Abstract
Neonatal MR imaging is invaluable in assessing the term born neonate who presents with an encephalopathy. Successful imaging requires adaptations to both the hardware and the sequences used for adults. The perinatal and postnatal details often predict the pattern of lesions sustained and are essential for correct interpretation of the imaging findings, but additional or alternative diagnoses in infants with apparent hypoxic ischaemic encephalopathy should always be considered. Perinatally acquired lesions are usually at their most obvious between 1 and 2 weeks of age. Very early imaging (<3 days) may be useful to make management decisions in ventilated neonates, but abnormalities may be subtle at that stage. Diffusion-weighted imaging is clinically useful for the early identification of ischaemic white matter in the neonatal brain but is less reliable in detecting lesions within the basal ganglia and thalami. The pattern of lesions seen on MRI can predict neurodevelopmental outcome. Additional useful information may be obtained by advanced techniques such as MR angiography, venography and perfusion-weighted imaging. Serial imaging with quantification of both structure size and tissue damage provides invaluable insights into perinatal brain injury.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neonatal encephalopathy is often but not exclusively due to perinatally acquired brain injury. Clinical signs include seizures, altered tone, feeding difficulties, decreased conscious level or irritability. MR imaging has a vital role to play in assessing the neonate with a perinatal encephalopathy. Unfortunately MR imaging of the sick neonate is not easy and experience is limited to relatively few centres, both in the practicalities of performing a successful examination and in the interpretation of the results. This review concentrates on perinatal brain injury in the term born neonate.
Practical issues
Sedation
Successful imaging of the neonatal brain requires careful preparation of the infant and close cooperation between radiologist, radiographer and neonatologist. Neonates may be successfully imaged during natural sleep, following a feed or under light sedation. We use chloral hydrate at a dose of 25–50 mg/kg orally, via nasogastric tube or rectally [1]. We keep the infant nil by mouth for at least 1 h prior to administration as this aids absorption of the chloral hydrate that we administer approximately 15 min prior to the start of the examination. Neonates will then usually sleep through an examination of 30–45 min. Severely encephalopathic neonates may not require sedation or may already be sedated by anticonvulsant medication. All neonates, sedated or not, should be monitored during scanning with MR-compatible pulse oximetry and ECG. A paediatrically qualified member of staff should be in attendance throughout the scan.
Safety
Excessive noise, particularly with fast sequences such as diffusion-weighted imaging (DWI) or perfusion-weighted imaging, may wake a sleeping infant or harm the developing auditory system and ear protection should be used. We use mouldable dental putty as individualized earplugs and neonatal earmuffs (Minimuffs, Natus, Chalgrove, UK). Infants may move even when asleep; moulded air bags or foam placed snugly around the infant’s head will keep this to a minimum. Swaddling the infants will keep them warm and also reduce movements. Full metal checks need to be carried out, with particular attention, in this population, to the presence of intravenous scalp lines, long lines, EEG electrodes, intraventricular shunts and metal fasteners on baby clothes [2]. All non-radiological staff involved in neonatal imaging need to be trained in MR safety.
Hardware and software adaptations
Good image quality requires a high signal-to-noise ratio and this can be maximized by using a closely fitting coil. In the absence of a dedicated neonatal head coil an adult knee coil may be used. This will normally accommodate an infant up to about 6 weeks post-term. Phased-array coils may provide improved benefit in terms of signal to noise even if designed for the adult brain. MR-compatible ventilator equipment may be required for the sickest infants, but in the absence of this a neonate can be safely hand bagged during a short MR examination. A larger adult-type coil may then be necessary to accommodate the endotracheal tube.
The majority of neonatal studies have been performed at 1 or 1.5 T, but 3-T scanners are now commercially available and may eventually replace many 1.5-T systems, particularly for brain imaging. Most MR sequences designed for imaging the adult brain need to be adapted to obtain good quality images of the immature brain with its higher water content. The exact imaging parameters depend on the specific system and magnet strength being used. Our parameters for neonatal brain imaging at 1.5 T are shown in Table 1. We would routinely perform the following sequences:
-
T1-weighted (T1-W) sequence acquired in the transverse plane. This is ideal for assessing the basal ganglia and thalami and provides the best views of the posterior limb of the internal capsule (Fig. 1).
-
T2-weighted (T2-W) sequence acquired in the transverse plane. This is better than T1-W imaging for identifying early ischaemic change and provides excellent grey/white matter contrast in the very immature brain (Fig. 1).
-
T1-W sequence acquired in the sagittal plane.
-
A volume acquisition is ideal as it provides thin slices and can be reformatted into any plane. It can be used for absolute quantification of brain structures.
-
DWI is ideal for early (<1 week) identification of ischaemic parenchymal tissue.
Normal MR appearances of the term neonatal brain. a T1-W inversion recovery. Myelin is seen as high-signal intensity within the posterior limb of the internal capsule (arrow). b T2-W sequence. Myelin in the posterior limb of the internal capsule is seen as a smaller region of low-signal intensity (arrow)
In addition we may add the following:
-
A venogram to exclude the presence of sinus thrombosis and differentiate this from subdural haemorrhage.
-
Intravenous contrast medium (gadolinium dimeglumine gadopentetate at a dose of 0.2 ml/kg) in suspected infection, e.g. herpes encephalitis.
-
Angiography to look at both cerebral and neck vessels, which may be abnormal in focal stroke.
Clinical presentation
The pattern of injury sustained by a neonate may be influenced by their gestation and predicted by the clinical history and the clinical presentation [3, 4]. In some cases a specific insult may be recognized such as hypoglycaemia or an acute hypoxic-ischaemic event such as a uterine rupture, but in many cases the aetiology is not clear. Infants who develop neonatal seizures, but who do not require aggressive resuscitation at delivery, are more likely to have white matter (WM) and cortical lesions such as a focal stroke or parasagittal infarction [3] (Fig. 2). Within this group there are also more likely to be neonates with alternative or additional pathologies, e.g. cerebral malformations, metabolic disorders or hypoglycaemia [4]. Infants with a global hypoxic-ischaemic insult, particularly if it is acute, are likely to sustain basal ganglia and thalamic (BGT) lesions (Fig. 3). In infants born following a sentinel event, e.g. a placental abruption, BGT lesions are likely to be isolated, but additional WM lesions may be found in a large proportion of those presenting without an apparent precipitating event [5] (Fig. 4). The clinical presentation therefore serves as a guide to the lesions that will have been sustained. To this end the radiologist needs to request as much information as possible from the neonatologist. This should include date of delivery, gestational age, antenatal history, type of delivery, Apgar scores, resuscitation, neonatal course and family history.
a–c A neonate with a perinatal left-sided middle cerebral artery infarct. d–f A neonate with extensive parasagittal WM infarction. In both infants, the perinatal WM infarction is imaged during the first week after delivery (a, d T1-W images; b, e T2-W images; c, f DWI). There is relatively subtle loss of grey-white matter differentiation on T1-W images although the T2-W image shows a more obvious abnormality. DWI shows obvious high-signal intensity consistent with restricted diffusion in areas of infarction
Timing of scans
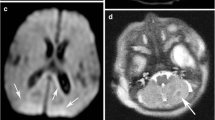
Perinatal brain lesions are at their most visually obvious between 1 and 2 weeks from delivery (Fig. 5). This is convenient as neonates may be clinically stable and off assisted ventilation by this time. Neonatologists may request earlier scanning in order to make a diagnosis or to assist in clinical management. Imaging within the first couple of days may show only subtle abnormalities in the presence of significant brain injury. Early image examinations should always include DWI. DWI should identify any infarcted WM (Fig. 2), but is not always so reliable at detecting significant injury to the basal ganglia and thalami (Fig. 6). As for adult stroke, the DWI visual appearances of infarcted tissue are obvious very early and last for approximately 1 week, by which time conventional imaging should be obviously abnormal [6, 7]. Sometimes visual analysis of the DWI is unremarkable or difficult to interpret (Fig. 7), even in the presence of severe damage. Most scanners have the software necessary to obtain apparent diffusion coefficients (ADC) from the trace diffusion images. It is recommended that ADC values are measured in all infants even when the DWI appears normal (Fig. 7). During the first week from injury ADC values are usually decreased in the presence of ischaemic WM, but may be reduced, normal or elevated in clinically significant basal ganglia lesions. Difficulties in interpreting DWI findings have led to studies using diffusion tensor techniques to explore tissue microstructure further (see advanced techniques).
Left panel DWI in three orthogonal planes of sensitisation does not show any focal high-signal intensity with ADC values. The actual ADC values (×10−3 mm−2 s−1) in tissue with normal values in parentheses. b Follow-up imaging showing extensive infarction in both white and central grey matter. Measuring the ADC values will correctly identify ischaemic tissue when there is widespread abnormality
Lesion evolution
Knowledge of not only the range of perinatally acquired lesions but also their evolution is important. There is a predictable evolution of perinatal brain lesions (Figs. 4, 5 and 8) and so serial conventional imaging may allow the timing of injury to be assessed. Repeat imaging may also be useful to document atypical evolution of imaging abnormalities, particularly when an additional or different diagnosis such as a metabolic disorder is suspected. In some metabolic disorders there are additional congenital malformations of the brain such as agenesis of the corpus callosum in non-ketotic hyperglycinaemia. A normal scan or an isolated delay in myelination in an infant with persisting seizures should raise the possibility of a metabolic disorder.
a–c Moderate BGT lesions: early (a) and late (b, c) imaging. Low-signal intensity in the posterior putamen and lateral thalami is more obvious as high-signal intensity on the T2-W images (c). Moderate BGT are associated with athetoid or dystonic quadriplegic cerebral palsy. Cognition may be normal. Head growth is relatively preserved. d, e Severe BGT lesions: early (d) and late (e) imaging. At 8 months (e) there is WM atrophy, delayed myelination, and atrophied BGT with abnormal, persisting high-signal intensity. Infants with severe BGT develop spastic quadriplegic cerebral palsy, cognitive impairments and severe persistent feeding difficulties. Head growth is poor with a secondary microcephaly developing over the first few weeks and months form delivery
Scan interpretation
The correct interpretation of images requires a thorough knowledge of the normally developing brain and of the range of perinatally acquired lesions and their evolution. Perinatal injury is often symmetrical and may be confused with normal appearances and vice versa by those not experienced in neonatal imaging. It may be appropriate to send images to a centre that regularly performs neonatal examinations for a confirmatory report or second opinion. There is so much invaluable information in a good quality set of neonatal images that benefits infant, parents and medical staff that it is worth ensuring that they have been correctly interpreted.
Patterns of injury and prediction of outcome
MR imaging provides detailed information about the pattern of lesions following perinatal brain injury and conventional sequences are able to provide excellent predictions of outcome for many infants.
In neonates with a global hypoxic-ischaemic insult resulting in hypoxic-ischaemic encephalopathy (HIE), lesions are usually detected within the basal ganglia and thalami with abnormal signal intensity in the intervening posterior limb of the internal capsule (PLIC) (Fig. 3). Abnormal signal intensity within the PLIC is an excellent predictor of abnormal outcome in term infants with HIE [8]. BGT lesions give rise to motor impairment in the form of cerebral palsy. The severity of the BGT lesions dictates the severity and nature of the cerebral palsy (Fig. 3) [5–9]. Lesions in the BGT are often accompanied by injury to the cortex and subcortical WM, most typically around the central sulcus. These changes are most obvious after the first week from injury (Fig. 5). In approximately 50% of neonates with BGT lesions there will be more extensive WM abnormalities [4, 5, 8, 10] (Figs. 4 and 7). The motor outcome for these children is still dictated by the BGT lesions, but WM involvement may exacerbate any cognitive deficit. However, infants with severe BGT lesions have severe cognitive impairment regardless of the severity of additional WM involvement.
Neonate presenting with HIE. a T1-W image shows normal basal ganglia but bilateral white-matter infarction (arrows). b Follow-up FLAIR image shows bilateral increased signal intensity consistent with glial formation within previously infarcted tissue (arrow). This infant had no motor deficit, but was microcephalic and had cognitive impairment
In some infants who present with what is thought to be HIE there is no BGT involvement but only WM lesions (Figs. 2d–f and 9). There may be a history of hypoglycaemia or infection. These WM lesions may be haemorrhagic. These lesions give rise to tissue atrophy and later cognitive impairment; the more severe the WM lesions the worse the cognitive outcome [10]. It is important to note therefore that in some children who later show isolated cognitive impairment this may have arisen as a result of a perinatal injury. However, there would normally be an abnormal perinatal history usually with seizures and the child is likely to be microcephalic. In addition, in children who have a term perinatally acquired WM injury later follow-up images may be indistinguishable from what would normally be called periventricular leukomalacia (Fig. 10). The injury may then be wrongly attributed to antenatal damage at an earlier gestation. Once again, in an infant with signs of periventricular leukomalacia on imaging and/or clinically, who was born at term, it is probably only reasonable to implicate perinatal events if there were neonatal symptoms such as encephalopathy or seizures.
Term-born neonate presenting with HIE. a Term T2-W image shows haemorrhagic WM lesions. b, c At 16 months, follow-up imaging shows WM atrophy, ventricular dilation, irregular angulated ventricles and some periventricular glial tissue (arrow). These changes would typically be called periventricular leukomalacia and be attributed to an injury to an immature brain. In a term-born infant, therefore, they would have been attributed to an antenatal injury
Post-mortem imaging
Infants with severe encephalopathy should always have brain imaging. This may be difficult and may not be possible before an infant dies. In such circumstances, and particularly if no autopsy is performed, post-mortem MR imaging should be considered. It may allow confirmation of abnormalities consistent with a hypoxic-ischaemic insult or suggest an alternative diagnosis. This would clearly have important genetic and medicolegal implications.
Advanced MR techniques
Diffusion-weighted imaging
Most modern scanners have the ability to perform DWI. Images may be obtained very quickly and are probably best left to the end of the imaging examination as the noise may wake the sleeping infant. There are relatively few studies using DWI in infants with HIE and these have had conflicting results [11–14]. As for unilateral infarction DWI will usually normalize by the end of the first week, both visually and in terms of ADC values. In infants with HIE, ADC values are significantly reduced in the first week following severe injury to either the WM or BGT (P<0.0001), but values have normalized by the end of the first week and then increase during the second week. We have found that ADC values <1.1×10−3/mm2 are always associated with WM infarction and values <0.8×10−3/mm2 with thalamic infarction [5].
Early visual analysis may be particularly misleading when there has been widespread injury to the WM and BGT, probably because there is no normal tissue for comparison (Fig. 7). A visual clue may be found by observing the appearance of the usually normal-appearing cerebellum (Fig. 11). In these infants, measuring the ADC values will correctly detect the presence of ischaemic tissue. Worrying, however, is that both the early visual appearances of DWI and the ADC values may be normal in the presence of isolated but clinically significant BGT lesions. We have shown that ADC values are either normal or increased in moderate BGT and WM lesions when compared to controls [5]. Moderate WM abnormalities are associated with relatively good outcome: normal motor development, but higher risk of cognitive impairment. Moderate BGT lesions are usually associated with significant motor impairment in the form of quadriplegic cerebral palsy. It is, therefore, very important to be able to correctly detect both these types of moderate lesions. More sophisticated diffusion techniques may improve our ability to identify moderate abnormalities.
Term-born neonate with HIE. a There is cerebellar ‘sparing’ despite extensive infarction at a few weeks of age. DWI shows widespread high-signal intensity, but ADC values have to be measured to appreciate the extent of injury. d The cerebellum has relatively low signal intensity (arrow) and normal ADC values. Whilst later cerebellar growth may not be optimal in the presence of supratentorial lesions, the presence of early cerebellar abnormalities in neonates with HIE is suggestive of an additional or alternative aetiology
Diffusion tensor imaging
Diffusion tensor imaging (DTI) may improve the ability to increase the detection of abnormal tissue by providing another parameter: the anisotropy or directional diffusivity within a tissue. Anisotropy increases with age as increasing myelination decreases radial diffusivity perpendicular to WM tracts. Anisotropy is variably deranged following acute focal infarction in adults. We have shown that during the first week from delivery, fractional anisotropy (FA) values in the WM are significantly decreased not only in infants with severe abnormality, but also in those with moderate abnormality [15]. Of interest, given the phenomenon of pseudonormalization with ADC values, is that FA values in severe WM lesions are also significantly reduced during the second and third weeks.
In addition, anisotropy is significantly decreased in the first week throughout the BGT and become progressively more abnormal within the region of the ventrolateral nuclei. FA is therefore significantly abnormal in both severe and moderate WM and BGT lesions with no pseudonormalization. This suggests that a combination of ADC and FA values derived from DTI combined with visual analysis of conventional imaging offers a good approach for identifying and timing all abnormal tissue in perinatal brain injury. This combination needs to be instituted on a large group of infants with neonatal encephalopathy imaged early during the first week. It is unlikely that this approach, however, could serve as a useful basis for identifying infants for potential early intervention such as hypothermia. Hypothermia has been associated with a decrease in BGT lesions in neonates with HIE [16]. Current trials are starting hypothermia within 6 h of delivery and it is unrealistic at present to expect infants to have safely undergone a good quality MR scan within this time. It may, however, provide a useful method for monitoring the effects of treatment both during and after interventions.
Potential therapies at present concentrate on acute intervention, but there is evidence to suggest ongoing injury in the neonate with HIE that may be amenable to later treatments. In a study of infants who had sustained BGT lesions perinatally, we found that WM appearances, which were initially normal with normal ADC values, deteriorated during the second week. ADC values instead of decreasing within the WM as would normally happen with increasing postnatal age, actually increased [17]. This phenomenon can be witnessed when looking at serially obtained conventional images when WM eventually atrophies (Fig. 8). These late changes are consistent with delayed injury occurring as a consequence of an initial insult to the BGT and this finding suggests that there may be some value in intervening within the first few days after a perinatal hypoxic-ischaemic insult. These interventions may have to be targeted towards the WM and possibly to different mechanisms of cell death, e.g. apoptosis and necrosis.
Further imaging advances include MR angiography that may detect anomalies that predispose infants to injury. Angiograms in infants with neonatal stroke will be asymmetrical once an infarct is established, but may show increased vessel outgrowth into infarcted regions. MR venography may detect abnormalities in sinus flow consistent with thrombosis. Thrombosis within the sagittal sinus results in haemorrhagic infarction of cortex and superficial WM. Perfusion-weighted imaging of the neonatal brain has been reported using a contrast technique [18], but arterial spin labelling may provide a more attractive alternative, particularly if advantage is taken of the improved signal to noise offered by imaging at 3 T [19]. The latter demonstrates improved resolution for T2-W scans and the improved signal to noise provides additional advantages for performing DTI, angiography and functional studies.
The field of neonatal brain imaging is growing rapidly, but access to basic clinical imaging remains difficult for many neonatologists. Those involved in clinical imaging research need to continue to exploit developments in MR techniques for the benefit of the neonatal population and ensure that these advances are translated into the clinical setting.
References
Cowan FM (1998) Sedation for magnetic resonance scanning of infants and young children. In: Whitwam JG, McCloy RF (eds) Principles and practice of sedation. Blackwell, London, pp 206–213
Pennock J (2002) Patient preparation, safety and hazards in imaging infants and children. In: Rutherford M (ed) MRI of the neonatal brain. Saunders, London, pp 3–16
Mercuri E, Cowan F, Rutherford M, et al (1995) Ischaemic and haemorrhagic brain lesions in newborns with seizures and normal Apgar scores. Arch Dis Child Fetal Neonatal Ed 73:67–74
Cowan F, Rutherford M, Groenendaal F, et al (2003) Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 361:713–714
Rutherford MA, Counsell S, Allsop J, et al (2004) Diffusion weighted MR imaging in term perinatal brain injury: a comparison with site of lesion and time from birth. Pediatrics 114:1004–1014
Kuker W, Mohrle S, Mader I, et al (2004) MRI for the management of neonatal cerebral infarctions: importance of timing. Childs Nerv Syst 20:742–748
Mader I, Schoning M, Klose U, et al (2002) Neonatal cerebral infarction diagnosed by diffusion-weighted MRI: pseudonormalization occurs early. Stroke 33:1142–1145
Rutherford MA, Pennock JM, Counsell SJ, et al (1998) Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 102:323–328
Rutherford MA (2002) The asphyxiated term infant. In: Rutherford M (ed) MRI of the neonatal brain. Saunders, London, pp 99–128
Cowan F, Dubowitz L, Mercuri E, et al (2003) White matter injury can lead to cognitive without major motor deficits following perinatal asphyxia and early encephalopathy. Dev Med Child Neurol Suppl 93:14
Forbes KP, Pipe JG, Bird R (2000) Neonatal hypoxic-ischemic encephalopathy: detection with diffusion-weighted MR imaging. AJNR 21:1490–1496
Wolf RL, Zimmerman RA, Clancy R, et al (2001) Quantitative apparent diffusion coefficient measurements in term neonates for early detection of hypoxic-ischemic brain injury: initial experience. Radiology 218:825–833
McKinstry RC, Miller JH, Snyder AZ, et al (2002) A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 59:824–833
Vermeulen RJ, Fetter WP, Hendrikx L, et al (2003) Diffusion-weighted MRI in severe neonatal hypoxic ischaemia: the white cerebrum. Neuropediatrics 34:72–76
Ward P, Counsell S, Allsop J, et al (2006) Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics DOI: 10.1542/peds.2005-0545
Rutherford MA, Azzopardi D, Whitelaw A, et al (2005) Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics 116:1001–1006
Rutherford MA, Counsell SJ, Allsop JM, et al (2004) Delayed abnormalities in diffusion following perinatal hypoxia-ischaemia to the brain: a marker for secondary injury and a late therapeutic window? Early Hum Dev 77:119–122
Tanner SF, Cornette L, Ramenghi LA, et al (2003) Cerebral perfusion in infants and neonates: preliminary results obtained using dynamic susceptibility contrast enhanced magnetic resonance imaging. Arch Dis Child Fetal Neonatal Ed 88:525–530
Rutherford M, Malamateniou C, Zeka J, et al (2004) MR imaging of the neonatal brain at 3 Tesla. Eur J Paediatr Neurol 8:281–289
Acknowledgements
We would like to thank all the staff of the Robert Steiner MR Unit, Imaging Sciences Department, Hammersmith Hospital and the neonatal units of Hammersmith and Queen Charlottes Hospital. We are also grateful to the Medical Research Council, the Academy of Medical Sciences, the Health Foundation and Philips Medical Systems for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rutherford, M., Srinivasan, L., Dyet, L. et al. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol 36, 582–592 (2006). https://doi.org/10.1007/s00247-006-0164-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-006-0164-8