Abstract
Background: Plexiform neurofibromas are a frequent complication of neurofibromatosis type 1. This article discusses MR imaging findings and distribution of plexiform neurofibromas in the abdomen and pelvis. Objective: To define the most prevalent patterns of involvement and MR imaging findings in abdominopelvic neurofibromatosis type 1. Materials and methods: We reviewed the MR appearance of abdominopelvic lesions in 23 male and 20 female patients (median age: 16 years) with type 1 neurofibromatosis. The patients were part of a multi-institutional study of 300 patients. Imaging included coronal or sagittal, and axial short tau inversion recovery images. Results: The most common abdominopelvic involvement was in the abdominopelvic wall (n=28, 65%) and lumbosacral plexus (n=27, 63%). Retroperitoneal involvement was frequent (n=15, 35%). Lesions were less often intraperitoneal (21%) (P=0.001). Pelvic disease (n=27, 63%), neural canal involvement (n=18, 42%), and hydronephrosis (n=4, 9%) were also noted. Target-like appearance of plexiform lesions was noted in more than half the patients. Conclusion: Abdominopelvic involvement in neurofibromatosis type 1 is primarily extraperitoneal. Although lesions are most prevalent in the abdominopelvic wall and lumbosacral plexus, retroperitoneal and pelvic involvement is common and usually affects important organs. MR imaging added information in the initial and follow-up clinical evaluation of these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neurofibromatosis type 1 (NF1), an autosomal dominant disorder, has a highly variable natural history and phenotypic expression. Plexiform neurofibromas can cause significant morbidity because of disfiguring lesions, bone deformities, and involvement of adjacent structures. The risk of sarcomatous degeneration is low, between 3 and 5% [1, 2]. Malignant change typically occurs in young adults, with a male predominance [2]. Abdominopelvic involvement in NF1, although rare, can result in obstruction and dysfunction of the gastrointestinal tract and genitourinary tract. Sometimes these abdominopelvic symptoms can be the presenting findings in neurofibromatosis.
The MR imaging appearance of abdominopelvic neurofibromatosis has only been described in sporadic cases [3–5]. We report the findings in a series of 43 patients who are part of a larger cohort of nearly 300 patients with NF1 being studied within a multicenter trial to determine the natural history of plexiform neurofibromas. In the subset with abdominopelvic involvement, our goals are to describe the major patterns of the tumors and to document the effects of neurofibromas on abdominal and pelvic structures.
Materials and methods
Subjects
We reviewed the MR appearance of abdominopelvic lesions in 23 male and 20 female patients, with a median age of 16 years (age range, 4–52 years). All patients fulfilled diagnostic criteria for NF1 [6]. The initial protocol was to have serial MR imaging examinations three times during a 3-year observation period. In our population, 21 patients have had one study, 19 have had two studies, and 3 have had three studies, for a total of 68 MR imaging examinations. The study is ongoing. As part of the plexiform neurofibroma multi-institutional study, MR imaging data of 300 patients were sent to a central location for analysis. Of these patients, 43 who had MRI scans of the abdomen were selected for analysis of abdominal lesions. The human research committees of the 14 participating institutions approved the study.
MR imaging
All MR imaging was performed at high field strength, with 35 magnets operating at 1.5 T and one magnet at 1.0 T. Coil and field of view selection varied according to the location of the lesion. The protocol included coronal and contiguous axial short tau inversion recovery images (TR/TE 6,000/35; inversion time, 150 ms; echo-train length 8). Axial images were used for volumetric measurements. Slice thickness was 10 mm for the abdomen and pelvis. A 256×256 or 512×160 matrix was used. Contrast medium agent was not administered to any patient.
Image analysis
Involved regions were classified as the abdominopelvic wall, nerve plexi, pelvis, and abdomen. Nerve plexus involvement was classified into sacral, lumbar, celiac, and para-aortic. Sciatic nerve involvement was noted in the lumbosacral plexus abnormalities, which were also graded based on their size. Lesions less than 1 cm in greatest diameter were classified as grade 0, those 1–5 cm as grade 1, and those greater than 5 cm as grade 2. Lesions were evaluated for the presence or absence of the typical target-like appearance, defined as a well-circumscribed central area of low signal intensity and peripheral high signal intensity.
The affected abdominal and pelvic muscle groups were individually evaluated, and the frequency of involvement was studied for rectus abdominis, erector spinae, and gluteus. Genitourinary organs including the kidneys and ureter, the penis and scrotum in males, and the uterus in females were evaluated for patterns of involvement and for complications such as hydronephrosis and hydroureter. Secondary changes of the spine and pelvic bones such as neural foraminal widening, greater sciatic notch widening, and spinal cord compression were evaluated. Lesion signal intensity characteristics were analyzed. The serial progression of the tumor was quantitatively studied by volumetric analysis. Two radiologists jointly analyzed the images without knowledge of the patient’s history or the rate of clinical growth, and graded the lesions by consensus.
Statistical analysis
The significance of differences in prevalence of various findings was determined using the two-sample test of proportion (Intercooled Stata 7.0, Stata Corporation, College Station, TX, USA). A result with a P value less than 0.05 was thought to be significant.
Results
The plexiform neurofibromas were primarily extra-peritoneal. The most common regions of involvement were the abdominopelvic wall (n=28/43; 65% of patients), lumbosacral plexus (n=27/43; 63% of patients), and pelvis (n=27/43; 63% of patients). Abdominopelvic wall muscle involvement occurred primarily in the glutei (n=12), iliopsoas (n=11), and erector spinae (n=5) muscles. This was because of the involvement of the nerve roots in the intermuscular planes. These lesions were predominantly hyperintense with areas of heterogeneity. Posterior abdominopelvic wall involvement (n=13) (Fig. 1) was more frequently noted compared to anterior (n=7) and lateral (n=8) abdominopelvic wall involvement (P = 0.01). Lumbosacral plexus (n=27) involvement was combined lumbosacral (n=16), isolated sacral (n=6), or isolated lumbar (n=5). In 17 patients, the involvement extended into the sciatic nerve (Fig. 2). Lesions were either diffuse expansions of the nerve roots causing a chain-like appearance of nerves (Fig. 3) or large high signal intensity soft-tissue lesions causing mass effect on the surrounding abdominal structures, iliac vessels, sacrum, and iliac bones. Enlargement of sacral foramina was found in all 17 sciatic nerve lesions. Sciatic nerve tumors were also of high signal intensity and caused diffuse enlargement of the lower limb. Based on their size, lumbosacral plexus lesions were most commonly greater than 5 cm (n=17/27), less often between 1 cm and 5 cm (n=9/27), and seldom less than 1 cm (n=1). Extension into iliopsoas (n=11) and posterior abdominopelvic wall musculature was noted in cases of lumbar plexus involvement.
Pelvic lesions were either extensions of sacral lesions or primary lesions arising from the pelvic plexus of nerves (n=2) causing perirectal invasion. Bladder involvement was noted in eight patients in the form of mass effect (n=1) and diffuse or circumferential infiltration (n=7). Genital involvement occurred in six patients, with involvement of the penis or scrotum (n=2) and uterus or vagina (n=4). A patient with vulvar neurofibroma demonstrated a large lesion with extension into the perirectal and periuterine region (Fig. 4). Rectal involvement (n=13) was noted in the form of mass effect (n=4) and diffuse perirectal infiltration (n=9).
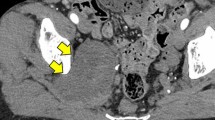
Retroperitoneal lesions were distant from the lumbosacral plexus and originated from the autonomic plexus in the celiac and para-aortic region. The regions frequently involved were the para-aortic (n=12), iliac (n=3), and celiac (n=2) regions. Diffuse celiac plexus involvement with infiltration into the peripancreatic region was noted in two patients. Hydronephrotic changes (n=2) were a result of retroperitoneal nerve plexus involvement causing ureteric obstruction. The hydronephrosis was severe in one patient (Fig. 5) with significant renal enlargement and thinning of parenchyma.
Retroperitoneal lesion causing hydronephrosis. a Axial STIR image (6,000/35); retroperitoneal neurofibromas (arrow) adjacent to the ureteropelvic junction. There is hydronephrosis of the left kidney. b Coronal STIR image (6,000/35). Retroperitoneal neurofibromas (arrows) causing ureteric obstruction and hydronephrosis of the left kidney.
Lesions were less commonly intraperitoneal (P=0.001), being located in the mesentery (n=7), spleen (n=1), and liver (n=1). Multiple plexiform lesions, which studded the mesenteric folds, were encountered in one patient, the hyperintense neurofibromas highlighted in the dark background of the visceral peritoneum on the STIR sequence (Fig. 6). The remaining six patients with mesenteric involvement had either focal primary mesenteric lesions (n=4) or secondary lesions extending from the retroperitoneum (n=2). Bony involvement included lumbosacral neural foraminal widening (n=20), greater sciatic foramina widening (n=2), and neural canal involvement (n=18).
Target-like appearance was noted in 53% (n=23/43) of patients. No subject showed loss of target-like appearance on follow-up imaging.
Discussion
We have found that, in the abdomen and pelvis, plexiform neurofibromas primarily affect the abdominopelvic wall and retroperitoneal regions. Plexiform neurofibromas are a frequent complication of NF1 1 [7–10]. With the emergence of new treatment protocols for treatment of plexiform neurofibromas [11], there is an increasing need to evaluate and classify these tumors.
Classification and analysis of complications of abdominal plexiform neurofibromatous lesions evaluated by MRI has not been reported. Abdominopelvic wall lesions are primarily posterior, probably because of the proximity of the posterior wall to the lumbosacral plexus and a lesser abundance of nerves in the anterior abdominopelvic wall. Lumbosacral plexus involvement resulted in significant secondary changes, such as spinal cord compression and obstructive hydronephrosis. Sacral plexus involvement produced mass effect on pelvic organs. Infiltration surrounding pelvic organs including the uterus and rectum was difficult to distinguish from primary involvement of the organ. Autonomic plexus involvement was also found to be common. Liver and spleen neurofibromas are extremely rare [12]; we found only one case of each.
Abdominal plexiform disease distribution has been evaluated with CT but not with MR imaging. Bass et al. [13] described the CT appearances of abdominal lesions in NF1. They studied 16 patients and found that almost all had retroperitoneal plexiform neurofibroma with predominant involvement of the psoas major muscle. This agrees with our findings that extraperitoneal lesions were more common than intraperitoneal. Eight of their 16 patients had lumbosacral plexus lesions, similar to our experience (n=27/43, 63%).
Biondetti et al. [14] emphasized the symmetric homogeneous appearance of paraspinal plexiform neurofibromas. Lumbosacral plexus tumors were asymmetric in 16 of 27 of our patients. Lesions were more likely to be symmetrical in the sacral plexus (n=10) than in the lumbar (n=1). The patients studied by Tonsgard et al. [2] had a high frequency of involvement of the sacral plexus, usually a result of a plexiform neurofibroma of the sciatic nerve that continued into the sacral plexus. Most of our patients also had predominant sacral plexus involvement with extension into the sciatic nerve (n=17/22). According to Tonsgard et al. [2], rectal and bladder involvement usually occurs by invasion by a large lesion. We found that in most cases, there was diffuse and intrinsic involvement of the walls of the bladder (n=7/8) and the rectum (n=10/13).
Retroperitoneal plexiform neurofibromas can cause complications such as mass effect on the spinal cord. We did not find any cases of sarcomatous degeneration. The small and large bowel can be affected by infiltration from retroperitoneal lesions, and these patients might present clinically with signs of bowel obstruction, but again, this was not seen. Ureteric obstruction and hydroureteronephrosis caused by retroperitoneal neurofibromas is a rare but important complication.
Huson et al. [8] studied the incidence of plexiform neurofibromas and found that 26.7% of individuals with NF1 had plexiform neurofibromas on physical examination. Tonsgard et al. [2] analyzed 126 individuals with NF1 using CT imaging and found an incidence of 20% in the thorax and 44% in the abdomen and pelvis.
Patients with plexiform neurofibromas can be asymptomatic when the lesions involve the viscera or the retroperitoneum. The clinical challenge and significance lies in the diagnosis and serial evaluation for above-mentioned complications. Surgical resection is seldom performed because the tumor cannot be isolated from the nervous plexi. Surgery is performed primarily when there is a suspicion of malignant degeneration or when the lesion is disfiguring or incapacitating.
None of our patients had imaging evidence of malignant degeneration. MR imaging findings suggestive of degeneration include hemorrhage and necrosis [15] and loss of the target sign [16]. Clearly, patients presenting with masses of evidence of obstruction should be imaged. However, abdominal wall involvement, a common finding in NF1, is often an isolated abnormality and does not require further imaging. The timing of the follow-up imaging will be clarified when the data from the natural history of the disease become apparent. MR imaging is more valuable than CT studies because it delineates the imaging morphology and extent of these lesions better.
Treatment of plexiform neurofibromas in general remains a surgical challenge. Surgery is usually performed for cosmetic reasons and in lesions with rapid growth. When disfiguring lesions are operated on, patients remain recurrence-free for an average of 6.8 years [15]. Medical treatment using anti-angiogenic drugs and farnesyl transferase inhibitors is being evaluated [11]. The unpredictable natural history of these lesions causes challenges in treatment response evaluation.
This was a retrospective study, and although the cohort is relatively large, there is variability in the data. Despite a standardized protocol, there were substantial differences in coverage of the area.
In summary, abdominal and pelvic plexiform neurofibromas are significant because they often have serious clinical implications. Our study shows that abdominopelvic involvement in NF1 1 primarily affects the abdominopelvic wall and the lumbosacral plexus and less frequently the retroperitoneum and pelvis. MR provides good contrast definition between neurofibromas and the surrounding soft tissues and thus allows characterization of the extent of these lesions.
References
Ducatman BS, Scheithauer BW, Piepgras DG, et al (1986) Malignant peripheral nerve sheath tumors: a clinicopathologic study of 120 cases. Cancer 57:2006–2021
Tonsgard JH, Kwak SM, Short MP, et al (1998) CT imaging in adults with neurofibromatosis-1: frequent asymptomatic plexiform lesions. Neurology 50:1755–1760
Niku SD, Mattrey RF, Kalota SJ, et al (1995) MRI of pelvic neurofibromatosis. Abdom Imaging 20:176–178
Ros PR, Eshaghi N (1991) Plexiform neurofibroma of the pelvis: CT and MRI findings. Magn Reson Imaging 9:463–465
Topsakal C, Erol FS, Ozercan I, et al (2001) Presacral solitary giant neurofibroma without neurofibromatosis Type 1 presenting as pelvic mass—case report. Neurol Med Chir (Tokyo) 41:620–625
Neurofibromatosis (1988) Conference statement. In: National institutes of health consensus development conference. Arch Neurol 45:575–578
Enzinger FM, Weis SM (1995) Benign tumors of peripheral nerves. In: Gray SM, Gery L (eds) Soft tissue tumors, 3rd edn. Mosby, St. Louis, pp 1130–1132
Huson SM, Harper PS, Compston DA (1988) Von Recklinghausen neurofibromatosis. A clinical and population study in south-east Wales. Brain 111(Pt6):1355–1381
Riccardi VM, Kleiner B (1977) Neurofibromatosis: a neoplastic birth defect with two age peaks of severe problems. Birth Defects Orig Arctic Ser 13:131–138
Sorensen SA, Mulvihill JJ, Nielsen A (1986) Long-term follow-up of von Recklinghausen neurofibromatosis. Survival and malignant neoplasms. N Engl J Med 314:1010–1015
Packer RJ, Gutmann DH, Rubenstein A, et al (2002) Plexiform neurofibromas in NF1: toward biologic-based therapy. Neurology 58:1461–1470
Imbert JP, Pilleul F, Valette PJ (2002) Value of MRI in hepatic plexiform neurofibromatosis. Case report. Gastroenterol Clin Biol 26:791–793
Bass JC, Korobkin M, Francis IR, et al (1994) Retroperitoneal plexiform neurofibromas: CT findings. AJR 163:617–620
Biondetti PR, Vigo M, Fiore D, et al (1983) CT appearance of generalized von Recklinghausen neurofibromatosis. J Comput Assist Tomogr 7:866–869
Korf BR (1999) Plexiform neurofibromas. Am J Med Genet (Semin Med Genet) 89:31–37
Lannicelli E, Rossi G, Almberger M, et al (2002) Integrated imaging in peripheral nerve lesions in Type 1 neurofibromatosis. Radiol Med 103:332–343
Acknowledgments
We thank Meera Gupta, project manager, Susan Yan of Worldcare Inc. for help in the co-ordination of sites, and Noemi Chavez for help with the manuscript preparation. Supported by U.S. Army Grant No. NF70002, “Natural History of Plexiform Neurofibromas in NF1”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zacharia, T.T., Jaramillo, D., Poussaint, T.Y. et al. MR imaging of abdominopelvic involvement in neurofibromatosis type 1: a review of 43 patients. Pediatr Radiol 35, 317–322 (2005). https://doi.org/10.1007/s00247-004-1352-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-004-1352-z