Abstract
Background
Structural size in the neonatal brain is of clinical importance. Cranial ultrasonography (cUS) is the primary method used for evaluating the neonatal brain and it is important to know whether linear measurements made using this technique are accurate.
Objective
To compare linear measurements of different cerebral structures made from neonatal cUS and contemporaneous MRI.
Materials and methods
Preterm and term infants studies with cUS and MRI on the same day were studied. Linear measurements made using both techniques from many cerebral structures were compared using a paired t-test.
Results
A total of 44 sets of scans from 26 preterm and 8 term infants were assessed. Small but significant differences between the cUS and MRI measurements (P<0.05) were found for the ventricular index, the posterior horn depth of the lateral ventricle, the extracerebral space and interhemispheric fissure, and the cortex of the cingulate gyrus. No significant differences were found for any other measurements.
Conclusion
Linear measurements from cUS are accurate for most neonatal cerebral structures. Significant differences compared to MRI were found for a few structures, but only for the cortex were the absolute differences marked and possibly of clinical importance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Measurement of cerebral structures in the newborn infant brain can be of clinical importance for diagnosing cerebral abnormalities. Structural brain abnormalities can be quantified by performing linear measurements. Neuroimaging studies have shown that preterm infants at term-equivalent age have more subtle reduction in the size and maturation of many cerebral structures and an increased size of the lateral ventricles and cerebrospinal fluid volumes compared to term-born infants [1–4]. These differences between the preterm and term-born infant brain persist and are associated with less favourable neurodevelopmental outcomes for the preterm infant [1–5]. Several cranial ultrasound (cUS) and MRI studies on linear and volume measurements of different anatomical structures and tissues in the newborn infant and fetal brain are available in the literature [6–27]. These studies describe different techniques for performing the measurements on both cUS and MRI and give reference values for various cerebral structures [6–27].
It is generally considered that the measurements performed on cUS are consistent with those obtained from MRI, but few systematic studies have compared cUS with MRI and/or histological measurements of cerebral structures in the newborn infant or fetal brain [24, 26, 28]. Linear measurements of the height of the cerebellar vermis performed on cUS were not found to be consistent with those performed on MRI [24]. As sequential cUS is still the most commonly used and preferable technique for the evaluation of the newborn infant brain in the neonatal period and linear measurements can easily be done at the bedside, it is important to know whether the linear measurements of cerebral structures performed on cUS are accurate.
The aim of this study was, therefore, to explore whether the linear measurements of different cerebral structures performed on neonatal cUS are accurate, by comparing them with those obtained from contemporaneous MRI in the preterm and term-born infant brain.
Materials and methods
Patients
During the study period (February 2005 to February 2006) preterm and term-born infants who underwent a cUS and MRI examination on the same day were included. All infants were examined prior to scanning [29, 30] and infant growth characteristics were recorded. The cUS was performed just before or after the MRI examination as part of the routine assessment of the infant. Ethical approval for studying the newborn infant brain using MRI was given by the Hammersmith Hospitals Research Ethics Committee and parental consent was obtained in each case. The MRI examinations were performed as part of ongoing research cohort studies of apparently well preterm and normal term-born infants. Other infants were scanned for different clinical indications, including moderate ventriculomegaly, hypoxic-ischaemic encephalopathy and suspected metabolic and neuromuscular disorders.
Exclusion criteria were major congenital anomalies or serious acquired abnormalities of the central nervous system, chromosomal disorders, metabolic disorders, neonatal meningitis, severe hypoxic-ischaemic damage and neonatal stroke. Thus we did not include infants with major cerebral abnormalities.
Neuroimaging
Ultrasonography
All cUS scans were performed by the same observer (L.M.L.), using a Siemens Antares US scanner with a multifrequency transducer (Siemens Medical Solutions, Bracknell, UK). Scanning was done in at least six coronal and five sagittal planes, using the anterior fontanelle as the acoustic window. The transducer frequency was set at 7.3 MHz. For detection of cortical and/or subcortical abnormalities, higher frequencies were used. For deeper structures or in relatively large infants, a lower frequency was used. cUS scans were evaluated during and immediately after the procedure by the examiner and all scans were digitally stored and later evaluated by L.M.L. and F.C. Measurements were made later from the digitally stored images.
MRI
MRI was performed according to a standard MRI protocol for newborn infants using a 3.0-T Philips MR system (Philips, Best, The Netherlands). If needed, infants were given a sedative (oral chloral hydrate, 20–30 mg/kg), a safe and effective regimen [31]. They were laid supine and snugly swaddled. Ear protection comprised both individually moulded earplugs of a silicone-based putty (President putty, Coltene/Whaledent, Mahwah, N.J.) placed in the external ear and commercially available neonatal ear muffs (Natus MiniMuffs, Natus Medical, San Carlos, Calif.) placed over the ear. The head was stabilized using a pillow filled with polystyrene beads, from which the air was evacuated. The infant’s temperature, heart rate and oxygen saturation were monitored throughout the procedure, during which a neonatologist experienced in resuscitation and MR procedures was always present. For this study, images obtained in the sagittal plane acquired using T1-weighted MPRAGE sequences were used, but for all infants T2-weighted (T2-W), diffusion-weighted and often other sequences were also obtained. All MRI examinations were evaluated by two experienced observers (L.S. and M.R.) by consensus.
Linear measurements
All linear measurements on cUS were performed by L.M.L. using the software program Escape Medical Viewer (Escape Medical Imaging, Thessaloniki, Greece). The MR images were assessed using the software program ViewForum (Philips, Best, The Netherlands). With this program, different planes, including coronal and sagittal planes as obtained with cUS scanning, can be reconstructed, which enabled comparison of the cUS and MRI examinations. All linear measurements on MRI were performed by L.S. T1-weighted MPRAGE images were used as they are 3D volume scans and can be reconstructed in any plane and have been shown to be more sensitive for accurate structural measures. For the linear measurements, digital callipers capable of measuring to within 0.1 mm for cUS and 0.2 mm for MRI were used. For comparing the measurements, the MR image most closely akin to the cUS plane in which the cUS measurement was performed was used. We accept that neither MRI nor cUS can be considered as absolute measurements for in vivo tissue structures, but MRI is probably the optimal imaging modality we have for this purpose. Histological measurements are not available and post-mortem tissue is likely to be altered by change secondary to the cause of death and post-mortem artefacts.
cUS and MRI measurements were made on the following structures:
-
The corpus callosum: the width of the genu, body and splenium and length in the mid-sagittal plane (Fig. 1).
-
The cerebellum: the height and AP width of the vermis in the mid-sagittal plane and the maximum cerebellar hemispheric width in a coronal plane (Figs. 1 and 2).
-
The pons: the AP width in the mid-sagittal plane (Fig. 3).
-
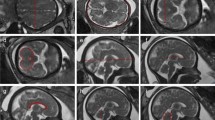
The lateral ventricles: the ventricular index (VI) in the mid-coronal plane and the depth (thalamo-occipital distance) of the posterior horns in a parasagittal plane (Figs. 4 and 5).
-
The cavum septum pellucidum: height and width in the mid-coronal plane (Fig. 6).
-
The fourth ventricle: width in the mid-sagittal plane (Fig. 3).
-
The interhemispheric fissure (IHF): width (maximum horizontal distance between the hemispheres, measured from the depth of sulci) in the mid-coronal plane (Fig. 6).
-
The extracerebral space (ECS): height (shortest vertical distance between the calvarium and the surface of the cerebral cortex) in the mid-coronal plane (Fig. 6).
-
The cortex: depth along the cingulate gyrus in a parasagittal plane, where the cortex is well defined running above and parallel to the mid of the corpus callosum (Fig. 7).
-
The basal ganglia: maximum transverse width in the mid-coronal plane (Fig. 8).
-
The caudate heads: maximum diagonal width in the mid-coronal plane (Fig. 8).
Most linear measurements on cUS and MRI were performed as described previously for newborn infants and fetuses [6–14, 19, 20, 22, 24]. For several linear measurements, no description was available in the literature. The width of the basal ganglia was measured from the midline of the brain to the most lateral border of the basal ganglia (i.e. lentiform nucleus), considered to be the most lateral border of the echogenic area on cUS and the high signal intensity area on MPRAGE images (Fig. 8). The caudate head was measured as the maximum diagonal width from the margin of the lateral ventricle to the outer border of the caudate head, considered to be the outer border of the echolucent area on cUS and the high signal intensity area on MPRAGE images (Fig. 8). The cortex was measured as the depth of the echolucent rim along the cingulate gyrus on cUS and the depth of the high signal intensity line on MPRAGE images (Fig. 7); the echolucent rim and the high signal intensity line are considered to reflect the cortex on, respectively, cUS and MRI. For each anatomical structure, three measurements were performed and, subsequently, the mean was recorded (in millimetres).
Data analysis
All linear measurements on contemporaneous cUS and MRI images were first performed simultaneously to optimize the measuring procedure and to obtain the most accurate comparison of cUS and MRI planes. Subsequently, the measurements on cUS were repeated blinded to the measure from MRI to determine whether there were significant differences between the cUS measurements performed simultaneously with MRI and those performed blinded. No significant differences or systematic errors (P>0.05) were detected between the simultaneous and blinded cUS measurements. The measurements obtained from the MRI were then used as reference values to explore the accuracy of the cUS measurements.
Statistical analyses were performed using StatsDirect statistical software (StatsDirect, Altrincham, UK). In order to explore the accuracy of cUS, as compared to MRI, for linear measurements of different cerebral structures a paired t-test and analysis of variances were performed for each parameter. To explore whether there is a correlation between the difference in cUS and MRI measurement and size of the cerebral structure and between the difference in cUS and MRI measurement and various infant growth characteristics, linear regression analyses were performed. The level of significance was taken at 0.05.
Results
Patients
Included in the study were 26 preterm infants (13 male) and 8 term-born infants (all male) who had a cUS and MRI examination on the same day. Mean gestational age (GA) and birth weight were, respectively, 29.5 weeks (range 24.4–32.7 weeks) and 1,230 g (range 586–1,848 g) for preterm infants and 39.4 weeks (38.0–40.6 weeks) and 3,218 g (2,986–3,400 g) for term-born infants. None had major pathology on the cUS or MRI examination. In 9 of the 26 preterm infants and 1 of the 8 term infants, two cUS and MRI examinations were performed on different days. The mean time interval between scans was 7.4 weeks (range 3.7–14.9 weeks). In total, 44 sets of examinations were analysed. Mean corrected GA (CGA) and weight on the day of scanning were, respectively, 37.5 weeks (range 27.1–57.0 weeks) and 2,296 g (range 870–4,210 g) for preterm infants and 42.9 weeks (38.9–49.0 weeks) and 4,007 g (3,127–5,450 g) for term-born infants.
cUS measurements and difference between cUS and MRI
The mean cUS measurements and the differences between the cUS and MRI measurements for the different cerebral structures are shown in Table 1. Significant differences between the cUS and MRI measurements (P<0.05) were found for the VI (left and right), the depth of the posterior horn of the lateral ventricle (left and right), the width of the IHF, the height of the ECS and the depth of the cortex along the cingulate gyrus. For all other measurements the P-value for the differences between the cUS and MRI measurements was >0.05. No difference in accuracy of the cUS measurements or the MRI measurements was found between the preterm and term-born infants. All cUS measurements of the different cerebral structures were appropriate for CGA on the day of scanning, as compared with reference values reported in the literature.
Difference in cUS and MRI measurement and size of cerebral structure
No significant positive or negative correlation was found between the differences in cUS and MRI measurements and size of the measured cerebral structure.
Difference in cUS and MRI measurement and infant growth characteristics
No significant positive or negative correlation was found between the differences in cUS and MRI measurements and GA, weight and head circumference at birth and CGA, weight and head circumference at day of scanning for any infant.
Discussion
Our study showed that linear measurements made from cUS are accurate as compared with measurements made from MR images for most cerebral structures and thus cUS can be reliably used for this purpose. Measurements of the size of the lateral ventricles, including the VI and the depth of the posterior horn, the height of the ECS, the width of the IHF and the depth of the cortex were significantly different from those made using MRI but the absolute differences were unlikely to be of clinical importance. The difference in cortical depth was consistent and more marked but so far this measure on cUS has not been used in a clinical context.
No significant differences in accuracy of the cUS measurements were found between preterm and term-born infants or in relation to their growth characteristics. All our cUS measurements were consistent with reference values in the literature [6, 8, 9, 11–25, 27]. Our study infants were heterogeneous with respect to GA at birth and CGA at scanning and the number of infants was relatively small, so our measurements cannot be used as normative reference values.
The inconsistency between cUS and MRI for measuring the lateral ventricles and the cortical depth was unexpected as these measurements appeared to be easy to make on both cUS and MRI. The difference in measurements of the ECS and IHF were less unexpected as the landmarks are more variable.
The VI of the lateral ventricles [6] measured on cUS is used to diagnose ventriculomegaly and to monitor ventricular size. By comparing measurements of the VI with expert clinical judgement of ventricular size in preterm infants with normal or mild to severely dilated lateral ventricles, Grasby et al. [32] showed that the VI correlates poorly with clinical judgement and has a low sensitivity for mild dilatation. The measurements of the ventricular height in a parasagittal plane, as described by London et al. [33], and the diagonal width of the frontal horns in a coronal plane, as described by Morony et al. [34], correlate well with expert judgement [32], probably because ballooning of the ventricles is an early sign of dilatation that is not identified by the VI. In addition, Horsch et al. [26] have shown that the ventricular height and frontal horn measurements on cUS correlate well with ventricular volume measurements on MRI. These measurements may therefore be more appropriate for assessing ventricular dilatation than the VI.
The depth of the posterior horn of the lateral ventricle has been suggested as a valuable alternative to the VI, especially as the posterior horn is the first to enlarge in posthaemorrhagic ventricular dilatation [35, 36] and usually the part of the ventricle most dilated antenatally. Consistent with the findings of Horsch et al. [26], we found a significant though small difference between cUS and MRI in measurements of the posterior horn of the lateral ventricles from the back of the thalamus to the furthest point of the occipital horn in an oblique parasagittal plane. The posterior horn was often difficult to visualize on MRI because of partial volume effects, and on MRI an oblique parasagittal plane is usually not obtained and is difficult to reconstruct; consequently, the full length of the posterior horn was difficult to measure. The apparent poor accuracy of cUS may partly have been due to the inaccuracy of the MRI measurements.
The inaccuracy of cUS for measuring the ECS and IHF is less surprising. Widening of the subarachnoid spaces (including ECS and IHF) can lead to difficulty in interpretation; it may be an indicator of cerebral pathology (e.g. atrophy), potential pathology (e.g. external hydrocephalus), or a normal developmental variant. Horsch et al. [4] have shown that preterm infants with signs of brain atrophy, including widening of the IHF, on cUS at discharge have a poorer neurodevelopmental outcome at 3 years of age. This makes accurate measurements of the ECS and IHF all the more important. We frequently encountered difficulties with cUS measurements of the ECS, mainly caused by loss of signal and presence of artefacts close to the transducer. Govaert et al. [7] used a higher frequency (10 MHz) and Libicher and Troger [9] magnified their cUS images to overcome this difficulty. We were also able to overcome this difficulty by using a higher frequency probe, and suggest using higher scanning frequencies when performing measurements of the ECS and IHF.
The measurements of the cortical depth on cUS were significantly and consistently greater than on MRI, despite the fact that the cortex seemed relatively easy to define on both cUS and MRI at the site we chose (Fig. 7). MRI studies on cortical depth have shown regional variation in preterm and very-low-birth-weight infants [37]. In addition, cortical surface area and volume are smaller in these infants than in term infants and associated with suboptimal neurodevelopment [37, 38]. Although the medial margin of the cortex, the echolucent rim, mostly appears to be clear on cUS, the subplate and possibly part the subcortical white matter may not be distinguished from the cortex (personal observation). Measuring the cortical depth on MRI has proven difficult and time-consuming [39, 40], and no easy and accurate method has been described. Measurements on MRI may also underestimate the cortical depth.
A possible explanation for the inconsistency between cUS and MRI measurements found in our study may be that cUS and MRI images are obtained at different angles and, consequently, identical planes cannot be obtained. On cUS coronal and sagittal images are obtained through the anterior fontanel angling out from the midline, whereas on MRI images are obtained in parallel horizontal and vertical planes. Most measurements were performed in the mid-sagittal and mid-coronal plane and MR images were reconstructed to obtain planes that closely resembled the cUS planes. A difference in angle could explain the inconsistency between measurements obtained in parasagittal planes, particularly the posterior horn depth. It could also explain the (non-significant) inconsistency between measurements of cerebral structures with an ovoid shape, such as the lateral ventricles, basal ganglia and caudate heads, or measurements with an ovoid structure as anatomical landmark, such as the thalamus for the measurement of the posterior horn depth. The ovoid shape of several cerebral structures is an intrinsic limit to complete reproducibility of the measurements of these structures.
Inconsistencies in ventricular measurements might result from differences in head position, inducing a shift of cerebrospinal fluid between ventricles. However, Davies et al. have showed that head position has no effect on the size of the lateral ventricles [13]. Therefore, this is an unlikely explanation for the inconsistency between ventricular measurements, but might partly account for differences in ECS measurements.
cUS and MRI are based on completely different methodologies and, consequently, the intrinsic contrast resolution of both techniques is completely different. This may account for some difference in the appearance of anatomical landmarks used for the measurements and difficulties in precisely defining the landmarks, particularly for the measurements of the cortical depth, ventricular width, height of the ECS and width of the IHF. To explore this, histological correlates would be necessary.
For several measurements, including the width of the basal ganglia and the diagonal width of the caudate heads, no reference values have, as yet, been reported. Atrophy of the deep grey matter occurs secondary to basal ganglia and/or white matter damage and is associated with neurodevelopmental delay and is thus of clinical importance. Although the basal ganglia and caudate heads are ovoid structures and therefore reproducible measurements may be more difficult, our experience was that the margins of these structures were not difficult to define visually either with cUS or MRI and that cUS was reliable compared to MRI for measuring the width of the basal ganglia and caudate heads, and we suggest that cUS can be used for early detection of deep grey-matter atrophy.
Anderson et al. [28] have suggested that measurements performed on cUS are limited in reflecting variations in cerebral structure. However, they compared and correlated linear measurements on two-dimensional cUS with volume measurements on three-dimensional MRI, which is difficult for various practical and technical reasons. In addition, they compared cUS and MRI examinations that were performed with a median time interval of 4 days (range 0–28 days) between scans, which might partly have caused the poor correlation between cUS and MRI for several measurements.
Limitations of our study were that sometimes exactly the same planes could not be compared and that the methodologies of the two imaging tools are different, making direct correlation of cUS and MRI for several measurements difficult. Secondly, we had no direct histological correlates and MRI measurements were used to explore the accuracy of cUS. However, we believe that as the MRI measurements were consistent with reference values in the literature, correlating cUS measurements with MRI measurements is valid for most cerebral structures. We did not perform cUS using a higher frequency in all infants, which might have overcome the limited accuracy of cUS in measuring the ECS. Finally, we did not include infants with major pathology, and it is important to determine that the consistency in measurements holds true when structures are abnormal. We have compared cUS and MRI measurements in eight infants, not included in this study, with hypoxic-ischemic encephalopathy associated with moderate cerebral abnormalities and found cUS to have an accuracy similar to that in infants with a normal brain.
Conclusion
Although more work is needed to optimize the linear measurements for which a limited accuracy of cUS was found, and reference values for several cerebral structures have, as yet, not been established, we found cUS a reliable tool for measuring most clinically important structures in the newborn infant brain. Not only is this useful for diagnosis of more major cerebral developmental abnormalities but it also allows the bedside assessment of more subtle alterations in growth that affect the preterm infant brain.
References
Cooke RW, Abernethy LJ (1999) Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch Dis Child 81:F116–F121
Peterson BS, Vohr B, Staib LH et al (2000) Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284:1939–1947
Inder TE, Warfield SK, Wang H et al (2005) Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294
Horsch S, Muentjes C, Franz A et al (2005) Ultrasound diagnosis of brain atrophy is related to neurodevelopmental outcome in preterm infants. Acta Paediatr 94:1815–1821
Lodygensky GA, Rademaker K, Zimine S et al (2005) Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 116:1–7
Levene MI (1981) Measurements of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 56:900–904
Govaert P, Pauwels W, Vanhaesebrouck P et al (1989) Ultrasound measurement of the subarachnoid space in infants. Eur J Pediatr 148:412–413
Mott SH, Bodensteiner KB, Allan WC (1992) The cavum septi pellucidi in term and preterm newborn infants. J Child Neurol 7:35–38
Libicher M, Troger J (1992) US measurement of the subarachnoid space in infants: normal values. Radiology 184:749–751
Iai M, Tanabe Y, Goto M et al (1994) A comparative magnetic resonance imaging study of the corpus callosum in neurologically normal children and children with spastic diplegia. Acta Paediatr 83:1086–1090
Anderson N, Wells E, Hay R et al (1996) Cerebellar vermis measurement at cranial sonography for assessing gestational age in the newborn weighing less than 2000 grams. Early Hum Dev 44:59–70
Jou HJ, Shyu MK, Wu SC et al (1998) Ultrasound measurement of the fetal cavum septi pellucidi. Ultrasound Obstet Gynecol 12:419–421
Davies MW, Swaminathan M, Chuang SL et al (2000) Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child 82:F218–F223
Makhoul IR, Goldstein I, Epelman M et al (2000) Neonatal transverse cerebellar diameter in normal and growth-restricted infants. J Matern Fetal Med 9:155–160
Davies MW, Swaminathan M, Betheras FR (2001) Measurement of the transverse cerebellar diameter in preterm neonates and its use in assessment of gestational age. Australas Radiol 45:309–312
Achiron R, Achiron A (2001) Development of the human fetal corpus callosum: a high-resolution, cross-sectional sonographic study. Ultrasound Obstet Gynecol 18:343–347
Lam WW, Ai VH, Wong V et al (2001) Ultrasonographic measurement of subarachnoid space in normal infants and children. Pediatr Neurol 25:380–384
Malinger G, Ginath S, Lerman-Sagie T et al (2001) The fetal cerebellar vermis: normal development as shown by transvaginal ultrasound. Prenat Diagn 21:687–692
Goldstein I, Makhoul IR, Tamir A et al (2002) Ultrasonographic nomograms of the fetal fourth ventricle: additional tool for detecting abnormalities of the posterior fossa. J Ultrasound Med 21:849–856
Zalel Y, Seidman DS, Brandt N et al (2002) The development of the fetal vermis: an in-utero sonographic evaluation. Ultrasound Obstet Gynecol 19:136–139
Serhatlioglu S, Kocakoc E, Kiris A et al (2003) Sonographic measurement of the fetal cerebellum, cisterna magna, and cavum septum pellucidum in normal fetuses in the second and third trimesters of pregnancy. J Clin Ultrasound 31:194–200
Achiron R, Kivilevitch Z, Lipitz S et al (2004) Development of the human fetal pons: in utero ultrasonographic study. Ultrasound Obstet Gynecol 24:506–510
Chavez MR, Ananth CV, Smulian JC et al (2004) Fetal transcerebellar diameter measurement with particular emphasis in the third trimester: a reliable predictor of gestational age. Am J Obstet Gynecol 191:979–984
Triulzi F, Parazzini C, Righini A (2005) MRI of fetal and neonatal cerebellar development. Semin Fetal Neonatal Med 10:411–420
Anderson NG, Laurent I, Cooke N et al (2005) Growth rate of corpus callosum in very premature infants. AJNR 26:2685–2690
Horsch S, Bengtsson J, Nordell A et al (2006) Lateral ventricular size in extremely premature infants: 3D MRI confirms 2D ultrasound measurements. Proceedings of the Annual Meeting of the Pediatric Academic Societies, San Francisco, CA
Anderson NG, Laurent I, Woodward LJ et al (2006) Detection of impaired growth of the corpus callosum in premature infants. Pediatrics 118:951–960
Anderson NG, Warfield SK, Wells S et al (2004) A limited range of measures of 2-D ultrasound correlate with 3-D MRI cerebral volumes in the premature infant at term. Ultrasound Med Biol 30:11–18
Mercuri E, Guzzetta A, Laroche S et al (2003) Neurologic examination of preterm infants at term age: Comparison with term infants. J Pediatr 142:647–655
Dubowitz L, Ricciw D, Mercuri E (2005) The Dubowitz neurological examination of the full-term newborn. Ment Retard Dev Disabil Res Rev 11:52–60
Cowan FM (1998) Sedation for magnetic resonance scanning of infants and young children. In: Whitwam JG, McCloy RF (eds) Principles and practice of sedation. Blackwell Healthcare, London, pp 206–213
Grasby DC, Esterman A, Marshall P (2003) Ultrasound grading of cerebral ventricular dilatation in preterm infants. J Paediatr Child Health 39:186–190
London DA, Carroll BA, Enzmann DR (1980) Sonography of ventricular size and germinal matrix hemorrhage in premature infants. AJNR 1:295–300
Morony S, Marshall P, Langlois S (1984) Periventricular haemorrhage and ventricular dilatation detected by real time ultrasound in infants <1500 g birthweight. Aust Paediatr J 20:252
Silverboard G, Horder MH, Ahmann PA et al (1980) Reliability of ultrasound diagnosis of intracerebral hemorrhage and posthemorrhagic hydrocephalus: comparison with CT. Pediatrics 66:507–514
Brann BS, Qualls C, Wells L et al (1991) Asymmetric growth of the lateral cerebral ventricle in infants with posthemorrhagic ventricular dilatation. J Pediatr 118:108–112
Martinussen M, Fischl B, Larsson HB et al (2005) Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain 128:2588–2596
Ajayi-Obe M, Saeed N, Cowan FM et al (2000) Reduced development of cerebral cortex in extremely preterm infants. Lancet 356:1162–1163
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055
Kabani N, Le Goualher G, MacDonald D et al (2001) Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage 13:375–380
Acknowledgements
This research was undertaken with financial support from The Doctor Catharina van Tussenbroek Foundation, The Academy of Medical Science, The Health Foundation, Philips Medical Systems, and the March of Dimes Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leijser, L.M., Srinivasan, L., Rutherford, M.A. et al. Structural linear measurements in the newborn brain: accuracy of cranial ultrasound compared to MRI. Pediatr Radiol 37, 640–648 (2007). https://doi.org/10.1007/s00247-007-0485-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-007-0485-2