Abstract
We present an infant with macrocrania, who initially demonstrated prominent extra-axial fluid collections on sonography of the brain, compatible with benign infantile hydrocephalus (BIH). Because of increasing macrocrania, a follow-up sonogram of the brain was performed; it revealed progressive enlargement of the extra-axial spaces, which now had echogenic debris. Color Doppler US showed bridging veins traversing these extra-axial spaces, so it was initially thought that these spaces were subarachnoid in nature (positive cortical vein sign). However, an arachnoid membrane was identified superior to the cortex, and there was compression of true cortical vessels beneath this dural membrane. An MRI of the brain showed the extra-axial spaces to represent bilateral subdural hematomas. The pathogenesis of spontaneous development of the subdural hematomas, in the setting of BIH, is discussed. We also emphasize that visualizing traversing bridging veins through extra-axial spaces does not necessarily imply that these spaces are subarachnoid in origin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many infants are referred for US of the brain because of macrocephaly. Most sonograms performed for macrocephaly are normal and demonstrate mildly prominent extra-axial spaces [1]. Dilatation of the extra-axial spaces with normal-size ventricles and no other brain abnormality is termed “benign infantile hydrocephalus” (BIH). These infants are generally followed by clinical parameters to document stability of head growth. The development of a subdural hematoma in the setting of BIH is a known, but rare phenomenon [2].
Making a distinction between a subarachnoid and subdural collection, using sonography, can be challenging. A positive cortical vein sign has been used as a sign of a subarachnoid collection [3, 4]. The positive cortical vein sign refers to observing bridging veins traversing an extra-axial space from the cortical surface to the arachnoid, and these vessels can also be traced into the superior sagittal sinus. A positive cortical vein sign implies that the extra-axial fluid is subarachnoid in origin. Not visualizing such traversing veins through extra-axial fluid collection implies that it is subdural in origin.
We present an infant with progressive macrocephaly, who ultimately developed bilateral subdural hematomas in the setting of BIH. In addition, these subdural hematomas were found to have bridging veins traversing them on color sonography.
Case report
A 3.5-month-old boy was referred for a head US because of progressive increase in head circumference. Medical history included spontaneous vaginal delivery at 32-week gestational age secondary to premature rupture of membranes. He spent 12 days in the Neonatal Intensive Care Unit, where he required continuous positive air-pressure treatment for 1 day. When he was 6 days old, he was diagnosed with grade I germinal matrix hemorrhage, which resolved on follow-up US examinations prior to discharge.
The patient was referred by his primary health care provider for a follow-up head US at 2 months of age because of a large head circumference that showed mildly prominent subarachnoid spaces. Cortical veins traversing the extra-axial spaces were noted (Fig. 1). The sonographic findings were thought to be compatible with BIH. The infant was subsequently seen at his primary pediatric clinic for a routine well-baby visit. During a 6-week period, his head circumference had increased from 32 to 42 cm. At this time, the US revealed larger extra-cerebral fluid collections, which had increased from the prior sonogram. These extra-axial collections also demonstrated diffuse low-level internal echoes and an arachnoid membrane superior to the cortex (Fig. 2). There also appeared to be mass effect upon the adjacent cerebral cortex. Color Doppler interrogation and spectral tracings demonstrated venous structures traversing these extra-axial fluid collections (Fig. 3), but they appeared to be fewer in number than noted on the prior sonogram. In addition, there were cortical veins noted to be trapped beneath the dural membrane (Fig. 3). Despite the fact that veins were noted to traverse these extra-axial fluid collections, the other sonographic findings were not compatible with BIH. The patient was admitted to the hospital, and a brain MRI was ordered. No history of trauma was elicited. A full evaluation for non-accidental trauma was performed, including a skeletal survey and ophthalmologic examination, but no definitive evidence for abuse was found. Evaluation for coagulopathy disorders did not reveal abnormalities.
Coronal sonogram of the brain. a Color Doppler shows mild prominence of the extra-axial spaces and traversing vessels to the cortex of the brain. The ventricles are of normal size. b Color Doppler spectral tracings demonstrate that the traversing extra-axial vessels have venous wave forms, a positive cortical vein sign
Coronal and sagittal color Doppler images taken at the same time as Fig. 2. a, b Note enlarged extra-axial spaces with traversing vessels, proved to be bilateral subdural hematomas on subsequent MRI. c Color spectral Doppler shows venous tracings of the vessels traversing the subdural hematoma. d Coronal color Doppler image shows entrapment of the true cortical vessels beneath the arachnoid membrane, compatible with compression by the subdural hematomas
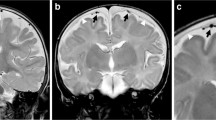
The MRI revealed bilateral subdural collections with layering hemorrhagic components (Fig. 4). It was also noted on the MRI that there were linear structures traversing the subdural collections bilaterally, which appeared to correlate with the traversing veins demonstrated on sonography. In addition, the MRI demonstrated cortical vessels trapped beneath the dural membrane, as seen on sonography (Fig. 4).
T-2 axial MRI images of the brain. a Bilateral subdural hematomas with layering of hemorrhagic components posteriorly. b Entrapment of vessels beneath an arachnoid membrane can also be seen. c Linear structures traversing the subdural hematomas (arrows) in the same location as those seen on the sonogram are thought to represent bridging veins or vascularized subdural membranes
The surgically drained subdural collections were composed of bloody fluid. The patient was discharged home with close follow-up by his primary care pediatrician.
Discussion
Babcock et al. [1] first coined the term benign macrocrania to describe infants with macrocephaly, normal to slightly enlarged ventricles, and bilaterally symmetric subarachnoid collections. These infants generally are 2–7 months of age, have normal neurological development and no other brain abnormalities. This condition has also been termed BIH, and is thought to be the result of inadequate absorption of CSF by the arachnoid villi. BIH generally resolves by 2 years of age [1, 5]. In addition, infants with BIH often have a family history of macrocrania [6]. The BIH is a diagnosis of exclusion. Other causes of enlarged extra-axial fluid spaces include passive dilatation of the subarachnoid space because of brain atrophy, subdural hygroma/hematoma, and subdural abscess secondary to meningitis [4].
It is widely believed, though difficult to prove, that distention of the subarachnoid spaces caused by BIH might predispose patients to subdural bleeding [5]. Mori et al. [2] studied 20 infants with BIH who were followed over an 8-year period. Three of these infants developed spontaneous subdural hematomas. The etiology of the spontaneous development of subdural hematomas is most likely secondary to arachnoid ruptures or tearing of bridging veins by progressive enlargement of the subarachnoid spaces [2, 5]. It is also possible that bleeding into the subdural space in the setting of BIH might be the result of minor trauma to distended subdural veins. The amount of trauma necessary for this to happen is probably less than in the infant population without BIH [5]. Ravid and Maytal [7] reported on three healthy infants who spontaneously developed subdural hematomas in association with BIH and who had no other risk factors for subdural development. The researchers also postulated that there might be stretching and rupture of subdural veins with BIH. In 2000, a theoretical mathematical model of the cranial vault was produced by Papasian and Frim [8]. The authors proposed a relationship between venous stretch and the width of the extra-axial spaces, which supports the predisposition of extra-axial bleeding with only minimal trauma in infants with BIH.
Distinction between a subdural collection and the subarachnoid space can be difficult using ultrasonography. McCluney et al. [3] and, subsequently, Chen et al. [4] described the “cortical vein sign” to distinguish these two extra-axial spaces. The cortical vein sign refers to visualization of cortical veins within extra-axial fluid collections at the cerebral convexities. If cortical veins traverse the extra-axial collection, then a positive cortical vein sign is present and is compatible with a subarachnoid collection. The absence of cortical veins traversing the extra-axial spaces favors a subdural collection. Subdural collections, if large enough, have been described as causing displacement of brain tissue, as well as entrapment of the cortical veins within the subarachnoid space. In our case, we noted bridging veins traversing the extra-axial spaces, but these collections proved to be subdural in nature. We observed, however, that these veins appeared to be fewer than usually seen with subarachnoid collections. Another clue on the sonogram that pointed to a subdural collection was the identification of an inferiorly displaced arachnoid membrane (Fig. 2), a finding not present with subarachnoid collections. Bridging veins that traversed the subdural spaces extended to this membrane. Close inspection also showed compression of cortical vessels beneath this membrane. Other findings that were suggestive for the presence of a subdural collection included the extra-axial space progressively enlarging from the initial sonogram, compression of brain tissue, and the presence of echogenic material in the collection. This echogenic material subsequently proved to be hemorrhage.
Therefore, our case illustrates that visualization of veins traversing an extra-axial collection does not necessarily imply a subarachnoid collection. The exact nature of this observation is not certain. It is most likely that these vessels represent engorged and stretched veins within the subdural space. Bridging vessels have been seen in subdural collections on MRI as reported by Swift and McBride [5]. In addition, several authors have proposed that subdural membranes in time become highly vascularized by the ingrowth of numerous small vessels. These vessels can easily be torn in the setting of minor trauma and predispose the patient to chronic re-bleeding into the subdural space [9, 10]. Hemorrhage from new vessels might then increase angiogenesis factors in the subdural fluid, resulting in a cycle of hemorrhage and new vessel formation. It is possible that these bridging veins represent vascularized subdural membranes.
In conclusion, subdural hematomas can arise spontaneously in infants with enlarged subarachnoid fluid collections. Although clinical follow-up might be sufficient in the majority of infants with BIH, imaging follow-up of infants with progressive macrocrania is appropriate. One should also be alerted to the fact that bridging veins can also traverse the subdural space, and the presence of such vessels does not necessarily imply a subarachnoid collection. These vessels might represent stretched subdural veins or possibly vascularized subdural membranes. Other signs of a subdural collection include observation of an arachnoid membrane, compression of true cortical vessels beneath this membrane, compression of brain tissue, and a complex appearance of the extra-axial fluid.
References
Babcock D, Han B, Dine M (1988) Sonographic findings in infants with macrocrania. AJR 150:1359–1365
Mori K, Sakamoto T, Nishimura K, et al (1993) Subarachnoid fluid collection in infants complicated by subdural hematoma. Childs Nerv Syst 9:282–284
McCluney K, Ueakley J, Festermacher M, et al (1992) Subdural hygroma versus atrophy on MR brain scans: “the cortical vein sign.” AJNR 13:1335–1339
Chen C, Chou T, Zimmerman R, et al (1996) Pericerebral fluid collection: differentiation of enlarge subarachnoid spaces from subdural collections with color Doppler US. Radiology 201:389–392
Swift D, McBride L (2000) Chronic subdural hematoma in children. Neurosurg Clin N Am 11:439–443
Swischuck L (2004) Imaging of the newborn, infant, and young child, 5th edn. Lippincott Williams and Wilkins, p 993
Ravid S, Maytal J (2003) External hydrocephalus: a probable cause for subdural hematoma in infancy. Pediatr Neurol 28:139–141
Papasian NC, Frim DM (2000) A theoretical model of benign external hydrocephalus that predicts a predisposition toward extra-axial hemorrhage after minor head trauma. Pediatr Neurosurg 33:188–193
Nakamura S (1989) Extraction of angiogenesis factor from chronic subdural hematomas. Significance in capsule formation and hematoma growth. Brain Inj 3:129–136
Yamashima T, Yamamoto S (1984) How do vessels proliferate in the capsule of a chronic subdural hematoma? Neurosurgery 15:672–678
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amodio, J., Spektor, V., Pramanik, B. et al. Spontaneous development of bilateral subdural hematomas in an infant with benign infantile hydrocephalus: color Doppler assessment of vessels traversing extra-axial spaces. Pediatr Radiol 35, 1113–1117 (2005). https://doi.org/10.1007/s00247-005-1503-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-005-1503-x