Abstract
Background
Quantitative CT shows promise as an outcome measure for cystic fibrosis (CF) lung disease in infancy, but must be accomplished at a dose as low as reasonably achievable.
Objective
To determine the feasibility of ultra-low-dose CT for quantitative measurements of airway dimensions.
Materials and methods
Two juvenile pigs were anesthetized and their lungs scanned at 25 cm H2O face-mask pressure in apnoea using beam currents of 5, 10, 20, 40 and 100 mAs. The lumen diameters and wall thicknesses of matched airways (n=22) at each dose were measured by two observers using validated software. Measurement variability at each dose was compared to that at 100 mAs (reference dose) for large and small airways (lumen diameter <2.5 mm).
Results
Lowering CT dose (mAs) affected measurement variability for lumen diameter of small and large airways (P<0.001) and for wall thickness of small (P<0.001), but not large (P=0.63), airways. To obtain the same measurement variability at 5 mAs as at 100 mAs, four to six small airways or one to three large airways have to be measured and averaged.
Conclusion
Quantitative airway measurements are feasible on images obtained at as low as 5 mAs, but more airways need to be measured to compensate for greater measurement variability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Airway abnormalities can be visualized and quantified by CT in children [1, 2] and infants [3] with cystic fibrosis (CF). Recent studies of CT in children with CF have demonstrated greater sensitivity of CT in identifying airway abnormalities compared to pulmonary function tests [4, 5], which suggests that CT will have an important role as a surrogate outcome measure in CF lung disease [6, 7]. The increasing use of routine CT scanning in children has raised concerns about the risks of cumulative radiation exposure [8, 9]. Infants and children are more susceptible to radiation than adults, and therefore CT examinations must be acquired at as low a radiation dose as reasonable achievable [10–13]. The radiation risk per CT scan is closely related to the beam current in milliamperes per second (mAs).
The long-term success of CT as an outcome measure depends on shifting the emphasis from qualitative to quantitative assessment to make the data more reproducible and amenable to statistical analysis. There is reason to believe that quantitative assessments can be performed at ultra-low radiation doses; however, the relationship between mAs and the measurement variability of quantitatively measured airway and lumen dimensions is unknown.
The purpose of this study was to assess the feasibility of ultra-low-dose quantitative measurements of wall and lumen dimensions of large and small airways in a piglet infant animal model.
Materials and methods
Acquisition of CT scans
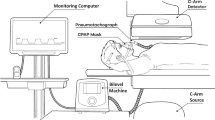
Two pigs of approximately 3,500 g were used for this study according to an approved IACUC (Institutional Animal Care and Use Committee) protocol. The pigs were pre-anaesthetised with intramuscular tiletamine/zolazepam (4.4 mg/kg) and xylazine (2.2 mg/kg). Once an adequate level of anaesthesia was obtained, the pigs were intubated and the sedation was maintained by inhalation with isoflurane (1–3% inhalation) supplemented with vecuronium. Heart rate and oxygen saturation were monitored throughout the procedure. CT scans were performed on a GE Light Speed Plus scanner (General Electric Medical Systems, Milwaukee, Wis.) using the helical technique with eight detectors. All scans were acquired at 120 kVp, slice collimation of 1.25 mm, gantry rotation 0.5 s, and table speed of 13.5 mm/s. Scans were obtained at 100, 40, 20, 10 and 5 mAs (Fig. 1). Between each scan, the pigs were manually ventilated using the existing endotracheal tube. When scanning, lungs were held at full inflation near total lung capacity with a system incorporating a pressure-release valve to control distending pressure at 25 cm water pressure as previously described to obtain chest CT scans in infants [14]. All images were reconstructed at 1.25 mm using a high-frequency bone algorithm (General Electric Medical Systems) and at 5 mm in a standard algorithm with a field of view (FOV) of 250 mm, with a corresponding pixel size of 0.49×0.49 mm. The 1.25-mm images were used for airway measurements and the 5-mm images were used for the measurement of intrapulmonary gas volume.
Quantitative CT analysis
All clearly visible airways on the 100-mAs scan series which were round with cross-sections perpendicular to the z-axis were measured and served as reference scans. The same airways on the 40, 20, 10 and 5-mAs series were matched to those on the reference scans (see Fig. 1). Airway wall area (WA) and lumen area (LA) were measured using custom-validated software by two independent observers for each airway [15–17]. Briefly, the software works as follows. First, a cross-sectional cut airway or artery is identified and enlarged. Next, a seed-point is placed in the centre of the airway or arterial lumen. From this point, 64 radial rays are automatically cast from the centre of the lumen outward. The computer allows the observer to delete rays that are evidently too long or too short. Airway wall and lumen edges are defined using the full-width at half-maximum principle and assessed from 64 radial rays cast from the centre of the lumen into the parenchyma on cross-sectional cut airways [15–17]. The software calculates WA and LA that we converted into more meaningful and easier to understand dimensions, airway wall thickness (AWT) and airway lumen diameter (LD), using the following formulas: LD=2*square root(LA)/π and AWT=0.5*((2*square root(WA+LA)/π)−(LD)) [18]. Gas volume was measured from CT using a previously described method [19, 20]. Briefly, the lung was segmented from the chest wall and surrounding structures and the lung volume was calculated by summing the voxels within the lung.
Statistical analysis
Interobserver variability was calculated for airway wall thickness and lumen diameter by expressing the difference between the two observers as a percentage of the average of the two observers. This percentage was plotted against the mean lumen diameter to detect systematic errors depending on airway size [21].
Measurement variability between airway wall thickness and lumen diameter at the various doses (5, 10, 20 and 40 mAs) relative to 100 mAs was determined using variance analysis models. The measurement variability was expressed as standard error of the mean (SEM) ± standard error (SE), and was also expressed as the number of airways that would need to be measured for there to be equivalent variability at different doses. Airways were grouped into small airways (lumen diameter smaller than or equal to 2.5 mm) and large airways (lumen diameter greater than 2.5 mm). Therefore variance models were performed separately for large and small airway groups for both airway wall thickness and lumen diameter. For all models the average of the measurements of the two observers was used. Data were analysed using Proc Mixed in SAS (version 8e, SAS Institute, Cary, N.C.). Significance was assumed for P-values less than 0.05.
Results
The volume of gas in the lungs at total lung capacity was 491 and 365 ml. We could match 11 airways on both lower and higher dose images. These 11 airways were measured in each pig. The mean±SD lumen diameter of the 22 airways was 2.7±1.7 mm (range 0.96–6.6 mm).
Interobserver variability
Figure 2 shows the interobserver variability against airway size at the various doses (5, 10, 20, 40 and 100 mAs) for airway wall thickness and lumen diameter. The mean (SD) interobserver differences for airway lumen diameter were 0.49% (2.95), 0.66% (3.11), 0.45% (4.28), 2.41% (9.86) and 0.34% (4.65) for the 100, 40, 20, 10 and 5 mAs doses, respectively. The mean (SD) interobserver differences in airway wall thickness were 0.89% (6.01), 3.15% (5.8), 2.88% (8.35), 5.85% (14.2) and 2.90% (9.95) for the 100, 40, 20, 10 and 5 mAs doses, respectively.
Airway lumen measurement variability
Measurement variability in lumen diameters was directly related to dose for both small airways (P<0.001) and large airways (P<0.001; Table 1). The same measurement variability as in the 100 mAs series for lumen diameter measurements of small airways was obtained by measuring and averaging 5.4, 6.6, 6.0 and 2.8 small airways at 5, 10, 20 and 40 mAs, respectively. To obtain the same measurement variability for lumen diameter measurements of large airways relative to the 100 mAs series 2.0, 3.0, 0.2 and 1.6 large airways had to be measured and averaged at 5, 10, 20 and 40 mAs, respectively.
Airway wall measurement variability
The variability in measurements of wall thickness was directly related to dose for small airways (P<0.001), but not for large airways (P=0.63; Table 2). The same measurement variability as in the 100 mAs series for airway wall thickness measurements of small airways was obtained by measuring and averaging 6.0, 4.7, 2.4 and 1.4 small airways at 5, 10, 20 and 40 mAs, respectively. To obtain the same measurement variability as in the 100 mAs series for airway wall thickness of large airways relative to 100 mAs, the same numbers of large airways had to be measured at 5, 10, 20 and 40 mAs, respectively.
Discussion
If CT is to be used as an outcome measure for CF lung disease in children, ultra-low-dose protocols will be mandatory to diminish the long-term risk of secondary radiation-induced malignancy [8]. We tested the feasibility of ultra-low-dose CT by examining the resulting variance of measured airway structures over a range of radiation doses. This necessitated an animal model. In our animal model, we used the same CT scanning technique that we currently employ in infants where motion-free images are obtained at full inflation using face-mask pressure during induced apnoea as previously described [14]. The intrapulmonary gas volumes at these pressures were similar to those in human infants [22].
The major finding of this study is that airway measurements were feasible for both observers over the range of doses studied, 100 to 5 mAs. Although image noise increased with decreasing dose the observers were able to resolve and measure the lumen of the airways even at 5 mAs. The only requirement of the quantitative measurement program is the selection of the lumen of cross-sectioned airways, which was feasible at all doses used in this study.
As expected, measurement variability of airway lumen diameter and wall thickness increased with decreasing dose, except for wall thickness measurements in the larger airways. The increase in measurement variability became most pronounced at doses of 20 mAs or less. There was some uncertainty as to whether or not measurement variability in wall thickening significantly increased with dose in the larger airway subgroup (>2.5 mm). This may have been related to the relatively high interobserver variability for the airway wall measurements. Another factor may have been that our methodology assumed that the airway was a perfect circle, which is not the case in reality. The error associated with non-linearity may be greatest for wall thickness measurements of larger airways. Therefore, such a larger measurement error might have masked largely the effect of dose. Because this was a quantitative study, we were able to estimate the effect of this variability in practical terms that could be used in determining power calculations for research studies. At the lowest dose of 5–20 mAs, approximately four to six airways in the <2.5 mm subgroup would have to be averaged to obtain the same measurement variability for lumen diameter and wall thickness as at 100 mAs.
Most current protocols for high-resolution CT in infants utilize a kVp of 120 and mAs of 20 [23], although a decade ago mAs could reach 120. Our data suggest that a fourfold dose reduction to 5 mAs may be feasible. This additional reduction could be important to further develop CT measurements as a surrogate endpoint in therapeutic trials, especially for infants and children with CF. Since we aimed in this study to determine the relationship between noise and mAs we used 100 mAs, and not 20 mAs, as a reference dose for statistical analysis.
Study limitations
Measurement variability will be affected by a number of factors, and these should be kept in mind when considering the results of this study. The most important factor is the resolution of the CT scanner. Resolution depends on available slice collimation, FOV, pitch, reconstruction algorithm and presence of respiratory motion, in addition to radiation dose. We used a high-resolution scan protocol that we routinely employ for clinical studies in infants. Since this study was performed, CT scanners with double the resolution have become available. The results for ultra-low-dose imaging for the small airways would be expected to improve with these higher resolution CT scanners.
Measurement variability also depends on the lung volumes at which measurements are made. There is likely some variability inherent in our data from slight differences in lung volume between scan series; however, this is likely limited because the images were acquired near total lung capacity and without respiratory motion. We did not test measurement variability during quiet breathing. With the fast scan times of the newest generation of multidetector CT scanners that dampen the effects of respiratory motion, it is conceivable that accurate measurements could be obtained even at low doses, but this has not been investigated. One problem that would be anticipated in making measurements during quiet breathing (volumes near functional residual capacity) is that the airway lumens may not be adequately distended, inducing inaccuracy that would be difficult to control for.
In this study, kVp was kept constant and only mAs was varied. In clinical practice, both kVp and mAs may be varied. The effect on measurement accuracy of lowering kVp in addition is not known but we would anticipate that its effect would be directly related to the applied radiation exposure of whatever combination of kVp and mAs is used. In fact, some institutions changed infant CT protocols to 100 kVp and 80 kVp after we had finished our experiments. Nevertheless, our data do demonstrate a relationship between noise and mAs, which was our aim.
Conclusion
In conclusion, we showed that small and large airway wall and lumen measurements are feasible on CT scans obtained at doses as low as 5 mAs. In order to obtain the same measurement variability, however, a larger number of airways have to be measured.
References
de Jong PA, Ottink MD, Robben SG, et al (2004) Pulmonary disease assessment in cystic fibrosis: comparison of CT scoring systems and value of bronchial and arterial dimension measurements. Radiology 231:434–439
de Jong PA, Nakano Y, Hop WC, et al (2005) Changes in airway dimensions on computed tomography scans of children with cystic fibrosis. Am J Respir Crit Care Med 172:218–224
Long FR, Williams RS, Castile RG (2004) Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr 144:154–161
de Jong PA, Lindblad A, Rubin L, et al (2006) Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax 61:80–85
de Jong PA, Nakano Y, Lequin MH, et al (2004) Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J 23:93–97
Brody AS, Tiddens HA, Castile RG, et al (2005) Computed tomography in the evaluation of cystic fibrosis lung disease. Am J Respir Crit Care Med 172:1246–1252
Robinson TE (2004) High-resolution CT scanning: potential outcome measure. Curr Opin Pulm Med 10:537–541
de Jong PA, Mayo JR, Golmohammadi K, et al (2006) Estimation of cancer mortality associated with repetitive computed tomography scanning. Am J Respir Crit Care Med 173:199–203
de Jong PA, Nakano Y, Lequin MH, et al (2006) Dose reduction for CT in children with cystic fibrosis: is it feasible to reduce the number of images per scan? Pediatr Radiol 36:50–53
Brenner D, Elliston C, Hall E, et al (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR 176:289–296
Brenner DJ (2002) Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol 32:228–233; discussion 242–244
Mayo JR, Aldrich J, Muller NL (2003) Radiation exposure at chest CT: a statement of the Fleischner Society. Radiology 228:15–21
Slovis TL (2002) The ALARA concept in pediatric CT: myth or reality? Radiology 223:5–6
Long FR, Castile RG, Brody AS, et al (1999) Lungs in infants and young children: improved thin-section CT with a noninvasive controlled-ventilation technique – initial experience. Radiology 212:588–593
Nakano Y, Whittall KP, Kalloger SE, et al (2002) Development and validation of human airway analysis algorithm using multidetector row CT. Proceedings of SPIE 4683:460–469
Nakano Y, Muro S, Sakai H, et al (2000) Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 162(3 Pt 1):1102–1108
Nakano Y, Wong JC, de Jong PA, et al (2005) The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 171:142–146
de Jong PA, Long FR, Wong JC, et al (2006) Computed tomographic estimation of lung dimensions throughout the growth period. Eur Respir J 27:261–267
Coxson HO, Mayo JR, Behzad H, et al (1995) Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol 79:1525–1530
Coxson HO, Rogers RM, Whittall KP, et al (1999) A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med 159:851–856
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Castile RG, Iram D, McCoy KS (2004) Gas trapping in normal infants and in infants with cystic fibrosis. Pediatr Pulmonol 37:461–469
Long FR (2005) Imaging evolution of airway disorders in children. Radiol Clin North Am 43:371–389
Acknowledgements
The authors want to express their thanks to Robert Short and Lisa Stout for assistance with the CT scanning and Harvey Coxson and Ahn-Tuan Tran of the Vancouver General Hospital (Vancouver, BC, Canada) for their invaluable expertise with quantitative CT analysis and help with measuring airway–artery pairs. The authors are also indebted to Gary Phillips of the Biostatistical Department of Ohio State University who performed the statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Jong, P.A., Long, F.R. & Nakano, Y. Computed tomography dose and variability of airway dimension measurements: how low can we go?. Pediatr Radiol 36, 1043–1047 (2006). https://doi.org/10.1007/s00247-006-0264-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-006-0264-5