Abstract
Although the Cone procedure has improved outcomes for patients with Ebstein´s anomaly (EA), neither RV systolic function recovery in long-term follow-up nor the best echocardiographic parameters to assess RV function are well established. Thus, we evaluated RV performance after the Cone procedure comparing two-dimensional (2DEcho) and three-dimensional (3DEcho) echocardiography to cardiac magnetic resonance (CMR). We assessed 27 EA patients after the Cone procedure (53% female, median age of 20 years at the procedure, median post-operative follow-up duration of 8 years). Echocardiography was performed 4 h apart from the CMR. RV global longitudinal strain (GLS), fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), myocardial performance index and tissue Doppler S’ velocity were assessed using 2DEcho, whereas 3DEcho was used to evaluate RV volumes and ejection fraction (RVEF). Echocardiographic variables were compared to CMR-RVEF. All patients were in the NYHA functional class I. Median TAPSE was 15.9 mm, FAC 30.2%, and RV-GLS -15%; median RVEF by 3DEcho was 31.9% and 43% by CMR. Among 2DEcho parameters, RV-GLS and FAC had a substantial correlation with CMR-RVEF (r = − 0.63 and r = 0.55, respectively); from 3DEcho, the indexed RV volumes and RVEF were closely correlated with CMR (RV-EDVi, r = 0.60, RV-ESVi, r = 0.72; and RVEF r = 0.60). RV systolic function is impaired years after the Cone procedure, despite a good clinical status. FAC and RV-GLS are useful 2DEcho tools to assess RV function in these patients; however, 3DEcho measurements appear to provide a better RV assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ebstein’s anomaly (EA) is characterized by a failure in the delamination of the tricuspid valve leaflets from the adjacent myocardium, resulting in various degrees of tricuspid valve abnormalities. The myocardium is invariably abnormal in EA, characterized by the so-called myopathy of the right ventricle (RV) [1,2,3]. There is evidence to support that RV dysfunction is present in EA patients before and after surgery, mainly using cardiac magnetic resonance imaging (CMR) [4, 5]. Two-dimensional echocardiography (2DEcho) is the most useful tool to assess RV performance in EA patients pre-operatively, [6] but there is paucity of data on long-term post-operative evaluation. Three-dimensional echocardiography (3DEcho) is mainly used to evaluate anatomical features in EA patients [7], and there are few studies that have used this method for functional RV evaluation [8]. However, surgical procedures for EA have largely evolved in the last two decades. Currently, the most frequently performed procedure is the Da Silva’s Cone procedure [9], in which the anterior and posterior/inferior tricuspid leaflets are detached and mobilized in a clockwise rotation in about 360° at the annular level, with longitudinal plication of the atrialized RV. Despite encouraging surgical results [10,11,12], some concern has been raised regarding recovery of RV systolic function and also about the patient’s clinical improvement. However, reports are contradictory regarding these concerns [13, 14], mainly due to the small number of patients studied and short duration of follow-up. Therefore, we sought to: (1) assess RV systolic function after the Cone procedure in long-term follow-up, and (2) establish the best 2DEcho and 3DEcho parameters to evaluate RV systolic function compared to CMR-EF.
Methods
Patient Population
We performed a prospective single center cross-sectional study of EA patients who underwent the Cone procedure between 1995 and 2014. Subjects underwent echo and CMR for the purposes of the research study from 10/2015 to 11/2016. Indications for operation included symptoms, or deteriorating exercise capacity, cyanosis (oxygen saturation < 90%), paradoxical embolism, progressive cardiomegaly (cardiothoracic ratio > 0.6 on chest X-ray), progressive RV enlargement (on echocardiography), and the onset or progression of atrial or ventricular arrhythmias.
We included EA patients operated on by two surgeons (JPS and LFS), aged ≥ 8 years old (in order to avoid sedation for CMR), all in regular cardiac rhythm. Exclusion criteria included permanent pacemaker, poor echocardiographic windows and inability to perform the CMR exam, such as extreme obesity or lack of cooperation.
NYHA functional class was assessed for all patients.
The study was approved by the Ethics Committee at Hospital Israelita Albert Einstein. Written informed consent was obtained from all patients or from parents when patients were under 18 years old.
Surgical Technique
The Cone procedure technique was performed as previously published [9]. Briefly, the anterior leaflet was partially detached from the tricuspid annulus. The incision was prolonged posteriorly, detaching the anterior and inferior tricuspid leaflets from their anomalous attachments in the RV as a single piece. The septal leaflet, whenever present, was detached and incorporated to the cone. The thin and atrialized RV free wall was plicated in a longitudinal fashion. The cone was completed by longitudinal fenestrations of the leaflets towards the apex of the RV. The true tricuspid annulus was plicated in a horizontal fashion to allow a tension-free re-attachment of the leaflets.
Usually, the atrial septal defect (ASD or PFO) was closed in a valved fashion. None of the patients received a prosthetic ring. If present, abnormal atrioventricular conduction tissues were treated surgically or by radio frequency after TV detachment.
Two-Dimensional Echocardiography
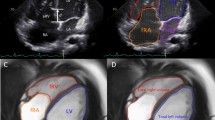
A comprehensive 2DEcho was obtained using a Philips iE33 or Epic 7 (Philips Medical Systems, Andover, MA) ultrasound system with a X5 matrix array transducer. Echocardiographic assessment was undertaken following a standardized protocol. Image acquisition started with subcostal views for the assessment of the atrial septum, followed by lateral decubitus for apical and parasternal views. ECG monitoring was used in all patients. The same experienced echocardiographer (ACL), performed all exams blinded to the CMR results. Following left ventricular (LV) functional and structural measurements, a complete assessment of the RV was performed, and the following measurements were obtained from the 4- chamber view: (1) tricuspid annular plane systolic excursion (TAPSE) measured by placing the M-mode cursor at the lateral tricuspid valve annulus, (2) S wave velocity with pulsed-wave tissue Doppler interrogation of the tricuspid lateral wall annulus, (3) fractional area change (FAC), calculated as: FAC (%) = 100 × (end-diastolic area –end-systolic area)/end-diastolic area, (4) myocardial performance index (MPI) using pulsed-wave tissue Doppler with a frame rate > 100 frames/s, calculated as: (isovolumic contraction time+isovolumic relaxation time)/ ejection time (Fig. 1), and (5) RV global longitudinal strain (RV-GLS), given as the average of six segments by speckle tracking echocardiography, including the RV free wall and the ventricular septum, keeping a minimum of 60 frames/s using a semi-automatic contour by selecting three landmarks (one at the lateral tricuspid annulus, another at the medial tricuspid annulus and the last one in the apex). All 2DEcho parameters were subsequently measured in the equipment, and the average of three measurements was used for analysis. RV free wall longitudinal strain (RV-FLS) was obtained off-line using 2D data set in the TomTec Image-Arena® software (version 5.5, Unterschleissheim, Germany). Normal values were obtained from the American and European Societies recommendations for cardiac chamber quantification [15, 16], as follows: TAPSE > 17 mm; S wave > 9.5 cm/s; FAC > 35%; Tissue Doppler MPI > 0.54; RV-FLS >- 20%; RV-GLS >- 17%.
Tricuspid valve anatomy and function were evaluated, including residual regurgitation (TR) and tricuspid valve stenosis (TS). TR was graded qualitatively from color flow mapping as none, trivial, moderate, or severe, using the width of the regurgitant jet and the extent of the jet into the right atrium [17].
Three-Dimensional Echocardiography
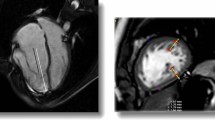
A Philips iE33 or Epic 7 ultrasound system (Philips Medical Systems, Andover, MA, USA) with a matrix X5 transthoracic transducer were used for image acquisition. Images were acquired during expiratory breath-holds (whenever possible) in the 4-chamber view, and we attempted to include the entire RV free wall throughout the cardiac cycle with frame rates optimized to > 20 frames/s. Multiple sets of full-volume data (four beats) were acquired from the apical 4-chamber view to maximize volume rate. The acquired raw datasets were transferred to TomTec Image-Arena® software for RV volumetric and functional off-line analysis by the same echocardiographer (ACL). The best dataset was chosen for each patient and 10 anatomical landmarks where manually defined as follows: the LV and RV long axes in 4 and 2-chamber views, followed by measurement of the LV outflow tract in the apical 3-chamber view, and finally, anterior and posterior junction points of the RV free wall with the ventricular septum and the longest RV cavity, both in a short-axis view. After the landmarks were defined, the software performed an automatic contour of the RV endocardial surface, which is tracked throughout the cardiac cycle, furnishing tridimensional models (Fig. 2) with end-diastolic volume (RV-EDV), end-systolic volume (RV-ESV) and ejection fraction (RVEF). Due to the patient’s wide age range, RV diastolic and systolic volumes were also indexed to body surface area. We considered 3D RVEF normal values > 45% [15].
Cardiac Magnetic Resonance (CMR)
All patients underwent CMR examination in a 1.5 T MRI scanner (General Electric Healthcare, Waukesha, USA) within 4 h of the echocardiogram.
The parameters included a repetition time of 2.7 ms, an echo time (TE) of 1.5 ms, a field of view (FOV) of 300–350 mm. A stack of 8 mm thick short-axis images with 2 mm gaps covered the LV and RV from the apex to the base. Also, a stack of 4-chambers view that covered the entire RV was acquired. Three long-axis images were obtained (2, 3, and 4-chamber views). Ventricular function and cardiac volumes were imaged using the steady state free procession sequence (SSFP) and were evaluated using the Simpson’s method on short-axis images.
All analyses were performed using a dedicated workstation (AW Server, GE healthcare). Inversion time was optimized to null normal myocardial signal.
Statistical Analysis
Continuous variables are described as medians and ranges. Categorical variables are presented as frequency distributions or proportions.
Pearson’s correlation coefficient was used to examine the association between 2DEcho, 3DEcho and CMR variables.
Intra-observer and inter-observer variability was performed by the same reader and another experienced echocardiographer (ACTR) in 12 patients. Intraclass correlation coefficients (ICC) was used to test the variability among the 2DEcho (TAPSE, FAC, S wave, MPI, RV-GLS and RV-FLS) and 3DEcho (RV diastolic and systolic volumes, and RVEF) parameters. Bland–Altman plots were generated for 3D parameters. We classified ICC as poor (0–0.19), weak (0.20–0.30), moderate (0.30–0.59), substantial (0.60–0.79) and strong (> 0.80). A p value < 0.05 was considered to be statistically significant. All analyses were performed using SPSS for Windows (version 22).
Results
There were 30 eligible subjects, of whom, 27 had CMR, 2DEcho and 3DEcho. Three patients were excluded due to inadequate 3DEcho images. Median age during testing was 20 years (range 8–54), and 14 patients were female (51.8%). Median post-operative time was 8 years (range 1–21); all patients were categorized in the New York Heart Association (NYHA) as functional class I for heart failure. Additional demographic and hemodynamic data are described in Table 1.
Two-Dimensional Echocardiography
TR was present in all patients: it was trivial in 81.5%, moderate in 14.8% and severe in only one patient (3.7%). No patient had TS. RV systolic function was overall impaired according to all 2DEcho parameters: median TAPSE was 15.9 mm (range: 6.8–30.0 mm), median FAC was 30.2% (range: 20.0–50.0%) and median RV-GLS was − 15.0% (range: − 24.0 to − 6.0%). Median RV-FLS was − 12.7% (range: − 20.7 to − 2.5%). Additional 2DEcho parameters are shown in Table 2.
Three-Dimensional Echocardiography
RV volumes were overall increased, with median 3DEcho RV-EDV of 130.4 ml (range: 58.7–323.5 ml), indexed RV-EDV (RV-EDVi) of 77.8 ml (range: 35.9–154 ml). RV-ESV was 90.6 ml (range: 33.2–264.9 ml), with indexed RV-ESV (RV-ESVi) of 49.7 ml (range: 21.3–125.3 ml); median RVEF was decreased (31.9%, range: 18.6–54.8%) (Table 2).
Cardiac Magnetic Resonance
Similarly to 3DEcho, RV volumes were overall increased, with a median CMR RV-EDV of 176.9 ml (range: 76.0–423.3 ml) and a median RV-EDVi of 112.1 ml (range: 46.7–197.7 ml). The median RV-ESV was 101.3 ml (range: 41.0–334.3 ml) and the median RV-ESVi was 65.0 ml (range: 24.2–158.1 ml). The median CMR-RVEF was 43.0% (range: 21.0–53.0%) (Table 3). When we grouped patients into better or worse RV systolic function according to the median CMR-RVEF (43%), there was no difference in age at Cone procedure, time from surgery, or any other demographic or operative characteristics.
Comparison Between 2DEcho Parameters and CMR-EF
RV-GLS and FAC correlated substantially with CMR-RVEF (r = − 0.63; p < 0.001 and r = 0.55; p = 0.003, respectively). (Figs. 3 and 4); RV-FLS correlated moderately with CMR-RVEF (r = − 0.4; p = 0.016); neither TAPSE, tissue Doppler S' velocity, nor MPI had significant correlations with CMR-RVEF. (Table 4).
Comparison Between 3DEcho Parameters and CMR
We found a substantial correlation between 3DEcho RV volumes and CMR (RV-ESVi: r = 0.60; p < 0.01, RV-EDVi: r = 0.72; p < 0.01), as well as 3DEcho RVEF and CMR-RVEF (r = 0.60; p = 0.01) (Table 5, Figs. 5, 6 and 7).
Intra- and Inter-Observer Results
We found excellent intra-observer (ICC = 0.99, 95% CI 0.97; 0.99) and inter-observer (ICC = 0.92, 95% CI 0.71; 0.97) reproducibility for 3DEcho en-diastolic volumes; similarly, end-systolic volumes had excellent intra-observer (ICC = 0.95, 95% CI 0.86; 0.98) and inter-observer (ICC = 0.93, 95% CI 0.75; 0.98) reproducibility. Greater variability was observed for 3DEcho RVEF analysis [intra-observer ICC = 0.61 (95% CI 0.17; 0.85), and inter-observer ICC = 0.43 (95% CI − 0.15; 0.79)]. Bland–Altman plots are shown in Fig. 8.
Bland–Altman graphs showing intra and inter-observer 3DEcho RVEF, RVEDV and RVESV variability. 3DEcho RVEF right ventricular ejection fraction estimated by three-dimensional echocardiography, 3DEcho RVEDV right ventricular end-diastolic volume estimated by three-dimensional echocardiography, 3DEcho RVESV Right ventricular end-systolic volume estimated by three-dimensional echocardiography
We also found excellent intra-observer variability for TAPSE (ICC 0.99, 95% CI 0.97; 0.99); S wave (ICC 0.99, 95% CI 0.98–0.99), FAC (ICC 0.93, 95% CI 0.78—0.98); MPI (ICC 0.93, 95% CI 0.78; 0.98), RV- GLS (ICC 0.97, 95% CI 0.91; 0.99) and RV-FLS (ICC 0.99, 95% CI 0.97; 0.99). Excellent inter-observer variability was achieved for all the previous parameters (TAPSE: ICC 0.99, 95% CI 0.98; 0.99. S wave: ICC 0.94, 95% CI 0.80; 0.98 and RV-GLS: ICC 0.97, 95% CI 0.91; 0.99). An adequate inter-observer variability was present in FAC (ICC 0.89, 95% CI 0.62, 0.97) and RV-FLS analysis (ICC 0.73, 95% CI 0.6, 0.92).
Discussion
Although CMR is the gold standard for evaluating global and regional RV function, echocardiography is the most frequently used method, primarily because of its wide availability, lower cost, and the possibility of conducting a real-time evaluation with high temporal and spatial resolution. To our knowledge, this is the first study to use multiple echocardiographic parameters, including 2D strain with speckle tracking echocardiography and 3DEcho parameters to compare with CMR parameters for functional evaluation of the RV years after the Cone procedure in EA patients. The main findings of this study are: (1) Systolic RV function is below reference values after the Cone procedure, which is indicated both by 2DECHO, 3DECHO and CMR, despite the mild degree of tricuspid regurgitation and the few clinical complaints of heart failure. (2) Among the 2DEcho parameters, RV-GLS and FAC had the best correlation to CMR-RVEF and (3) among 3DEcho parameters, volumes and EF were found to have a strong correlation with CMR RV EF.
A few previous studies have reported RV systolic dysfunction after the Cone procedure [13, 14], mostly with a shorter follow-up. In our study, CMR-RVEF was below the normal range in almost all (96%) patients, a finding also observed for 3DEcho EF and 2D-based parameters. EA patients without the Cone procedure were found to present signs and symptoms of heart failure and atrial arrhythmias, usually as a consequence of the extent of apical displacement of the TV and the resulting TR and RV dysfunction. By placing the tricuspid valve back into the anatomic annulus position and rotating the leaflets to give the valve a conical shape, the Cone repair allows for greater valvular coaptation, dramatically decreasing the degree of regurgitation [9]. Previous studies mentioned that RV systolic dysfunction could be a result of a decrease in TR, as well as due to myocardial stunning after cardiac bypass [13, 14]. A recent study retrospectively assessed EA patients before and after the Cone procedure and demonstrated that RV function was qualitatively reduced after a median follow-up of 6 months, with decreased FAC and TAPSE values in most patients [18]. In our study, RV dysfunction was present years after the Cone procedure, which leads us to hypothesize that the underlying EA myopathy is the main reason for persistent RV dysfunction, despite the excellent resolution of TR.[1,2,3], This phenomenon is possibly due to the atrialization of the entire RV inlet, preventing RV reverse remodeling following surgical correction [3].
RV-GLS and FAC showed the best 2DECHO correlation with CMR-RVEF. These are important findings because 2DEcho is used on a daily basis, mostly due to its availability, lower cost and the ease of the technique. Additionally, speckle tracking derived deformation measurements are less load-dependent, are reproducible and possibly reflect a more “global” and sensitive assessment of RV systolic function. RV-GLS has been shown to detect subclinical RV dysfunction [19]. Our findings are in agreement with the report by Kühn et al. [20], who found significant correlation between RV-GLS and CMR-EF in EA patients without operations; in that report, correlation was not so good, possibly due to different loading conditions (pre-operatively) resulting from significant tricuspid regurgitation. We also a found moderate correlation between RV-FLS obtained by TomTec software and CMR; however, we acknowledge that there are some methodological bias concerning different software and frame rate.
Our study did not identify a correlation between TAPSE and RV S’ velocity and CMR-RVEF. We believe that these two measurements, as opposed to FAC, are less accurate in this setting since they only assess longitudinal shortening in one segment of the RV. This is in consonance with other reports that assessed volume loaded RVs in congenital heart disease [20, 21]. Avitabile et al. [21] investigated patients with hypoplastic left heart syndrome after the Fontan procedure and compared TAPSE to CMR-EF, and observed no correlation for these examinations, suggesting these RVs could have a more radial and circumferential pattern of contraction, similar to other congenital heart defects [22, 23]. Our findings reiterate the concern of using TAPSE and RV S’ velocity after cardiac surgery, in particular involving tricuspid annular valvuloplasty and plication; although we believe TAPSE might be useful for monitoring RV function for the individual EA patient follow-up [21]. On the other hand, by including additional RV segments, FAC offers a better comparison to CMR-EF, which is also demonstrated in our study. In fact, RV function in operated EA seems to rely mainly on radial contraction, with significant participation of the apical segments in RV output [4]. These findings reinforce the importance to use two-dimensional measures that include more than one RV segment, such as RV-GLS and FAC, especially considering the abnormal pattern of myocardial contraction in EA myopathy.
Finally, we did not find a significant correlation between MPI and CMR-EF, most likely because MPI incorporates both diastolic and systolic function, using time intervals as a surrogate of RV function and also relies on RV basal segments manipulated in the surgical procedure.
We found strong correlations between 3DEcho parameters (volumes and EF) and CMR-RVEF. Similarly to our findings, other studies have shown excellent accuracy for 3DEcho-derived volumes [24, 25]. Van der Zwann et al. [24] showed a strong correlation between 3DEcho RV volumes and CMR RV derived volumes, while only a moderate correlation between RV 2DEcho dimensions and CMR RV-EDV, both for congenital heart disease patients and healthy individuals. Even with 3D echo, the RV remains a challenging chamber to visualize because it may be obscured by the sternum and ribs [26]. In our series, the best correlation was found for end-systolic volumes, possibly due to the smaller ventricular cavity size and better endocardial definition during systole, when the myocardium is thicker. Although end-diastolic volumes were also found to strongly correlate with volumes from CMR, it is important to mention that the lateral wall is relatively difficult to track in these particularly dilated RVs; this contributes to underestimating 3DEcho RV volumes compared to CMR RV and is likely responsible for the lower correlation between the two methods. Concern has been raised regarding the use of 3Decho in dilated RVs [27] since volumes are frequently underestimated when compared to CMR-derived volumes. When assessing dilated RVs, our practice has shown that manual adjustment is often necessary after the initial 3D automated analysis to assure that the entire chamber can be evaluated. In spite of this, some of the RV wall segments could not be adequately included in the dataset during diastole; accordingly, we excluded three patients from our analysis due to inadequate 3DEcho imaging. We believe this finding could explain the lower values for RVEF from 3Decho in this setting.
Finally, despite the long-term RV systolic dysfunction, patients had excellent resolution of TR and report very few symptoms, with significant improvement in clinical status compared to pre-operatively. This finding suggests that the use of standard parameters presented in this study and comparing them to normal controls might be inadequate to evaluate the RV in EA patients. Thus, development of EA-specific reference values should be needed.
Study Limitations and Strengths
The main strengths of our study are the prospective patient enrollment and concurrent acquisition of echocardiograms and CMRs, thus reflecting the same hemodynamic status. However, we acknowledge there are some limitations in this study. The degree of RV dilatation is an important limitation for the accuracy of 3DECho analysis, and influences the comparison of 3DEcho with CMR; however, the correlation coefficient between these variables was found to be high after the exclusion of technically inadequate imaging.
We excluded children under 8 years old due to these patients’ poor cooperation in obtaining both 3DEcho and CMR imaging without sedation, which limits the ability to extrapolate the results to a younger population.
Finally, our study included a small number of patients, given that many patients presenting for the Cone procedure live in remote Brazilian states, thus limiting our ability to enroll more patients in a prospective fashion. Even so, due to the rarity of the disease, this is possibly one of the largest follow-up studies after the Cone procedure comparing two–dimensional strain, 3Decho and CMR imaging.
Conclusion
RV dysfunction persists after the Cone procedure for EA, despite the clinical improvement. 3DEcho appears to offer better associations with CMR than 2D parameters. Among 2DEcho parameters, FAC and RV-GLS correlate better to CMR-EF and should be used in daily basis while TAPSE and S’ wave should be avoided. Efforts to develop EA-specific reference values should be pursued in future studies.
Abbreviations
- EA:
-
Ebstein’s anomaly
- RV:
-
Right ventricle
- 2DEcho:
-
Two-dimensional echocardiography
- 3DEcho:
-
Three-dimensional echocardiography
- RVEF:
-
Right ventricle ejection fraction
- CMR:
-
Cardiac magnetic resonance
- TR:
-
Tricuspid regurgitation
- RV-GLS:
-
Right ventricular global longitudinal strain
- RV-FLS:
-
Right ventricular free wall longitudinal strain
- FAC:
-
Fractional area change
- TAPSE:
-
Tricuspid annular plane systolic excursion
- MPI:
-
Myocardial performance index
- RV-EDV:
-
Right ventricle end-diastolic volume
- RV-EDVi:
-
Indexed right ventricle end-diastolic volume
- RV-ESV:
-
Right ventricle end-systolic volume
- RV-ESVi:
-
Indexed right ventricle end-systolic volume
- NYHA:
-
New York Heart Association
References
Brown ML, Dearani JA (2009) Ebstein malformation of the tricuspid valve: current concepts in management and outcomes. Curr Treat Options Cardiovasc Med 11:396–402
Brown ML, Dearani JA (2011) Ebstein anomaly. In: Gatzoulis MA, Webb GD, Daubeney PEF (eds) Diagnosis and Management of adult congenital heart disease, vol 39. Elsevier Saunders, Philadelphia, pp 288–294
Anderson KR, Lie JT (1979) The right ventricular myocardium in Ebstein’s anomaly: a morphometric histopathologic study. Mayo Clin Proc 54:181–184
Lee CM, Sheehan FH, Bouzas B, Chen SS, Gatzoulis MA, Kilner PJ (2013) The shape and function of the right ventricle in Ebstein’s anomaly. Int J Cardiol 167:704–710
Gutberlet M, Oellinger H, Ewert P, Nagdyman N, Amthauer H, Hoffmann T et al (2000) Pre- and post-operative evaluation of ventricular function, muscle mass and valve morphology by magnetic resonance tomography in Ebstein’s anomaly. Rofo 172:436–442
Therrien J, Henein MY, Li W, Somerville J, Rigby M (2000) Right ventricular long axis function in adults and children with Ebstein’s malformation. Int J Cardiol 73:243–249
Vettukattil JJ, Bharucha T, Anderson RH (2007) Defining Ebstein’s malformation using three-dimensional echocardiography. Interact Cardiovasc Thorac Surg 6:685–690
Lu X, Vyacheslav N, Bu L, Stolpen A, Ayres N, Pignatelli RH et al (2008) Accuracy and reproducibility of real-time three-dimensional echocardiography for assessment of right ventricular volumes and ejection fraction in children. J Am Soc Echocardiogr 21:84–89
da Silva JP, Baumgratz JF, da Fonseca L, Franchi SM, Lopes LM, Tavares GM et al (2007) The cone reconstruction of the tricuspid valve in Ebstein’s anomaly. The operation: early and midterm results. J Thorac Cardiovasc Surg 133:215–223
Anderson HN, Dearani JA, Said SM, Norris MD, Pundi KN, Miller AR et al (2014) Cone reconstruction in children with Ebstein anomaly: the Mayo Clinic experience. Congenit Heart Dis 9:266–271
Dearani JA, Said SM, O’Leary PW, Burkhart HM, Barnes RD, Cetta F (2013) Anatomic repair of Ebstein’s malformation: lessons learned with cone reconstruction. Ann Thorac Surg, 95:220–6; discussion 6–8
Vogel M, Marx GR, Tworetzky W, Cecchin F, Graham D, Mayer JE et al (2012) Ebstein’s malformation of the tricuspid valve: short-term outcomes of the ‘‘cone procedure’’ versus conventional surgery. Congenit Heart Dis 7:50–58
Lange R, Burri M, Eschenbach LK, Badiu CC, daSilva JP, Nagdyman N, et al (2015) Da Silva’s cone repair for Ebstein’s anomaly: effect on right ventricular size and function. Eur J Cardio Thorac Surg 48(2):316–320
Ibrahim M, Tsang VT, Caruana M, Hughes ML, Jenkyns S, Perdreau E et al (2015) Cone reconstruction for Ebstein’s anomaly: patient outcomes, biventricular function and cardiopulmonary exercise capacity. J Thorac Cadiovasc Surg 149(9):1144–1150
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L et al (2015) Recommendation for cardiac chamber quantification by Echocardiography in adults: an update from the American Society of Echocardiorgaphy and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39e14
Morris DA, Krisper M, Nakatani S, Kohncke C, Otsuji Y, Belyavskiy E et al (2017) Normal range and usefulness of right ventricular systolic strain to detect subtle right ventricular systolic abnormalities in patiens with heart failure: a multicentre study. Eur Heart J Cardiovasc Imaging 18(2):212–223
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Graysburn PA et al (2017) Recommendations for noninvasive evaluation of native valvular regurgitation. A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 30(4):303–371
Perdreau E, Tsang V, Hughes ML, Ibrahim M, Kataria S, Janagarajan K et al (2018) Change in biventricular function after cone reconstruction of Ebstein´s anomaly: an echocardiographic study. Eur Heart J Cardiovasc Imaging 19(7):808–815
Prakasa KR, Wang J, Tandri H, Dalai D, Bomma C, Chojnowski R et al (2007) Utility of tissue Doppler and strain echocardiography in arrythmogenic right ventricular dysplasia/cardiomyopathy. Am J Cardiol 100(3):507–512
Kühn A, Meierhofer C, Rutz T, Rondak I-C, Röhlig C, Schreiber C et al (2016) Non-volumetric echocardiographic indices and qualitative assessment of right ventricular systolic function in Ebstein’s anomaly: comparison with CMR-derived ejection fraction in 49 patients. Eur Heart J Cardiovasc Imaging 17:930–935
Avitabile CM, Whitehead K, Fogel M, Mercer-Rosa L (2014) Tricuspid annular plane systolic excursion does not correlate with right ventricular ejection fraction in patients with hypoplastic left heart syndrome after Fontan operation. Pediatr Cardiol 35(7):1253–1258
Pettersen E, Helle-Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B et al (2007) Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol 49:2450–2456
Sheehan FH, Ge S, Vick GW III, Urnes K, Kerwin WS, Bolson EL et al (2008) Three-dimensional shape analysis of right ventricular remodeling in repaired tetralogy of Fallot. Am J Cardiol 101:107–113
Van der Zwann HB, Geleijnse NL, McGhie JS, Boersma E, Helbing WA, Meijboom FJ et al (2011) Right ventricular quantification in clinical practice: two-dimensional vs* three-dimensional echocardiography compared with cardiac magnetic resonance imaging. Eur J Echocardiogr 12(9):656–664
Kühl HP, Schreckenberg M, Rulands D, Katoh M, Schäfer W, Schummers G et al (2004) High-resolution transthoracic real-time three-dimensional echocardiography: quantification of cardiac volumes and function using semi-automatic border detection and comparison with cardiac magnetic resonance imaging. J Am Coll Cardiol 43(11):2083–2090
Ostenfeld E, Carlsson M, Shahgaldi K, Roijer A, Holm J (2012) Manual correction of semi-automatic three-dimensional echocardiography is needed for right ventricular assessment in adults; validation with cardiac magnetic resonance. Cardiovasc Ultrasound. https://doi.org/10.1186/1476-7120-10-1
Crean AM, Maredia N, Ballard G, Menezes R, Whartson G, Foster J et al (2011) 3D Echo systematically underestimated right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease with moderate to severe RV dilatation. J Cardiovasc Magn Reson 13(1):78
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Dr. Mercer-Rosa is supported by grant NIH K01HL125521 and by Pulmonary Hypertension Association Supplement to K01HL125521.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lianza, A.C., Rodrigues, A.C.T., Mercer-Rosa, L. et al. Right Ventricular Systolic Function After the Cone Procedure for Ebstein's Anomaly: Comparison Between Echocardiography and Cardiac Magnetic Resonance. Pediatr Cardiol 41, 985–995 (2020). https://doi.org/10.1007/s00246-020-02347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-020-02347-6