalities; when gyral abnormalities were present, leukoencephalopathy could also be diffuse.
The aim of our study was to analyze brain MR abnormalities in congenital CMV infection taking into account the proposed MR criteria.
Materials and methods
Medical records of all newborns admitted to our Department of Pediatrics from January 1994 to December 2007 with symptomatic congenital CMV infection were considered after the approval by our University Department review board. The diagnosis of congenital infection was based on CMV isolation from urine during the first 3 weeks of life. A patient was defined as symptomatic if at least one of the following abnormalities was present at birth: intrauterine growth retardation, microcephaly (head circumference more than 2 standard deviation below the age-norm), hepatomegaly, splenomegaly, petechiae, jaundice, thrombocytopenia (platelet count < 75 × 103/uL), laboratory evidence of hepatitis (alanine aminotransferase level > 100U/L), chorioretinitis or neonatal deafness [1, 15]. MRI examinations were performed using 1.0 T and 1.5 T scanners and were reviewed by a single neuroradiologist with 10 years’ experience who was blind to clinical and other brain imaging findings (CT and ultrasound). Sagittal and axial spin-echo (SE) T1-weighted and axial and/or coronal Fast SE T2-weighted images were available in all patients; in some cases, T2* or inversion recovery T1-weighted sequences were performed for a more precise evaluation of parenchymal calcification or myelin abnormalities. FLAIR was routinely performed after the age of 2, when the myelination process is thought to be almost complete. White matter abnormalities (WMAs) were assessed on T2-weighted images. In order to graduate WMAs, we used the parameters proposed by van der Knaap for children with WM disorders [14], since most of the WM scores present in the literature are based on adults. Ventriculomegaly (frontal and occipital horn ratio greater than 0.4), periventricular cysts (parenchymal round lesions close to ventricle lining with cerebrospinal fluid-like signal), hippocampal dysplasia (incomplete rotation of the hippocampal formation), cortical malformations, cerebellar hypoplasia (marked reduction in size with reduced number of folia) and brain malformations were evaluated in all the available sequences. Cerebellar hypoplasia and cortical malformations were considered signs of an early vertical infection [12].
For the evaluation of the outcome, every child was examined by a pediatric neurologist who performed a clinical evaluation, verifying also the acquisition of neurodevelopmental milestones. In accordance with common clinical practice, the presence of cerebral palsy, epilepsy or mental retardation was considered a marker of poor outcome. Correlation between MR findings and clinical outcome was assessed by means of the Fisher exact test.
Results
In the study period, there were clinical files on 17 children with congenital CMV infection; we considered 14 of them since the remaining three did not undergo brain MRI assessment because their parents refused consent. Microcephaly, cerebral palsy and epilepsy were found in eight, six and seven patients respectively; in six children all these findings were concomitant. Twelve children developed SNHL, four in the absence of other neurologic sequelae. The mean follow-up was 59 months (range 10–168).
At the time of the first MRI, the patient age ranged between 5 and 54 months. MRI findings and outcome of patients are illustrated in Table 1. We could not evaluate WM abnormalities in two children because they were too young at the time of the MR examination (5 months). In these children, T1 sequences used for evaluating myelination at this age did not show any signal abnormality; noteworthy, spin-echo T2 sequences were not available, while fast spin-echo and FLAIR images are not considered reliable for this purpose [16]. WM abnormalities were observed in 11 of 12 children (Table 2, Fig. 1). According to van der Knaap’s WM abnormalities criteria, the distribution of lesions was bilateral and multifocal in 2/11 children; among seven children with concomitant cortical dysplasia, WM involvement was diffuse in three patients, extensive and confluent in two while in the last two patients it was undetermined because of age. The zone predominantly involved was the deep white matter in 5/11 while the location of the largest lesion was parietal in 6/11. Six children underwent MRI examination after 2 years of age; in this subgroup, WM abnormalities were extensive and confluent (4/6), bilateral and multifocal (1/6) or absent (1/6). Four children had more than one MRI examination and all showed a progression of the myelinating process (Fig. 2). Periventricular cysts were detected in 6/14 patients and were generally multiple; two children (patients 8 and 10) presented a single isolated temporal periventricular cyst.
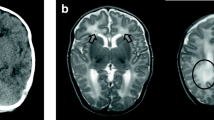
Brain MRI scans in patient 7 at 14 (a) and 24 (b) months of age. Axial T2-weighted images at the same level through the lateral ventricles show the progression of myelination (white arrows). White matter abnormalities are difficult to evaluate at a very young age due to incomplete or delayed myelination. This child, except for sensorineural hearing loss (SNHL), had a normal neurological examination
Besides WM abnormalities, frequent MR findings were ventriculomegaly (9/14), migration disorders (6/14 polymicrogyria and 1/14 pachygyria-lissencephaly), hippocampal dysplasia (6/14) and cerebellar hypoplasia (4/14) (Figs. 3, 4 and 5). In six children, the hippocampus was thinner and vertically oriented as if its development and rotation had been arrested in the early phases. White matter and cortical abnormalities of the anterior part of the temporal lobe were present in 13/14 children (Figs. 1 and 3). Among children with migration disorders, WMAs were diffuse in three while two presented extensive and confluent WMAs.
Brain MRI in a 4-year-old male with cerebral palsy, epilepsy and sensorineural hearing loss (SNHL) (patient 1). a Axial T2-weighted images show diffuse patchy deep and juxtacortical white matter abnormalities. b Coronal FLAIR images reveal bilateral temporal and left frontal cystic lesions (white arrows) and slight hippocampal dysplasia more evident on the right side (white arrowhead)
Brain MRI. a Axial T2-weighted image of a 2-year-old male (patient 9) discloses severe ventriculomegaly with poor operculisation (black arrows) and a pachigyric-lissencephalic appearance of the cortex; the residual juxtacortical frontal white matter is abnormal (white arrowheads). b Axial T1-weighted image of a 5-year-old male (patient 3). Near the large dysmorphic lateral ventricles, small parenchymal calcifications (white arrows) are recognizable. c Coronal T2-weighted image of a 15-month-old male (patient 2) with hippocampal dysplasia more evident on the right side (black arrows). Severe, deep and juxtacortical white matter signal abnormalities are also evident. d Sagittal T1-weighted image of patient 9 reveals microcephaly and severe cerebellar/brainstem hypoplasia (white arrowheads). All these children were clinically severely affected and presented cerebral palsy, sensorineural hearing loss (SNHL) and epilepsy
Brain MRI findings in axial T2-weighted images. a Four-month-old male affected by epilepsy (patient 14). The white matter burden is difficult to evaluate due to incomplete myelination while the concomitant perisilvian polymicrogyria is easily recognizable due to the apparent focal thickening of the cortex (white arrowheads). b Five-month-old female (patient 4) with severe cognitive impairment: diffuse cortical dysplasia and severe ventricular enlargement. c Four-month-old male (patient 13) presenting with faciopharyngoglossomasticatory diplegia (Foix-Chavany-Marie syndrome) due to diffuse bilateral polymicrogyria (white arrowheads) with sparing of the posterior regions
We found a significant correlation between the detection of migration disorders (p=0.02), ventriculomegaly (p=0.02) or hippocampal dysplasia (p=0.03) and the presence of cerebral palsy.
We found no correlation between migration disorders or hippocampal dysplasia and epilepsy. No correlation was found between the presence of WM abnormalities and poor clinical outcome or SNHL. The distribution of WM abnormalities according to the presence of SNHL shows that extensive and confluent lesions are frequent in patients with SNHL, although this pattern of WM involvement may also be observed in normal hearing patients. Signs of an early vertical infection (cerebellar hypoplasia and cortical malformations) were present in seven patients; all four patients with cerebellar hypoplasia also presented polymicrogyria or lissencephaly.
Discussion
About 1% of all newborns in developed countries are associated with congenital CMV infection and 10–15% of these are symptomatic at birth. Ninety percent of symptomatic children develop a neurological impairment which ranges greatly from isolated SNHL to severe mental and/or motor deficits [1, 17]. In this clinical context, early predictors of outcome are needed to tailor appropriate management and counselling. Several studies have shown that microcephaly has a high specificity as a predictor of neurological impairment [11, 18]. It can be considered an indirect sign of severe nervous tissue loss resulting from an early damage to the germinal matrix, which is known to be selectively vulnerable to CMV infection [12]. Fetal MRI was recently shown to be a valuable tool for visualizing the germinal matrix—normally hypointense in T2-weighted images—and its abnormalities, such as thickening due to migration disorders, which might be associated with early and severe brain damage [19, 20]. Moreover, fetal MRI was shown to have a higher sensitivity than ultrasound in detecting polar temporal lesions, microencephaly and cortical anomalies [20]. On the other hand, some authors have shown that fetal MRI should be considered complementary to ultrasound in order to confirm or rule out the presence of CMV-related fetal brain abnormalities [21], and that prenatal imaging might not be a reliable prognostic marker [22, 23]. Post-natal neuroradiological features have also been investigated; many authors have shown typical brain CT abnormalities in congenital CMV infection, such as WM hypodensity, cerebellar hypoplasia, cortical dysplasia, periventricular calcifications, ventriculomegaly and brain atrophy [10, 24, 25]. In particular, Noyola et al. described a score based on CT findings (calcifications, ventriculomegaly and brain atrophy) and showed a high correlation between their absence and a good clinical outcome [11]. On the other hand, few papers addressed the role of brain MRI in early congenital CMV infection [26, 27]; this technique is known to be very sensitive in identifying cortical dysplasia, gray matter heterotopias or brain malformations such as schizencephaly, while its multiplanar characteristics allow an easier detection of hippocampal abnormalities and periventricular cysts. Furthermore, MRI can reveal WM abnormalities, except in the first year of life when lesions and unmyelinated WM might share a similar hyperintensity. Van der Knaap et al. tried to identify a typical MRI pattern in a group of eight children with congenital CMV infection who were symptomatic at birth; all of them had bilateral and multifocal WM abnormalities, predominantly involving the deep WM, with the largest lesions in the parietal lobe. In those children with gyral abnormalities, WM abnormalities could be either bilateral-multifocal or diffuse [14]. According to van der Knaap et al., the presence of these WM abnormalities should direct the clinicians toward the diagnosis of congenital CMV infection [28, 29], thus avoiding inconclusive and expensive metabolic investigations or the creation of new entities of leukoencephalopathy [30]. Nevertheless, in our cohort of symptomatic congenital CMV infection, documented within the first three weeks of life, these MR criteria were entirely fulfilled in only 1/12 children (patient 8). Similarly, Suzuki et al. did not detect WM abnormalities in 6/16 MRI examinations of congenital CMV infected children, thus posing some doubts about the presence of pathognomonic WM patterns [31]. Moreover, WM evaluation in CMV infection can be hampered by the difficulty in assessing WM abnormalities burden in early life, since it might be hindered by a partial myelination, which continues after birth and ends at around 2 years of age. Actually, spin-echo T2-weighted images are known to be more sensitive and specific for WM abnormalities even before 8 months of age whenever the increase of signal intensity is marked; yet, more subtle abnormalities are difficult to separate from incompletely myelinated brain tissue. Myelination may be slowed in CMV congenitally infected children and might progress even after 2 years of age [26, 27]. In our series, no correlation was found between a specific pattern of WM involvement and the severity of neurological impairment or the presence of SNHL. Consequently, WM abnormalities appear to be difficult to assess in the first years of life and, if present, they are scarcely predictive of poor neurological outcome. Recently, diffusion tensor imaging has been proposed for quantitative objective analysis of the myelination process; however, conclusions about the underlying structural changes remain challenging and its usefulness in distinguishing delayed myelination from CMV-related changes remain to be proven.
On the other hand, gray matter and morphological brain abnormalities can be reliably detected even in early life by MRI, and some of these features, namely, cortical malformations, hippocampal dysplasia and ventriculomegaly, have been found to correlate with poor neurological outcome, in particular with cerebral palsy. Recently, Suzuki et al. found an increased prevalence of migration disorder and ventricular dilatation in children with epilepsy. This correlation was not confirmed in the present study; however, some of our non-epileptic children with migration disorders were very young (19 months of age in patient 12 and 10 months in patient 13) and a longer follow-up might change our data since the onset of epilepsy has been observed to range widely (2–37 months) [31].
In the neonatal period, MR and ultrasound seem to be the favorite tools for detecting coexisting brain alterations such as periventricular cysts, cerebellar hypoplasia and hippocampal dysplasia [13]. Periventricular cysts are considered suggestive of viral congenital infection since the selective vulnerability of the germinal matrix might result in germinolytic lesions [26, 32]; nevertheless, this feature seems to lack prognostic value, at least in our group of patients. Cerebellar hypoplasia is considered a sign of CMV infection [12] occurring in the first trimester of pregnancy and should therefore correlate with severe brain damage. Accordingly, all our patients with cerebellar hypoplasia had a poor neurological outcome, while the absence of cerebellar hypoplasia does not imply a good outcome, as observed in our cohort. Hippocampal dysplasia is a relatively frequent though nonspecific sign of CMV infection, characterized by a vertical orientation of the hippocampus. It is usually associated with ventricular enlargement of the contiguous temporal horn of the lateral ventricle [12]. This abnormality was found only in microcephalic patients and most likely represents a further marker of severe brain involvement. Despite the pivotal role of hippocampal formation in epileptogenesis, no significant correlation between hippocampal dysplasia and epilepsy was present in our cohort; however, epilepsy might have a later onset, and a longer clinical follow-up would be desirable before excluding this relationship.
In conclusion, our study clearly indicates that WM abnormalities in symptomatic congenital CMV infection are polymorphic, difficult to evaluate at a very young age and scarcely correlated with clinical outcome. On the contrary, cortical malformations, ventriculomegaly and hippocampal dysplasia are detectable even at a young age and have a strong clinical predictive value.
References
Boppana SB, Stagno S, Pass RF et al (1992) Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J 11:93–99
Ivarsson S-A, Lernmark B, Svanberg L (1997) Ten-year clinical, developmental, and intellectual follow- up of children with congenital cytomegalovirus infection without neurologic symptoms at one year of age. Pediatrics 99:800–803
Pass RF, Stagno S, Myers GJ et al (1980) Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 66:758–762
Stagno S (2001) Cytomegalovirus. In: Remington JS, Klein JO (eds) Infectious diseases of the fetus and newborn infant, 5th edn. Saunders, Philadelphia, pp 389–424
Fowler KB, Stagno S, Pass RF et al (1992) The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 326:663–667
Crumpacker CS (2000) Cytomegalovirus. In: Mandell GM, Bennett JE, Dolin R (eds) Principles and practice of infectious diseases, 5th edn. Churchill Livingstone, Philadelphia, pp 1586–1599
Behrman RE (1997) Il feto e il neonato. In: Giovannini M (ed). Trattato di pediatria Nelson, 15th edn. Minerva Medica, Torino, pp 539–540
Barbi M, Binda S, Caroppo S et al (2003) A wider role for congenital cytomegalovirus infection in sensorineural hearing loss. Pediatr Infect Dis J 22:39–42
Williamson WD, Demmler GJ, Percy AK et al (1992) Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics 90:862–866
Boppana SB, Fowler KB, Vaid Y et al (1997) Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics 99:409–414
Noyola DE, Demmler GJ, Nelson CT et al (2001) Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr 138:325–331
Barkovich AJ, Lindan CE (1994) Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. Am J Neuroradiol 15:703–715
De Vries LS, Gunardi H, Barth PG et al (2004) The spectrum of cranial ultrasound and magnetic resonance imaging abnormalities in congenital cytomegalovirus infection. Neuropediatrics 35:113–119
van der Knaap MS, Vermeulen G, Barkhof F et al (2004) Pattern of white matter abnormalities at MR imaging: use of polymerase chain reaction testing of Guthrie cards to link pattern with congenital cytomegalovirus infection. Radiology 230:529–536
Ross SA, Boppana SB (2004) Congenital cytomegalovirus infection: outcome and diagnosis. Semin Pediatr Infect Dis 16:44–49
van der Knaap MS (2005) Myelination and retarded myelination. In: Magnetic resonance of myelination and myelin disorders, 3rd edn. Springer, pp 40–41
Kenneson A, Cannon MJ (2007) Review and meta-analysis of the epidemiology of congenital cytomegalovirus infection. Rev Med Virol 17:253–276
Nelson CT, Demmler GJ, Istas AS et al (1993) Early prediction of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. Pediatr Res 39:180A
Salmaso R, Franco R, de Santis M et al (2007) Early detection by magnetic resonance imaging of fetal cerebral damage in a fetus with hydrops and cytomegalovirus infection. J Matern Fetal Neonatal Med 20:559–561
Doneda C, Parazzini C, Righini A et al (2010) Early cerebral lesions in cytomegalovirus infection: prenatal MR imaging. Radiology 255:613–621
Benoist G, Salomon LJ, Mohlo M et al (2008) Cytomegalovirus-related fetal brain lesions: comparison between targeted ultrasound examination and magnetic resonance imaging. Ultrasound Obstet Gynecol 32:900–905
Benoist G, Salomon LJ, Jacquemard F et al (2008) The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG 115:823–829
Guerra B, Simonazzi G, Puccetti C et al (2008) Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 198:380.e1–380.e7
Steinlin MI, Nadal D, Eich GF et al (1996) Late intrauterine cytomegalovirus infection: clinical and neuroimaging findings. Pediatr Neurol 15:249–253
Barkovich AJ (2005) Infections of the nervous system. In: Barkovich AJ (ed) Pediatric neuroimaging, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 802–806
Boesch J, Issakainen J, Kewitz G et al (1989) Magnetic resonance imaging of the brain in congenital cytomegalovirus infection. Pediatr Radiol 19:91–93
Sugita K, Ando M, Makino M et al (1991) Magnetic resonance imaging of the brain in congenital rubella virus and cytomegalovirus infection. Neuroradiology 33:239–242
Lopez-Pison J, Rubio-Rubio R, Ureña-Hornos T et al (2005) Diagnóstico retrospectivo de infección congenital por citomegalovirus en un caso clínico infantile. Rev Neurol 40:733–736
O'Rourke D, Bradley L, King MD et al (2010) Leukoencephalopathy with anterior temporal cysts due to congenital CMV infection diagnosed retrospectively. J Neuroimaging 20:292–293
Tatli B, Ozmen M, Aydinli N et al (2005) Not a new leukodystrophy but congenital cytomegalovirus infection. J Child Neurol 20:525
Suzuki Y, Toribe Y, Mogami Y et al (2008) Epilepsy in patients with congenital cytomegalovirus infection. Brain Dev 30:420–424
Guibaud L, Attia-Sobol J, Buenerd A et al (2004) Focal sonographic periventricular pattern associated with mild ventriculomegaly in foetal cytomegalic infection revealing cytomegalic encephalitis in the third trimester of pregnancy. Prenat Diagn 24:727–732
Acknowledgments
The authors thank Chiara Briani, M.D., for helpful comments in the preparation of this manuscript.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manara, R., Balao, L., Baracchini, C. et al. Brain magnetic resonance findings in symptomatic congenital cytomegalovirus infection. Pediatr Radiol 41, 962–970 (2011). https://doi.org/10.1007/s00247-011-2120-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2120-5