Abstract
Spica MRI is a fast and effective tool to assess morphology after closed reduction for developmental dysplasia of the hip (DDH) without the need for sedation. The multiplanar capabilities allow depiction of coronal and axial reduction of the hips. Due to MRI’s inherent ability to delineate soft tissue structures, both intrinsic and extrinsic obstacles to failed reduction may be identified. Technical and interpretative challenges of spica MRI are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Developmental dysplasia of the hip (DDH) denotes a wide spectrum of abnormalities in the relationship of the femoral head and acetabulum, ranging from subtle acetabular dysplasia to irreducible hip dislocation. The goal of treating DDH is to position the femoral head centered deep within the acetabulum, thereby influencing acetabular growth to “contain” the femoral head. Closed reduction of a child’s hip with DDH can be achieved with a passive abduction orthotic device applied in the outpatient setting with great success if applied before the child reaches an age at which they can overpower the device. As the child becomes older, a rigid abduction orthotic device may be utilized. Ultimately, a sedated closed reduction with spica casting or an open surgical reduction may be necessary to reduce the subluxed or dislocated hip [1].
After spica casting, determination of hip location can be achieved with multiple imaging techniques including radiographs, arthrography, ultrasound and CT. MRI is an established technique that may also determine successful reduction in the management of DDH without ionizing radiation and may be quickly performed with the vast majority of infants not requiring sedation [2]. The purpose of this article is to discuss various post-reduction spica imaging techniques with emphasis on MRI of patients with DDH. MRI has multiplanar capabilities that can provide an accurate assessment of the relationship of the femoral head and acetabulum and excellent soft tissue delineation to assist in identifying obstacles to successful reduction. With gadolinium enhancement, it can identify areas of decreased or irregular enhancement indicative of altered epiphyseal cartilage blood flow, which may be a sign of early ischemia [3].
Description
The technique for spica MR imaging is detailed in Tables 1 and 2. At our institution, A 1.5 and 3T Philips Achieva platforms are utilized (Philips, Amsterdam, Netherlands). We routinely perform pre- and postcontrast imaging for our patients. Because the child is immobilized in a cast, these studies can often be performed without the need for sedation. However, spica casting does not eliminate patient motion altogether. Therefore, strategies to soothe the infant, such as feeding the child prior to imaging, swaddling the infant in a blanket, and/or imaging when the infant is asleep should be considered. For an awake child who is moving, a diagnostic study can often be obtained but may require that multiple sequences are repeated. There may be significant motion artifact leading to an aesthetically suboptimal exam, but the motion artifact is often limited because the child is constrained by the spica cast. If the child is significantly agitated, the goal should be to obtain the best possible non-contrast sequences of the hips to determine reduction. We will often defer giving intravenous gadolinium if there is significant motion artifact on the precontrast sequences.
MR spica studies are scheduled to occur after operative reduction in the OR. After the child has been extubated and is ready to be discharged, he or she will then arrive at the radiology suite for MR spica imaging. These studies are given 15-min time slots if they are scheduled in advance. However, due to the short duration of the exam, these studies can be added on an unscheduled basis between routinely scheduled exams.
Discussion
MRI spica imaging and interpretation
Magnetic resonance imaging is an attractive alternative for post-reduction evaluation of the hip, given its multiplanar capabilities and lack of ionizing radiation to the gonadal region, particularly since these patients may require repeat studies. MRI offers excellent visualization of soft tissue and cartilaginous structures, such as the capital femoral epiphysis, allowing accurate assessment of the adequacy of reduction regardless of the presence of an ossific nucleus [4].
MRI is superior to CT in depiction of soft tissue abnormalities that may serve as an obstacle to reduction. The major intrinsic obstacles to successful reduction include abnormal femoral epiphyseal and acetabular shape, inverted labrum (Figs. 1, 2, 3), large pulvinar, and hypertrophy of the ligamentum teres and transverse acetabular ligaments (Fig. 4). The major extrinsic obstacles to a complete reduction include invagination of the iliopsoas muscle, shortening of the external rotators and adductor muscles, and adhesion of the capsule to the ilium [2].
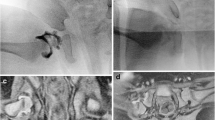
A 1-year-old with inverted superior labrum and aspherical femoral head. a, b PD coronal images show the superior labrum of the left hip is inverted and demonstrates abnormal increased intrasubstance signal (arrows). The normal superior labrum of the right hip (arrowheads). Note the normal spherical shape of the nondysplastic right femoral head, and the dysplastic aspherical and small left femoral head
An 8-month-old with inverted left anterior labrum. PD FS axial image shows inverted anterior labrum (thin arrow) and mildly posteriorly subluxed left femoral head. Compare with normal right anterior labrum (arrowhead). Changes related to left-sided adductor tenotomy (*with arrow). Note normal left posterior labrum (thick arrow)
An 8-month-old after successful closed reduction for left-sided DDH. PD coronal (a) and PD FS axial (b) show the near-complete reduction of left-sided DDH. There is mild posterior subluxation of the left femoral head, but this is within acceptable range. Note hypertrophied transverse ligament/ligametum teres complex (arrow) and minor edema related to adductor tenotomy (arrowhead)
Both coronal and axial sequences are used to determine whether the femoral head is concentrically reduced, subluxed (Fig. 5) or frankly dislocated.
A 1-month-old with posterior subluxation of the right femoral head after attempted closed reduction. PD FS axial (a) shows posterior subluxation of the right femoral head with abnormal posterior deviation of the posterior labrum (arrow). Note the normally located left femoral head with normal appearance of the posterior labrum (b, arrowhead)
With gadolinium enhancement, MRI also offers the ability to determine the extent of hip abduction and the vascularity of the femoral head after reduction in order to identify hips that may be at increased risk of ischemia and development of AVN at a time when it may be reversible, compared to the months it takes to manifest abnormalities on digital radiographs. The blood supply to the cartilaginous femoral head is derived primarily from the deep medial femoral circumflex vessels that enter the femoral epiphysis and supply numerous parallel, non-anastamosing vascular canals from which nutrients diffuse to supply the cartilage, normally producing a striped appearance of the epiphysis with prominent enhancement of the cartilaginous femoral head using gadolinium-enhanced MRI [5]. Placement of the spica cast with hips in excessive abduction can diminish the blood supply to the femoral head (Fig. 6), leading to decreased global enhancement of the femoral head, often with areas of focal decreased enhancement with sharp demarcations since the vascular canals do not anastomose [2, 3]. While the appropriate hip-abduction angle for a given patient varies, studies have suggested an association between hip-abduction angles greater than 55° and the development of AVN [2, 3].
A 6-month-old with abnormal perfusion defects throughout the left femoral head. T1 post-Gd FS (a) shows geographical areas of nonenhancement throughout the left capital femoral epiphysis (arrowheads). Note normal speckled enhancement pattern of the right capital femoral epiphysis. (b) The child was recast in less hip abduction (b) and T1 post-Gd FS imaging performed 1 day after post-casting shows normalization of capital femoral epiphyseal enhancement. Note hypertrophied ligamentum teres (arrows)
The clinical implications of decreased gadolinium enhancement of the cartilaginous capital femoral epiphysis on MRI after closed reduction have been evaluated in several studies. Jaramillo et al. describe abduction-induced ischemia of the femoral head in piglets, manifested as decreased global enhancement. The ischemia was reversible if corrected within 6 h, though the applicability of this finding to clinical practice is uncertain [6]. Tiderius et al. conducted a retrospective analysis of gadolinium-enhanced MRI as a predictor of AVN in 27 infants (28 hips) with DDH who underwent closed reduction with spica cast placement and were followed for a minimum of 1 year. Fifty percent of hips with radiographic evidence of AVN (3 of 6 hips) showed global decreased enhancement compared with the 2 of 22 hips without AVN [3]. Given the limited specificity of decreased global enhancement of the femoral head, further studies are required before such findings on MRI can be used to guide treatment decisions, such as a possible return to the operating room for revision closed reduction and casting.
Conclusion
MRI is the ideal imaging modality for evaluating adequacy of closed reduction for DDH. MRI does not expose the gonadal region to ionizing radiation, it can be performed quickly and immobilization in a spica cast often precludes the need for sedation. Given its superior soft tissue resolution compared with digital radiographs, ultrasound and CT, MRI may help identify intrinsic and extrinsic causes for failed reduction and, with gadolinium enhancement, may assist in the detection of hips at risk of developing AVN.
References
Vitale MG, Skaggs DL (2001) Developmental dysplasia of the hip from six months to four years of age. J Am Acad Orthop Surg 9:401–411
Jaramillo D, Villegas-Medina O, Laor T et al (1998) Gadolinium-enhanced MR imaging of pediatric patients after reduction of dysplastic hips: assessment of femoral head position, factors impeding reduction, and femoral head ischemia. AJR 170:1633–1637
Tiderius C, Jaramillo D, Connolly S et al (2009) Post-closed reduction perfusion magnetic resonance imaging as a predictor of avascular necrosis in developmental hip dysplasia: a preliminary report. J Pediatr Orthop 29:14–20
Duffy CM, Taylor FN, Coleman L et al (2002) Magnetic resonance imaging evaluation of surgical management in developmental dysplasia of the hip in childhood. J Pediatr Orthop 22:92–100
Barnewolt CE, Shapiro F, Jaramillo D (1997) Normal gadolinium-enhanced MR images of the developing appendicular skeleton: Part I. Cartilaginous epiphysis and physis. AJR 169:183–189
Jaramillo D, Villegas-Medina OL, Doty DK et al (1996) Gadolinium-enhanced MR imaging demonstrates abduction-caused hip ischemia and its reversal in piglets. AJR 166:879–887
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desai, A.A., Martus, J.E., Schoenecker, J. et al. Spica MRI after closed reduction for developmental dysplasia of the hip. Pediatr Radiol 41, 525–529 (2011). https://doi.org/10.1007/s00247-010-1965-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-010-1965-3









