Abstract
Background
MRI and FDG-PET may predict the histological grading of paediatric brain-stem gliomas.
Objective
To assess MRI findings and metabolic imaging using FDG-PET of brain-stem gliomas based on histological grading.
Materials and methods
Included in the study were 20 paediatric patients (age 3–14 years, mean 8.2 years) with brain-stem glioma (five glioblastomas, ten anaplastic astrocytomas and five low-grade astrocytomas). MR images were assessed for the anatomical site of tumour origin, focality, pattern of tumour growth, and enhancement.
Results
All glioblastomas were located in the pons and showed diffuse pontine enlargement with focally exophytic features. Eight anaplastic astrocytomas were located in the pons and demonstrated diffuse pontine enlargement without exophytic features. Low-grade astrocytomas were located in the pons, midbrain or medulla and showed focally exophytic growth features and peripheral enhancement. In 12 patients in whom FDG-PET was undertaken, glioblastomas showed hypermetabolic or hypometabolic lesions, anaplastic astrocytomas showed no metabolic change or hypometabolic lesions and low-grade astrocytomas showed hypometabolism compared with the cerebellum.
Conclusion
MRI findings correlated well with histological grading of brain-stem gliomas and MRI may therefore predict the histological grading. FDG-PET may be helpful in differentiating between anaplastic astrocytoma and glioblastomas among high-grade tumours.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brain-stem tumours constitute 10–20% of primary intracranial neoplasms in childhood and 25% of all posterior fossa tumours in childhood [1–4]; almost 90% are glial neoplasms [4–8]. Until 15–20 years ago, brain-stem gliomas were considered inoperable and patients were treated with steroids and radiation. The prognosis was uniformly very poor, with survival rarely exceeding 1–2 years, except in a very few exceptional cases [4].
With the advent of MRI, which is superior to CT for defining tumour borders, it has become evident that brain-stem gliomas are a heterogeneous group of neoplasms divisible into distinct subgroups and that some are surgically resectable with minor morbidity [4, 6, 8–10]. In recent years, the heterogeneity of this group of neoplasms has been recognized and prognostic factors have been identified. Poor prognostic features include symptom duration of less than 6 months, positive findings in two of three groups of neurological signs (cranial nerve deficits, long tract and cerebellar signs) and diffuse infiltration of the brain stem [11]. On CT scans, hypodensity prior to contrast medium administration has been considered a poor prognostic feature. More favourable prognostic features include symptom duration of greater than 12 months before diagnosis, exophytic location, and pathology suggesting a low-grade tumour [12].
There are few reports of the preoperative prediction of histological grading of brain-stem gliomas. The purpose of this study was to assess MR findings and metabolic imaging using 18F-fluorodeoxyglucose PET (FDG-PET) of brain-stem gliomas based on histological grading and to predict the prognosis of brain-stem gliomas preoperatively.
Materials and methods
Patient population
We reviewed the MRI and PET findings of 20 children who underwent surgery or stereotactic biopsy for brain-stem glioma at our hospital. We excluded thalamic or tectal gliomas. There were 14 boys and 6 girls with an age range of 3–14 years (mean 8.2 years). Preoperatively, MRI was performed in all patients and FDG-PET in 12 patients.
MRI
Standard MRI was performed on a 1.0-T unit (Expert, Siemens, Erlangen, Germany) or a 1.5-T unit (Vision, Siemens; or Signa, GE Medical Systems, Milwaukee, Wis.). T1-weighted (T1-W) images in the sagittal and axial planes and T2-weighted (T2-W) images in the axial plane were available in all patients. Images obtained after the intravenous administration of 10 ml gadolinium ditriamine penta-acetate (Gd-DTPA; Omniscan, Amersham Health, Cork, Republic of Ireland) were available in all patients.
PET
Axial raw data were obtained on a PET scanner (ECAT Exact; CTI/Siemens, Knoxville, Tenn.) 60 min after intravenous injection of 18FDG (3.7 MBq/kg). Acquisition time was approximately 20 min. Axial images were reconstructed with a Shepp-Logan filter (cut-off frequency, 0.35 cycles per pixel) and realigned in coronal and sagittal planes. Spatial resolution was 6.1×6.1×4.3 mm.
Image interpretation and data analysis
Blinded reinterpretation of MR images and PET scans was performed without knowledge of the results of clinical examination or other imaging findings. The diagnostic criteria for the two imaging methods were based on qualitative visual interpretation.
Experienced neuroradiologists interpreted the MR images. Both T1-W and T2-W images were assessed for the anatomical site of tumour origin (midbrain, pons, medulla), pattern of tumour growth (diffuse vs. focal), direction and extent of tumour growth (longitudinal infiltration beyond the brain-stem segment of origin; axial involvement of ventral, lateral and/or dorsal aspects of the brain stem), degree of brain-stem enlargement, direction and degree of exophytic growth if present, signal intensity of the tumour as compared with surrounding brain parenchyma, presence and degree of hydrocephalus, and the presence or absence of cysts, haemorrhage or necrosis. We assessed the presence or absence of enhancement and the pattern of enhancement (focal, ring or peripheral) if present. For the most part, our definitions were consistent with those suggested by Barkovich et al. [13]. A focal tumour was defined as one that was sharply marginated and occupies less than one half of the involved brain-stem segment, but could extend longitudinally to a single adjacent segment so long as it remained sharply marginated and appeared to compress and displace rather than infiltrate adjacent brain parenchyma. In contrast, a diffuse tumour was defined as one that was poorly marginated, involving more than one-half of the involved brain-stem segment, or infiltrating the segments of brain both superior and inferior to the segment of origin [3]. The degree of brain-stem enlargement and the degree of hydrocephalus were determined subjectively.
We assessed the metabolic imaging of tumours using FDG-PET and comparison with the cerebellum. Lesions showing increased isotope concentration on the PET images by comparison with surrounding cerebellum were characterized as hypermetabolic. Conversely, if the isotope concentration in the lesion was low, this was characterized as hypometabolic.
Results
Surgery and pathology
Surgical resections were performed in 7 children, and stereotactic biopsies were obtained in 13. The biopsy sites were targeted at solid, non-necrotic and sometimes enhancing areas. Histological grades were based on the degree of anaplasia or dedifferentiation, number of mitoses, and presence of necrosis. Histopathological studies confirmed low-grade astrocytomas (grade I/II) in five patients, anaplastic astrocytomas (grade III) in ten patients, and glioblastoma (grade IV) in five patients.
Clinical features
The patients’ symptoms included gait disturbance, headache, dysarthria and hemiparesis. No specific symptom was correlated to histological grade (Table 1). Symptom duration of all brain-stem gliomas was 6 days to 7 years (mean 190 days). Patients with glioblastoma had symptom durations of 6 days to 4 months (mean 32.6 days), those with anaplastic astrocytoma 10 days to 8 months (mean 59 days), and those with low-grade astrocytoma 2 months to 7 years (mean 608 days).
MR imaging
All the tumours showed high signal intensities similar to that of CSF on T2-W images and heterogeneous or homogeneous low signal intensity on T1-W images.
The glioblastomas (n=5) were located in the pons and showed diffuse pontine enlargement with focally exophytic features (Fig. 1). Four glioblastomas had internal ring enhancement with central necrosis (Fig. 1), and one had homogeneous signal intensity without enhancement (Table 1). Four glioblastomas had intratumoral necrosis.
Glioblastoma in a 5-year-old boy with gait disturbance (patient 1). a Axial T2-W image demonstrates bulbous enlargement of the pons, a cyst-like lesion in the posterior pons and irregular patchy high signal intensity in the anterior pons. b Axial contrast-enhanced T1-W image shows irregular ring-like enhancement in the posterior pons and diffuse non-enhancing low signal intensity in the anterior pons. Tumour extends dorsally into the fourth ventricle. c Axial FDG-PET image shows a focal intense hypermetabolic ‘C-shaped’ lesion on the right and posterior side of the pons at the site of gadolinium enhancement
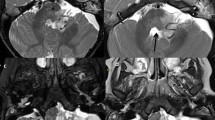
Eight anaplastic astrocytomas were located in the pons and demonstrated diffuse pontine enlargement without exophytic growth or necrosis (Fig. 2). Three of ten anaplastic astrocytomas demonstrated spotty or patchy enhancement within the tumour and seven showed no enhancement (Fig. 2). One anaplastic astrocytoma in the medulla grew dorsally with an exophytic component and showed peripheral enhancement with central necrosis. Hydrocephalus was present in three patients; intratumoral cyst and necrosis were present in one patient.
Anaplastic astrocytoma in a 10-year-old boy with ataxic gait and vomiting (patient 8). a Axial T2-W image demonstrates diffuse pontine enlargement with homogeneously high signal intensity. There is no focal lesion or exophytic feature. b Sagittal contrast-enhanced T1-W image shows a globule-shaped, diffuse low signal intensity lesion occupying the pons that does not enhance. c Axial FDG-PET image shows no hypermetabolism in the brain-stem mass. There is subtle, but not definite, hypometabolism in the brain stem
Low-grade astrocytomas were located in the pons (n=1), midbrain (n=1) or medulla (n=3). Low-grade astrocytomas had features of focally exophytic growth (Fig. 3) except one, and showed peripheral or inner ring enhancement (Fig. 3). The centre of the tumours was located peripherally in the brain stem in contrast to its location in glioblastomas and anaplastic astrocytomas, and enlargement of the brain stem was seen in only one patient. They grew toward the fourth ventricle or cerebellum resulting in hydrocephalus (n=3). Two patients had necrosis, one an intratumoral cyst and two showed intratumoral haemorrhage.
Low-grade astrocytoma in an 8-year-old boy with nausea, vomiting and headache (patient 20). a Axial T2-W image demonstrates a heterogeneous high signal intensity mass on the right side of the medulla. The tumour extends to the right cerebellum. b Sagittal contrast-enhanced T1-W image shows peripheral enhancement with internal ring enhancement. Necrosis is also seen. c Axial FDG-PET image shows a hypometabolic lesion in the right cerebellum and medulla
PET imaging
In 12 patients undergoing FDG-PET, the glioblastomas were hypermetabolic (n=2) (Fig. 1) or hypometabolic (n=2). The hypermetabolic glioblastomas showed focal ring enhancement and the hypometabolic tumours showed no or peripheral enhancement on MRI. Anaplastic astrocytomas showed no metabolic change (n=2) or hypometabolic lesions (n=4) (Fig. 2), while low-grade astrocytomas showed hypometabolism (n=2) (Fig. 3).
Discussion
The brain stem comprises the midbrain, pons and medulla oblongata, and performs several vital functions such as the control of respiration. Tumours in these locations are seldom biopsied and the majority of patients are treated with radiotherapy without histological confirmation [14].
Brain-stem gliomas most commonly originate in or involve the pons (54%), followed by the medulla (32%); infrequently they involve the midbrain. Brain-stem glioma is a progressive disease with a median survival of only 4–15 months. Most tumours (75%) occur before the age of 10 years, with the median age of presentation being approximately 6 years. Predominantly males are affected. Even with radiation and chemotherapy, the overall 5-year survival among those with histologically documented lesions is 5% [15]. Although the prognosis remains poor, there have been minimal improvements in the long-term survival of patients with low-grade brain-stem gliomas directly attributable to adjuvant therapy [9, 16].
High-grade brain-stem gliomas tend to infiltrate diffusely in intimate association with the existing neurons and blood vessels. Histological grade is based on the degree of anaplasia and dedifferentiation, the number of mitoses, capillary endothelial proliferation, and the presence of necrosis [15, 17, 18]. Anaplastic astrocytomas tend to be more cellular and to have significant nuclear pleomorphism and atypia. The most important criteria for the diagnosis of anaplasia is the presence of necrosis and endothelial proliferation [19].
The management of focal astrocytoma is difficult, because the tumour is biologically benign and potentially curable, but is located entirely in the brain stem, making surgery very dangerous. There is agreement in the literature that diffuse tumours should not be treated with surgery because this does not improve outcome and is associated with high mortality and morbidity. Biopsy of the tumour (open or stereotactic) is also not indicated, since the diagnosis can usually be made on the basis of the clinical history and radiological investigations [4].
There are many reports of the correlation of MRI appearance with clinical outcome [1, 4, 5]. However, there are few reports of the correlation of MRI appearance with histological grade of brain-stem gliomas. Fishbein et al. [3] categorized brain-stem gliomas by assessing the primary level of origin and the pattern of growth. They proposed six groups: focal midbrain tumours, diffuse midbrain tumours, tectal tumours, focal pontine tumours, diffuse pontine tumours, and cervicomedullary tumours. The diffuse pontine glioma is the most common form of brain-stem tumour and carries the poorest prognosis [3]. The tumour has its epicentre in the pons and infiltrates the brain-stem axis without involving the fourth ventricle [5]. Our study also demonstrated high-grade astrocytomas, i.e. glioblastoma and anaplastic astrocytoma, which showed diffuse pontine enlargement and low-grade astrocytomas which showed focally exophytic growth without pontine enlargement.
Cervicomedullary, exophytic and cystic gliomas should be treated with surgery, since these are mainly low-grade astrocytomas that compress the neural structures but do not infiltrate them [4]. Brain-stem gliomas that have focal involvement or are exophytic may have a more favourable prognosis in that they tend to be low-grade and are often amenable to surgical resection [15, 17, 18, 20]. By definition, focal tumours are tumours of limited size (less than 2 cm) that are well circumscribed, without evidence of infiltration, and without oedema on MR imaging. Focal tumours may occur at any level in the brain stem but are most frequently seen in the midbrain and medulla [8]; in our study, three low-grade astrocytomas were located in the medulla, one in the midbrain and only one in the pons.
In a previous study, the presence or absence of enhancement after the administration of paramagnetic contrast medium had no significant correlation with outcome [3]. We consider that the type of enhancement is related to histological grade, and found that glioblastomas usually have internal dense ring enhancement, anaplastic astrocytomas have no or spotty enhancement, while low-grade astrocytomas show peripheral linear enhancement in the exophytic portion. We believe that the thick ring-like enhancement in glioblastomas is related to tumour necrosis.
There was a fairly random distribution of ages between histological types. However, in this study, patients with low-grade astrocytoma had a longer symptom history than those with anaplastic astrocytoma or glioblastoma (Table 1). Patients with glioblastoma had the shortest history. This finding is similar to those reported previously [11, 12]. Symptom duration of less than 6 months is a poor prognostic feature and a duration greater than 12 months is more favourable.
FDG-PET is a technique that permits measurement of regional cerebral glucose metabolism. It provides a unique opportunity for the study of physiological processes and is useful in the differentiation of low-grade and high-grade gliomas and in the determination of prognosis in patients with these lesions [21–24]. The glucose metabolic rate in these tumours seems to be a good predictor of their biological behaviour and aggressiveness. Quantification of absolute metabolic rate requires blood sampling, which is time-consuming and invasive. We performed visual and semiquantitative analysis of tumour uptake as compared with uptake of other brain structures. In the literature, PET images of anaplastic astrocytomas have shown hypermetabolism because they are high-grade gliomas [21–23]. However, in our study they showed no metabolic abnormality or hypometabolism. Our data showed hypermetabolism compared with surrounding structures only in glioblastomas. Two glioblastomas with no or peripheral enhancement on MRI showed hypometabolism.
Although the population of our study was small, MRI findings were well correlated with histological grade of brain-stem gliomas and MRI may predict the histological grade of brain-stem glioma. FDG-PET may be helpful in differentiation between anaplastic astrocytoma and glioblastoma.
References
Albright AL, Price RA, Guthkelch AN (1983) Brain stem gliomas of children. A clinicopathological study. Cancer 52:2313–2319
Farwell R, Dohrmann GJ, Flannery JT (1977) Central nervous system tumors in children. Cancer 40:3123–3132
Fischbein NJ, Prados MD, Wara W, et al (1996) Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg 24:9–23
Rubin G, Michowitz S, Horev G, et al (1998) Pediatric brain stem gliomas; an update. Childs Nerv Syst 14:167–173
Epstein FJ, Farmer JP (1993) Brain stem glioma growth patterns. J Neurosurg 78:408–412
Kahn AP, Hirsch JF, Vinchon M, et al (1993) Surgical management of brain stem tumor in children: results and statistical analysis of 75 cases. J Neurosurg 79:845–852
Moghrabi A, Kerby T, Tien R, et al (1995) Prognostic value of contrast-enhanced magnetic resonance imaging in brain stem gliomas. Pediatr Neurosurg 23:293–298
Freeman CR, Farmer JP (1998) Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys 40:265–271
Jallo GI, Biser-Rohrbaugh A, Freed D (2004) Brainstem gliomas. Childs Nerv Syst 20:143–153
Lesniak MS, Klem JM, Weingart J, et al (2003) Surgical outcome following resection of contrast-enhanced pediatric brainstem gliomas. Pediatr Neurosurg 39:314–322
Freeman CR, Bourgouin PM, Sanford RA, et al (1996) Long term survivors of childhood brain stem gliomas treated with hyperfractionated radiotherapy. Clinical characteristics and treatment related toxicities. The Pediatric Oncology Group. Cancer 77:555–562
Cohen ME, Duffner PK, Heffner RR, et al (1986) Prognostic factors in brain stem gliomas. Neurology 36:602–605
Barkovich AJ, Krischer J, Kun LE, et al (1990/91) Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg 16:73–83
Kaplan AM, Albright AL, Zimmerman RA, et al (1996) Brainstem gliomas in children. A Children’s Cancer Group review of 119 cases. Pediatr Neurosurg 24:185–192
Kane AG, Robles HA, Smirniotopoulos JG, et al (1993) Radiologic-pathologic correlation: diffuse pontine astrocytoma. AJNR 14:941–945
Bowers DC, Krause TP, Aronson LJ, et al (2001) Second surgery for recurrent pilocytic astrocytoma in children. Pediatr Neurosurg 34:229–234
Stroink AR, Hoffman HJ, Hendrick EB, et al (1987) Transependymal benign dorsally exophytic brain stem gliomas in childhood: diagnosis and treatment recommendations. Neurosurgery 20:439–444
Stroink AR, Hoffman HJ, Hendrick EB, et al (1986) Diagnosis and management of pediatric brain stem gliomas. J Neurosurg 65:745–750
Pollack IF, Hoffman HJ, Humphreys RP, et al (1993) The long-term outcome after surgical treatment of dorsally exophytic brain-stem gliomas. J Neurosurg 78:859–863
Young Poussaint T, Yousuf N, Barnes PD, et al (1999) Cervicomedullary astrocytomas of childhood: clinical and imaging follow-up. Pediatr Radiol 29:662–668
Delbeke D, Meyerowitz C, Lapidus R, et al (1995) Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low grade from high grade brain tumors with PET. Radiology 195:47–52
Padma MV, Said S, Jacobs M, et al (2003) Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol 64:227–237
De Witte O, Lefranc F, Levivier M, et al (2000) FDG-PET as a prognostic factor in high-grade astrocytoma. J Neurooncol 49:157–163
Fenton LZ, Madden JR, Foreman NK (2003) Brain stem glioma in a child: false diagnosis of radiation necrosis with FDG PET. Med Pediatr Oncol 40:260–262
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwon, J.W., Kim, IO., Cheon, JE. et al. Paediatric brain-stem gliomas: MRI, FDG-PET and histological grading correlation. Pediatr Radiol 36, 959–964 (2006). https://doi.org/10.1007/s00247-006-0256-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-006-0256-5