Abstract
Background
Heterotaxy with polysplenia is associated with many cardiovascular anomalies including the occasional occurrence of congenital extrahepatic portosystemic shunts (CEPS). Missing this anomaly can lead to inappropriate and ineffective therapy.
Objective
To emphasize the importance and associated anatomy of CEPS in conjunction with heterotaxy with polysplenia.
Materials and methods
Review of three young children who presented with cyanosis and pulmonary hypertension without a cardiac etiology. They were known (1) or discovered (2) to have heterotaxy with polysplenia.
Results
There was absence of the intrahepatic inferior vena cava (IVC) with azygos or hemiazygos continuation in all three cases. In spite of normal liver function, they were discovered to have large portosystemic shunts, splenorenal in location, along with diffuse peripheral pulmonary arterial dilatation suggestive of CEPS (Abernethy malformation) with hepatopulmonary or, more accurately, portopulmonary syndrome. All CEPS were ipsilateral to the spleens. Patency of the portal veins in these cases allowed for percutaneous shunt closure with resolution of cyanosis.
Conclusion
CEPS is associated with heterotaxy with polysplenia and can be symptomatic because of pulmonary arteriovenous (AV) shunting. Portal and hepatic vein patency are critical for determining feasibility of CEPS closure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heterotaxy with polysplenia is well known to be associated with a multitude of anomalies. The most common lesions include bilateral left-side bronchial branching and lung lobation and absence of the intrahepatic IVC with azygos or hemiazygos continuation. Accompanying cardiac anomalies might be simple intracardiac shunts or more complex anomalies such as complete atrioventricular septal defect (AVSD), anomalous pulmonary venous return and complete transposition or double-outlet right ventricle with a variable position of the cardiac apex [1, 2]. In the abdomen there is usually abnormal abdominal situs with either left- or right-side polysplenia and variable location of the liver, stomach and IVC/aorta. There is an increased incidence of bowel malrotation, preduodenal portal vein, and biliary atresia [1].
Portosystemic shunts occur most often in the setting of chronic hepatic disease and accompanying portal hypertension. Hepatic encephalopathy (caused by elevated ammonia levels) and hepatopulmonary syndrome are both described in this situation. Hepatopulmonary syndrome consists of diffuse peripheral pulmonary arteriolar/capillary vasodilatation with arteriovenous (AV) shunting resulting in hypoxemia [3]. This typically occurs in patients with significant underlying liver dysfunction. The same pulmonary findings have been described in the absence of parenchymal liver disease in association with intra- or extrahepatic portosystemic shunting. This entity would be more accurately termed portopulmonary rather than hepatopulmonary syndrome to reflect the absence of hepatic abnormality. Intrahepatic portosystemic shunts include a persistent patent ductus venosus, portal/hepatic venous fistula or chronic IVC or hepatic venous obstruction (Budd-Chiari) with collateral vessels [4, 5]. The presence of a congenital extrahepatic portosystemic shunt (CEPS) is known as the Abernethy malformation, divided into subtypes depending on the absence (type 1) or presence (type 2) of the portal vein [3, 6]. Several cases of CEPS have been described in children with heterotaxy and polysplenia [5, 7]. Pulmonary hypertension is an additional pulmonary vascular problem that occurs in association with portal hypertension (usually) with or without accompanying liver disease; this has been termed portopulmonary hypertension [8].
We illustrate three children with heterotaxy with polysplenia whose major clinical problem was portopulmonary syndrome caused by CEPS. Recognition of this rare entity is frequently delayed but is essential for appropriate management. This study was approved as needed by the Institutional Review Board of each participating institution.
Materials and methods
Three young girls from different institutions (ages 9 months, 1.5 years, and 3 years) had persistent tachypnea and hypoxemia requiring oxygen supplementation. One child, known to have polysplenia and heterotaxy, had been progressively symptomatic for 18 months (case 1). The other two children presented with acute respiratory distress and pneumonia with unexplained respiratory symptoms persisting after treatment.
Results
Echocardiography revealed levocardia in all, with no major intracardiac abnormalities to account for their symptoms. One child had bilateral superior vena cavae, the left of which had drained to an unroofed coronary sinus, and had previously been occluded percutaneously (case 1). The second child had a small atrial septal defect (ASD) and ventricular septal defect (VSD) that had closed spontaneously (case 2). A small patent foramen ovale was present in the third child (case 3). All three children were found to have tricuspid regurgitation on echocardiographic examination with estimated pulmonary hypertension (based on the peak velocity of the tricuspid jet) in the moderate to severe range. An echocardiographic bubble study in one child (case 3) showed prominent appearance of bubbles in the left atrium after several heartbeats, suggestive of intrapulmonary shunting. Chest radiographs were nonspecific (case 3, Fig. 3).
Preoperative imaging findings are described in Table 1. Chest CT angiography was performed in two children. The CT images were obtained during dynamic injection of intravenous contrast agent (2 cc/kg). The images were reconstructed to 1- to 1.5-mm thickness. Abdominal CT venography (CTV) was performed in all three children, at the same time as chest CT in two of them. Abdominal CT images were obtained approximately 50–60 s after the start of the contrast injection; these were then reconstructed to 1- to 1.5-mm thickness. One child had a high-resolution noncontrast chest CT (1-mm thick, 5-mm apart, Fig. 1) to evaluate for possible interstitial lung disease, followed by an abdominal CT venogram several weeks later (case 1). The abdominal CT venograms were obtained specifically to evaluate for a liver or portal venous abnormality as the cause of pulmonary hypertension. The possibility of an underlying pulmonary AVM or portosystemic shunt was considered prior to CT imaging in one child on the basis of the US bubble study suggesting intrapulmonary shunting (case 3). The three children were studied at three different institutions with somewhat varying CT techniques: kVp used ranged from 100 to 120 and mAs from 60 to 150; overall CTDI vol including the chest, abdomen and pelvis ranged from 4.2 to 18 and DLP from 118 to 250.
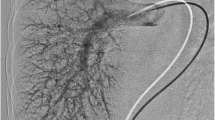
A 3-year-old girl, case 1. a An axial contrast-enhanced HRCT slice (1 mm) image, lung window at the lung bases, demonstrates diffusely prominent peripheral basilar vessels (arrows). Although the finding is subtle, pulmonary vessels normally taper peripherally and are not diffusely discernible within 5–10 mm of the visceral pleura. b Coronal thin maximum-intensity projection (MIP) reconstruction CT demonstrates the right-side stomach (ST) and polysplenia (SPL) and predominantly left-side liver. A large right portosystemic shunt (arrows) connects the splenic vein (SV) to the right renal vein (RV) at its junction with the IVC/azygos. Note that the portal vein is faintly seen (arrowhead). c Nuclear medicine pulmonary perfusion scan demonstrates marked uptake in the abdominal viscera and brain (arrows), indicating right-to-left shunting—calculated at 47%. d Left pulmonary angiogram—injection in left lower lobe artery demonstrates diffusely dilated peripheral arteriolar/capillary vessels, producing a spongy appearance. e Conventional angiogram (PA view) demonstrates injection of the right splenorenal shunt (arrows) via the right femoral vein to IVC catheter. The catheter has passed through the shunt with the catheter tip (*) in the shunt near the splenic vein connection. The large tortuous shunt is seen extending from the mid-splenic vein (SV) to the right renal vein (RV) at its junction with the IVC/azygos. The more proximal splenic vein on the right is not filled. There is faint filling of the right renal vein (RV). The portal vein (PV) (right branches fill better than left branches) and superior mesenteric vein (SMV) are patent. f Angiogram post-Amplatzer device occlusion of the upper portion of the shunt—lateral view. This demonstrates the residual lower part of the splenorenal shunt (arrow) connecting to the azygos vein (AZ) via a short segment of renal vein (RV) with no residual connection to the portal system
Chest CTs were unremarkable except for prominent peripheral pulmonary vessels abutting the visceral pleura, most marked at the lung bases, observed in two children (cases 1 and 3, Figs. 1 and 3).
Abdominal CTV revealed polysplenia in all three children (a new diagnosis in cases 2 and 3), right-side in one (case 1, Fig. 1) and left-side in the other two (Figs. 2 and 3). The stomach was located ipsilateral to the spleen and the bulk of the liver and liver hilum was contralateral in all (Figs. 1, 2, and 3). All had absence of the intrahepatic IVC with azygos (case 1) or hemiazygos (cases 2 and 3) continuation. Hepatic veins were patent in all cases draining to the right atrium via a patent suprahepatic IVC.
An 18-month-old girl, case 2. a Axial slice from a contrast-enhanced abdominal CT venogram demonstrates right liver (L) and left polysplenia (SPL). The portion of the splenorenal shunt connecting with the left renal vein (RV) is seen (arrow) behind the more proximal splenic vein (SV). This drains posteriorly to the hemiazygos vein (HZ). The entire course of the shunt cannot be seen on a single image. There is partial visualization of a retroaortic right renal vein, also draining to the hemiazygos vein. Ao = abdominal aorta. b CT 3-D volume-rendered coronal image demonstrates bilateral left-side bronchial branching in the lungs consistent with left isomerism. There is a large tortuous left splenorenal shunt (arrows) draining from the splenic vein (SV) proximal to the superior mesenteric vein (SMV) connection, extending superiorly and behind the more proximal splenic vein to the left renal vein (RV) and draining to the hemiazygos vein (HZ). The superior mesenteric vein (SMV) and portal vein (PV, arrowheads) are intact. c Conventional angiogram (oblique view). The catheter course is from the femoral vein to IVC to hemiazygos (HZ) to shunt via a small segment of left renal vein to splenic vein with the catheter tip (*) in the intrahepatic portal vein. Contrast injection fills the portal vein (PV, arrow), a small segment of the splenic vein (SV), tortuous splenorenal shunt (black arrows) draining to the left renal/hemiazygos vein (HZ). Note that the proximal splenic vein on the left, superior mesenteric vein, and most of the left renal vein are not opacified because of the direction of blood flow
A 9-month-old girl, case 3. a Chest radiograph, note cardiomegaly and nonspecific diffuse increased lung markings. b Contrast-enhanced CTA coronal MIP reconstruction of the chest demonstrates diffusely dilated peripheral vessels extending close to the visceral pleura, consistent with portopulmonary syndrome. No discrete arteriovenous malformations are seen. c Axial contrast-enhanced CTV slice demonstrates left polysplenia (SPL) and right-side liver (L). The confluence of the left splenorenal shunt to the hemiazygos vein (HZ) is shown (arrow). The right renal vein (arrowhead) drains to the IVC/azygos (arrowhead). C colon. d Oblique coronal CT thin MIP reconstruction demonstrates the large tortuous left splenorenal shunt (arrow) draining to both the hemiazygos (HZ) and azygos (AZ) veins. SV splenic vein. RV left renal vein
Large extrahepatic portosystemic shunts were seen on the abdominal CTV in all three cases (Table 1, Figs. 1, 2 and 3). The portosystemic connections occurred between enlarged native vessels rather than an aberrant vessel. In all cases the connection was splenorenal, extending from the ipsilateral renal vein at its junction with the azygos or hemiazygos vein to the splenic vein proximal to its junction with the superior mesenteric vein to form the portal vein. There was a right splenorenal shunt draining to the azygos vein (case 1, Fig. 1), a left splenorenal shunt draining to the hemiazygos vein (case 2, Fig. 2), and a left splenorenal shunt communicating with both the azygos and hemiazygos veins in the abdomen (case 3, Fig. 3). Splenic and superior mesenteric veins were patent in all three children, with variable size and location. A small main portal vein (Figs. 1 and 2) was thought to be present in all, although it was poorly defined by CT in two children (cases 1 and 3, Fig. 1). Because liver function tests and the imaging appearance of the liver were normal in all three children, a diagnosis of portopulmonary syndrome caused by a CEPS/Abernethy malformation type 2 (patent portal vein) was suggested.
Doppler US imaging was performed after the CT in one child (case 1) to clarify whether the portal vein was patent. This showed reversal of portal flow in a small main portal vein. The splenorenal shunt was poorly seen because of overlying bowel gas.
Nuclear medicine pulmonary perfusion study in case 1 revealed marked right-to-left shunting with 47% of the agent reaching systemic organs (Fig. 1). In the absence of an intracardiac shunt, this finding supported intrapulmonary shunting. A nuclear lung perfusion study in a second child (case 2) failed to confirm right-to-left shunting.
Conventional angiographic studies convincingly showed diffuse peripheral pulmonary arteriolar/capillary vasodilatation with early venous filling in all three cases (Fig. 1). No discrete arteriovenous malformations were seen on CT or conventional angiographic imaging.
All three children underwent angiographic confirmation of the portosystemic connections. The location of the portosystemic shunts on angiography coincided with the findings on CT but the connections were somewhat more difficult to determine because the proximal splenic, superior mesenteric, and most of the renal veins were not opacified with contrast agent, related to the position of the catheter and direction of flow of non-opacified blood (Figs. 1 and 2).
Identification of a patent, although small, portal vein on angiography (Figs. 1 and 2) enabled a decision that the portosystemic connections could be closed with rerouting of blood through the liver. Interventional occlusion of the splenorenal shunts was performed using an Amplatzer vascular plug (8 to 16 mm) after test balloon occlusion. No procedural complications were encountered. In one child (case 3) the shunt was only partially occluded by coil embolization initially, because of concern about the small size of the portal vein. When no clinical improvement in hypoxemia occurred, the shunt was completely occluded with an Amplatzer device during a second procedure 2 months later.
There was a prompt and sustained improvement in arterial oxygen saturation after shunt closure in all three children, consistent with probable decreased intrapulmonary shunting. All three children were gradually weaned off supplemental oxygen. Pulmonary hypertension, as evaluated echocardiographically, abated in two children but persisted in one.
Repeat pulmonary angiography in one child (case 1) 13 months after shunt occlusion still showed bilateral, diffuse pulmonary arteriocapillary dilatation. However, a repeat lung perfusion scan quantified only a 6% right-to-left shunt as compared to 47% previously (case 1).
Early follow-up abdominal Doppler US imaging in all three children demonstrated normal hepatopetal portal venous flow with a measurable increased extrahepatic portal vein caliber in one child (case 1). However, follow-up Doppler US in one child 1 year after CEPS closure showed occlusion of the portal vein at the liver hilum with cavernous transformation (case 3). This was confirmed by MR imaging. The portosystemic shunt remains closed and the child is currently asymptomatic, with no evidence of esophageal or gastric varices.
Discussion
CEPS and portopulmonary syndrome caused severe clinical symptoms with presentation at an early age in the three children described in this report. Although CEPS has been described in conjunction with heterotaxy with polysplenia [5, 7], it is not well known, is considered uncommon and is not usually specifically looked for in these individuals. Therefore, there is frequently a delay in reaching the correct diagnosis, as in our cases.
The incidence of CEPS in conjunction with polysplenia is unknown. The anecdotal experience of one of the authors (BN) of recently identifying large CEPS in two very young infants (ages 8 days and 1 month) with heterotaxy with polysplenia who underwent CTV (1) or MRA (1) suggests that this entity is not as rare as generally thought but rather underdiagnosed. The incidence of portopulmonary syndrome in association with CEPS is also unknown; however, recognition and understanding of this entity are important in making the correct diagnosis and choosing appropriate patient management. This is highlighted by several prior reports of children undergoing liver transplantation because of intrapulmonary shunting in the setting of biliary atresia and heterotaxy with polysplenia [7, 9, 10]. In most of these cases liver function was preserved, suggesting that the portosystemic connections that were present were more likely CEPS rather than secondary to underlying liver disease.
Additionally, in a recent report of a young girl with hypoxemia, polysplenia, and diffuse pulmonary AVMs [11], the authors did not mention the possibility of Abernethy malformation and hepato/portopulmonary syndrome and advocated lung transplantation as the only therapeutic option. Given our current knowledge regarding polysplenia and portopulmonary syndrome it is possible that a CEPS might have been the underlying problem in this case. Another report described diffuse microscopic pulmonary AVMs in association with polysplenia and although an abdominal portosystemic connection was recognized, its significance was apparently not appreciated [2].
Murray et al. [5] described three cases of CEPS (polysplenia in two) and reviewed 58 others (nine with polysplenia). They described the most common associations as nodular lesions of the liver, cardiac anomalies, encephalopathy, polysplenia, biliary atresia, and skeletal and renal anomalies. These authors did not mention hepato/portopulmonary syndrome, although one of their cases presented very similarly to our three cases with respiratory distress, pulmonary hypertension and right heart failure and possibly had unrecognized portopulmonary syndrome [5].
Imaging the pulmonary changes of hepatopulmonary/portopulmonary syndrome can be quite challenging. Chest radiographs (described in adults) tend to have mild, nonspecific bibasilar nodular or reticulonodular opacities easily mistaken for interstitial lung disease [12]. On CT these opacities can be shown to be related to distal vascular dilatation, best appreciated on thicker slices rather than thin-section high-resolution chest CT [12]. In our experience these findings were quite subtle on CT imaging (Figs. 1 and 3) and were much easier if the reader was specifically considering the diagnosis. No discrete arteriovenous malformations (AVMS) were seen on either CT or conventional angiography in our cases.
The pulmonary vascular abnormality in hepatopulmonary and portopulmonary syndrome is identical, most often consisting of diffuse arteriocapillary dilation (15–100 microns, normal 8–15 microns) with resultant rapid transit through the lung and incomplete oxygenation [3]. Our cases appeared very similar to previous angiographic descriptions of mild to extensive dilated peripheral vessels (spongy appearance), most marked at the lung bases with or without early venous filling [12–14]. These arteriocapillary dilations are thought to occur as a result of loss of the normal balance between pulmonary vasodilators and constrictors caused by an unknown agent absorbed from or produced in the gut and normally modified in the liver before reaching the pulmonary circulation.
There have been descriptions of multiple small peripheral isolated AVMs in some cases of hepatopulmonary/portopulmonary syndrome [14, 15]. The exact distinction between diffuse peripheral arteriocapillary dilatation and true tiny pulmonary AVMs appears to be blurred with both patterns described but not clearly distinguished. It has been suggested that actual arteriovenous connections represent a more advanced and possibly less reversible entity [14–16].
Intrapulmonary right-to-left shunting occurs in several conditions in addition to hepato/portopulmonary syndrome. In hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome), there are often multiple and sometimes diffuse, discrete pulmonary AVMs, frequently also present in the skin, mucous membranes, and viscera [1, 13, 17]. In bidirectional Glenn shunts (superior cavopulmonary anastomosis) intrapulmonary shunting/AVMs can occur; the mechanism is thought to be similar to that theorized for hepato/portopulmonary syndrome. In fact, it is not uncommon to proceed to total cavopulmonary anastomosis (Fontan) in hopes of resolving the shunting by returning IVC and hepatic venous flow to the lungs [16, 18]. Pulmonary AVMs also occur commonly when a superior cavopulmonary anastomosis is performed in the setting of absence of the intrahepatic inferior vena cava with azygos or hemiazygos continuation (typically in heterotaxy with polysplenia). This anatomic situation, where all of the systemic venous return, minus the portal/ hepatic venous circulation, is baffled to the lungs (termed Kawashima operation), emphasizes the concept that venous flow from the gut must pass through the portal circulation and liver and then reach the lungs to prevent the development of arteriocapillary dilatation/AVMs.
Both contrast echocardiography and nuclear perfusion imaging of the lung have been used to document intrapulmonary AV shunting. In the case of echocardiography, intravenously injected microbubbles, which are normally largely trapped in the lung capillaries, are able to pass through to the left heart. The timing of the bubbles arriving in the left atrium distinguishes intracardiac (immediate) from intrapulmonary shunting (after several beats); this technique was useful in one of our cases in suggesting intrapulmonary shunting (case 3). In nuclear lung perfusion imaging, injected technetium macroaggregated albumin particles (10–50 microns in size) are normally almost entirely trapped in pulmonary capillaries. With either intracardiac or intrapulmonary right-to-left shunting, the agent can be imaged in the systemic circulation and the amount of shunting can be quantified by measuring the activity in the brain and abdominal viscera relative to pulmonary activity [6, 10, 12]. Although both of these studies might be helpful in individual cases, their reliability is not completely known. In one of our children, nuclear perfusion scan was useful (case 1), documenting a large right-to-left shunt (Fig. 1), which decreased markedly after CEPS occlusion. In another child (case 2), for unknown reasons, the nuclear perfusion scan failed to confirm the pulmonary shunting that was evident on direct angiography.
In order to obtain good CT visualization of the portal venous system, we advocate a technique similar to CT angiography. Thin CT cuts (0.6–1.5 mm) are obtained (or reconstructed) 50–60 s after a rapid dynamic contrast bolus followed by a saline bolus, which can be delivered using an injector or by hand injection. The speed and mode of the intravenous injection depends on the size of the child, contrast volume and available IV access. One hundred kVp and a reference mAs of 65–150 (Siemens Sensation 64, Siemens Medical Solutions, Erlangen, Germany) are settings we typically use to obtain a good-quality reasonably low-dose study. The CTV of the abdomen can be obtained after a chest CTA using the same contrast bolus. The chest CTA uses similar parameters to those for the abdomen (usually slightly lower reference mAs) and is typically timed about 15 s into the contrast injection. We (BN) tend to use fluoroscopic triggering with monitoring slices at the level of interest and triggered for best visualization of the anatomic structures of concern.
In the children described in this report, the shunts were very large and the presence of the abdominal venous abnormality was readily apparent. However, the exact connections were more complex because the vessels were markedly tortuous. Details were best defined by directly interacting with the CT dataset on a separate workstation using 2-D and 3-D reconstruction techniques.
The abdominal venous anomalies could also be well displayed on abdominal MR angiography with similar reconstruction capability. Peripheral pulmonary vasculature and detailed views of the pulmonary parenchyma are less well seen on MR than CT. Careful US imaging might identify CEPS, although bowel gas could obscure the anatomy, as in one of our children (case1).
A variety of portosystemic communications can occur including portocaval, mesocaval and splenorenal. The location of the portosystemic connections as splenorenal was most easily defined on CT in our cases because of the advantage of opacification of all the veins and the ability to obtain multiplanar and 3-D reformations.
Evaluation of the hepatic and portal veins is crucial in determining management of symptomatic CEPS. CEPS can be successfully closed surgically or percutaneously, as long as the portal and hepatic veins are patent so that blood can be rerouted through the liver. Absence of the portal vein leaves liver transplantation as the only definitive therapeutic option.
In the presence of a large extrahepatic portosystemic shunt, the portal vein might be very small, possibly hypoplastic on a congenital basis versus small because of decreased flow. There were no imaging findings suggestive of cavernous transformation of the portal vein on any of the children described in this report prior to shunt closure, presumably because portal flow could readily decompress through the shunt.
Identifying an intact but small portal vein (Fig. 1) proved quite difficult on CT in two cases despite good-quality imaging. US imaging was helpful in identifying a patent portal vein as well as flow direction in one of our children (case 1). The hypoplastic portal veins were demonstrated with much greater confidence on conventional angiography. Other publications confirm the difficulty in identifying a very small portal vein and suggest that at least some of the previously reported cases of type 1 Abernethy malformation (absent portal vein) have really been type 2. Successful closure of CEPS in spite of a hypoplastic portal vein has been described [5, 19, 20]. The lower limit of when a portal vein would be too small to allow closure of the shunt is unclear. Test balloon occlusion with evaluation of flow and portal venous pressure has been employed to evaluate the feasibility of shunt occlusion. In one of our cases with a very small portal vein, portal flow normalized and enlargement of the vein was seen on US follow-up (case 1); however, another child with a hypoplastic portal vein developed cavernous transformation of the portal vein at the liver hilum 1 year after CEPS occlusion (case 3). The ultimate consequence of this is not yet known.
All of our cases were occluded utilizing an Amplatzer device, which is reported to be advantageous for occluding large vessels. Because the device can be repositioned in situ before being completely deployed, its use might result in fewer complications such as unintended portal or splenic vein occlusion [20]. Successful coil embolization has also been described. Shunt closure is usually followed by marked improvement of hepato/portopulmonary syndrome [18–20]. This was our experience with a rapid initial response and continued, progressive improvement over time with the exception of persistent pulmonary hypertension in one of our cases.
In children with end-stage liver disease and hepatopulmonary syndrome, liver transplantation leads to marked improvement, if not complete cure, of the pulmonary issues over time [7, 9, 18]. Spencer et al. [18], however, reported a child with polysplenia/biliary atresia and hypoxemia who showed an initial good response following liver transplantation. Recurrent hypoxemia developed 6 years later and a previously unrecognized large extrahepatic portosystemic shunt was found. After closure of this shunt, hypoxemia resolved. This report indicates that closure of the portosystemic shunt is important in the definitive management of this condition and should be performed even in conjunction with liver transplantation [18].
The literature is somewhat confusing in regard to the spectrum of pulmonary vascular problems associated with hepatic/portal abnormalities. Portopulmonary hypertension and hepato/portopulmonary syndrome are the major entities described, both thought to be related to an imbalance of pulmonary vasodilators and vasoconstrictors. Their interrelationships are unclear; they have been said to be mutually exclusive but have also been reported as occurring in the same patient either concurrently or subsequently [8, 15, 21, 22]. Portopulmonary hypertension is described as pulmonary vasoconstriction with arterial intimal fibrosis and muscular thickening leading to irreversible plexiform arteriopathy of the pulmonary vasculature (a final pathway for any cause of severe prolonged pulmonary hypertension) in association with liver dysfunction and portal hypertension [8, 15]. By contrast hepatopulmonary and portopulmonary syndrome produces diffuse pulmonary arteriocapillary vasodilatation and pulmonary AV shunting related to liver dysfunction, portal hypertension (not invariable) and portosystemic shunting in the case of hepatopulmonary syndrome, and intra- or extrahepatic portosystemic shunting without liver dysfunction in the case of portopulmonary syndrome [8, 15]. The coexistence of pulmonary hypertension and portopulmonary syndrome in our patients might reflect the concurrence of these incompletely understood entities.
Conclusion
Our cases highlight the association between CEPS and heterotaxy with polysplenia. If not specifically sought, CEPS is easily overlooked. The presence of CEPS might be responsible for prolonged and significant patient morbidity that can be avoided with appropriate investigation and treatment. Portopulmonary syndrome and pulmonary hypertension are associated with CEPS/Abernethy malformation and can be successfully treated with percutaneous closure of the shunt when the portal vein is patent or with liver transplantation along with shunt closure when the native portal vein is absent. We advocate that CEPS be one of the anomalies specifically sought when imaging patients with polysplenia. The vascular abnormalities can largely be defined by noninvasive means such as CT or MR. In the setting of unexplained pulmonary hypertension, with or without polysplenia, CT imaging should include the chest as well as a venous phase of the upper abdomen to evaluate for liver abnormality including intra- or extrahepatic portosystemic shunting as an underlying etiology.
References
Towbin A, Newman B (2007) Syndromes and chromosonal anomalies. In: Slovis T (ed) Caffey’s pediatric diagnostic imaging. Mosby Elsevier, Philadelphia, pp 1605–1610
Papagiannis J, Kanter RJ, Effman EL et al (1993) Polysplenia with pulmonary arteriovenous malformations. Pediatr Cardiol 14:127–129
Kinane TB, Westra SJ (2004) Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 31-2004. A four-year-old boy with hypoxemia. New Engl J Med 351:1667–1675
De BK, Sen S, Biswas PK et al (2002) Occurrence of hepatopulmonary syndrome in Budd-Chiari syndrome and the role of venous decompression. Gastroenterology 122:897–903
Murray CP, Yoo SJ, Babyn PS (2003) Congenital extrahepatic portosystemic shunts. Pediatr Radiol 33:614–620
Alvarez AE, Ribeiro AF, Hessel G et al (2002) Abernethy malformation: one of the etiologies of hepatopulmonary syndrome. Pediatr Pulmonol 34:391–394
Gupta NA, Abramowsky C, Pillen T et al (2007) Pediatric hepatopulmonary syndrome is seen with polysplenia/interrupted inferior vena cava and without cirrhosis. Liver Transpl 13:680–686
Hoeper M, Krowka MJ, Strassburg CP (2004) Portopulmonary hypertension and hepatopulmonary syndrome. Lancet 363:1461–1468
Fewtrell MS, Noble-Jamieson G, Revell S et al (1994) Intrapulmonary shunting in the biliary atresia/polysplenia syndrome: reversal after liver transplantation. Arch Dis Child 70:501–504
Barbe T, Losay J, Grimon G et al (1995) Pulmonary arteriovenous shunting in children with liver disease. J Pediatr 126:571–579
Gurses D, Ulger Z, Levent E et al (2006) A very rare case of polysplenia syndrome with congenital diffuse pulmonary arteriovenous fistulas. Turk J Pediatr 48:96–99
McAdams HP, Erasmus J, Crockett R et al (1996) The hepatopulmonary syndrome: radiologic findings in 10 patients. AJR 166:1379–1385
Oh KS, Bender TM, Bowen A et al (1983) Plain radiographic, nuclear medicine and angiographic observations of hepatogenic pulmonary angiodysplasia. Pediatr Radiol 13:111–115
Krowka MJ, Dickson ER, Cortese DA (1993) Hepatopulmonary syndrome. clinical observations and lack of therapeutic response to somatostatin analogue. Chest 104:515–521
Krowka MJ (2000) Hepatopulmonary syndromes. Gut 46:1–4
Srivastava D, Preminger T, Lock JE et al (1995) Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation 92:1217–1222
Newman B, Effmann E (2007) Lung masses. In: Slovis T (ed) Caffey’s pediatric diagnostic imaging. Mosby Elsevier, Philadelphia, pp 1300–1303
Spencer LT, Langham MR, Hoyer MH et al (2000) Resolution of hypoxemia in a liver transplant recipient after ligation of a portosystemic shunt. J Pediatr 137:575–577
Ikeda S, Sera Y, Ohshiro H et al (1999) Surgical indications for patients with hyperammonemia. J Pediatr Surg 34:1012–1015
Alonso J, Sierre S, Lipsich J et al (2004) Endovascular treatment of congenital portal vein fistulas with the Amplatzer occlusion device. J Vasc Interv Radiol 15:989–993
Jones FD, Kuo PC, Johnson LB et al (1999) The coexistence of portopulmonary hypertension and hepatopulmonary syndrome. Anesthesiology 90:626–630
Ioachimescu OC, Mehta AC, Stoller JK (2007) Hepatopulmonary syndrome following portopulmonary hypertension. Eur Respir J 29:1277–1280
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newman, B., Feinstein, J.A., Cohen, R.A. et al. Congenital extrahepatic portosystemic shunt associated with heterotaxy and polysplenia. Pediatr Radiol 40, 1222–1230 (2010). https://doi.org/10.1007/s00247-009-1508-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-009-1508-y