Abstract
Surgical re-implantation of the left coronary artery (LCA) is the treatment of choice in anomalous origin of left coronary artery from pulmonary artery (ALCAPA). Despite normalization of left ventricular function after surgery, residual coronary lesions or myocardial fibrosis may be found. The aim of this study was to detect regional left ventricular dysfunction predictive of coronary lesions or residual myocardial fibrosis using speckle tracking. We enrolled ten patients treated with surgical re-implantation of LCA for ALCAPA. All patients were asymptomatic, and ejection fraction (EF) was normal. Using S-SR imaging, we studied longitudinal, radial, and circumferential function. A cardiac MRI was performed to assess myocardial fibrosis and the anatomy of the coronaries. In case of suspected coronary stenosis, a coronary angiography was performed. Finally, 20 normal subjects were enrolled as control group. Median age at surgery was 188 days, and mean follow-up was 8.7 ± 4.7 years. Longitudinal and circumferential functions were reduced in LCA territory compared to RCA territory and normal. MRI showed LCA stenosis in three of ten patients, confirmed by coronary angiography: these patients had the lowest longitudinal strain values in LCA territories (−11.7, −14.7 and −14.8%). Radial strain was preserved (Normal 45.6 ± 12.1, ALCAPA 43.5 ± 10.7%, p = ns), while circumferential strain was mildly depressed (−23.5 ± 3.8 vs. −20.3 ± 2.0%, p < 0.05). After LCA re-implantation, ALCAPA patients showed a residual sub-endocardial damage in LCA territories. Despite a normal systolic and diastolic function, the prevalence of residual coronary lesions was high. A mean longitudinal strain >−15% in LCA territories was able to identify those patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) is a rare congenital heart defect, occurring one in every 300,000 live birth with an incidence of 0.25–0.5% in children with congenital heart defects [1, 2]. It usually presents in infancy with myocardial ischemia and congestive heart failure, and if left untreated results in 90% mortality in the first year of life. However, 5–10% of cases can go beyond infancy into adulthood with risk of myocardial infarction, left ventricular dysfunction, mitral regurgitation, arrhythmia, cardiac insufficiency, silent myocardial ischemia, and sudden cardiac death [3].
Successful revascularization sets in motion the process of reverse left ventricular (LV) remodeling which in turn accounts for the dramatic improvement in LV function and resolution of mitral regurgitation. However, some degree of chronic impairment from pre-operative ultra-structural abnormalities may persist. Some workers have demonstrated variable degree of fibrosis with altered but viable myocytes, endocardial, and sub-endocardial fibrosis along with patchy myocardial necrosis resulting in incomplete reverse LV remodeling, and this may account for the few patients with persistent decrease in LV functions as late complications and late sequel of severe mitral valve regurgitation requiring valvular replacement [4–6].
Although most patients show good functional recovery after successful surgical correction, there is still the possibility of subtle persistent functional disability which is thought to exist in asymptomatic survivors of ALCAPA repair. Also the possibilities of persistent or recurrent myocardial damage, persistent subclinical ischemia, and new onset of transmural myocardial scaring, even after successful repair with subsequent recovery of LV function, have been documented [7, 8]. The knowledge of these facts brings to light the importance of long-term follow-up and consideration of the modalities most appropriate for the evaluation of survivors of ALCAPA repair. The optimal means of evaluation of myocardial function for asymptomatic long-term survivors of ALCAPA repair remains to be determined.
Myocardial strain (S) and strain rate (SR) measurement is an established echocardiographic technique that allows reliable analysis of LV deformation of all myocardial segments in radial, circumferential, and longitudinal directions and in addition provide information on myocardial torsion [9–12].
The aim of this study is to detect regional left ventricular dysfunction predictive of coronary lesions or residual myocardial fibrosis using echo-derived S.
Methods
Study Population
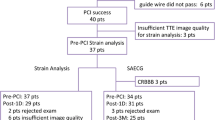
Between 2000 and 2010, in our Institution, 20 patients affected by ALCAPA were repaired using surgical LCA re-implantation. Between all, two patients died over the follow-up; three were lost to the follow-up; one presented severe mitral regurgitation and LV dilatation; four continued the follow-up in other Institutions and refused to participate to the study; and ten patients continued the follow-up in our Institution and accepted to participate to this study. After obtaining written informed consent, for all the patients, an echocardiography and a Cardiac MRI were planned on the same day. If coronary lesions were suspected, a coronary angiography was planned for diagnostic or interventional purposes.
Twenty healthy subjects with similar anthropometric characteristics were identified as control group just to compare echocardiographic data.
Local Ethical Committee approved the study (prot. N. 0015783—University of Padova).
Echocardiographic Data
Transthoracic echocardiographic recording was performed using a GE Vivid 9 ultrasound machine (GE Vingmed Ultrasound AS, Horten, Norway). A standard parasternal long-axis view was used to derive M-mode measurements of the LV end-systolic and end-diastolic dimensions and the right ventricular (RV) diastolic dimension.
The LV ejection fraction was calculated according to the modified Simpson rule. The global longitudinal ventricular function was assessed from standard apical views, with measurement of lateral, septal, and inferior mitral ring segments (LV function) as well as lateral tricuspid ring (RV function) displacement by conventional M-mode methods. The mitral valve inflow pattern was measured using peak E-wave, peak A-wave, and E-wave deceleration time.
To record mitral annulus velocities by Tissue Doppler imaging (TDI), a two-dimensionally guided 5-mm Doppler gate was placed at the site of the septal and the lateral mitral annulus and the lateral tricuspid annulus. The parameters measured were peak velocities (cm/s) during early diastole (E′ wave) for each site.
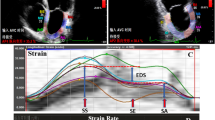
Speckle tracking evaluation was performed offline with the Echopac11software (GE, Echopac, Horten, Norway). Left ventricular longitudinal S and SR were assessed in the apical four-chamber view, the apical three-chamber view, and the apical two-chamber view. Left ventricular segments were divided in right coronary artery (RCA) and left coronary artery (LCA) territories taking in account the pre-operative coronary angiography, performed in all the patients enrolled. Measurements of radial and circumferential S and SR were calculated in the parasternal short-axis view at the level of the mitral valve and apex. The timing of any cardiac event was estimated from the blood-pool Doppler recordings, with end systole defined as aortic valve closure.
After manual tracing of the endocardial border in the end-systolic frame of a two-dimensional image and selection of the appropriate wall thickness, the software automatically determined six segments in each view. For longitudinal function, the apical, mid, and basal segments of the ventricular septum and lateral walls were determined from the apical four-chamber view; the apical, mid, and basal segments of the inferior and anterior walls from the apical two-chamber view; and the apical, mid, and basal segments of the posterior and antero-septal walls from the apical three-chamber view. For radial and circumferential function, the septal, antero-septal, anterior, lateral, posterior, and inferior segments were determined from the parasternal short-axis views.
In each region of interest, the motion of the acoustic markers in the myocardial tissue during the cardiac cycle was tracked automatically (speckle tracking). The tracking quality was scored as valid or poor. Segments with poor tracking were subjected to manual readjustments of the region of interest to improve the tracking score, and if this was not achievable, they were excluded from the analysis. Longitudinal, radial, and circumferential strain curves and peak systolic strain values then were created automatically and displayed by the Echopac software. All measurements were obtained at end expiration and averaged from three cardiac cycles. Inter- and intra-observer variability was tested in ten patients for each group.
Cardiac- MRI
CMR studies were performed on a 1.5 T scanner (Philips Achieva, Philips Healthcare, Best, The Netherlands) and a five-channel surface coil. Biventricular end-diastolic volume, end-systolic volume, and ejection fraction were measured from a stack of cine steady-state free precession short-axis images from atrioventricular junction to apex.
Free-breathing steady-state free precession cine cardiac magnetic resonance with respiratory navigator gating was performed to assess coronary origins and at least proximal tract anatomy.
Assessment of the presence of late gadolinium enhancement (LGE) imaging was performed in ventricular short-axis planes and in four-chamber planes, 10–15 min after contrast administration using a standard two-dimensional breath-hold phase-sensitive inversion recovery sequence with the inversion time selected to null the myocardial signal.
The presence of LGE was classified into a) number of cardiac segment involved; b) extension of the fibrosis: transmural, sub-endocardial or intra-myocardial fibrosis.
Coronary Angiography
An invasive selective coronary angiography by right femoral artery was performed in all the patients with suspected coronary lesion by echo or by C-MRI. The exam was performed in deep sedation and local anesthesia.
Statistical Analysis
Statistical analysis was performed using the software SPSS for windows version 18 (SPSS, Chicago, IL, USA). One-tailed unpaired Student’s t-test was used to compare left coronary artery and right coronary artery territories. Two-tailed unpaired student t-test was used to compare ALCAPA patients and control group. Continuous variables are expressed as mean ± standard deviation. A p value ≤ 0.05 was considered as statistically significant. Receiver operating characteristic curves of peak systolic strain defined by STE were calculated for the distinction of patients with and without myocardial fibrosis defined by LGE CMR.
Speckle tracking reproducibility was determined in 20 randomly selected subjects (ten patients and ten control subjects). Inter- and intra-observer variability was examined using both Pearson’s bivariate two-tailed correlations and Bland–Altman analysis.
Results
General characteristics of the patients are shown in Table 1. All the patients at diagnosis presented a severe dilated cardiomyopathy; the coronary angiography showed a significant left to right shunt due to the relevant collateral vessels by RCA to LCA. The surgery was planned within 1 week from the diagnosis. Median age at surgery was 188 days (range 54 days–8 years), and mean follow-up was 8.7 ± 4.7 years. Echocardiographic image quality was at least sufficient for all the patients, and all segments were successfully analyzed. Intra- and inter-observer variability was <5% for both the comparisons.
ALCAPA patients presented a mean LVEF similar to normal (65 ± 5% for both groups). Diastole was normal in all patients (E/A ratio 1.8 ± 0.5; E/E′lat ratio 7.2 ± 2.4). Mitral regurgitation was mild or trivial in all the patients.
Longitudinal and circumferential functions were reduced in LCA territories compared to RCA territories and normal subjects (Longitudinal S: LCA normal −21.3 ± 1.1%, LCA ALCAPA −16.4 ± 2.4% p < 0.0001; RCA normal −21.3 ± 1.1%, RCA ALCAPA −21.6 ± 1.1%, p = 0,49; Circumferential S: −23.5 ± 3.8 vs. -20,3 ± 2.0; p = 0.019) (Fig. 1; Table 2), while radial strain was preserved (Normal 45.0 ± 12.5%, ALCAPA 40.1 ± 20.2%, p = ns). C-MRI showed LCA stenosis in three of ten patients: these patients had the lowest longitudinal strain values in LCA territories (−11.7, −14.7 and −14.8%). Late gadolinium enhancement showed the presence of fibrosis in all patients: postero-lateral papillary muscle was constantly involved. Patients with LCA stenosis showed a higher extension of fibrosis (27 over 170 segments involved in ten patients, between patients with LCA occlusion the number of LV segments involved were 4, 5, and 8, respectively). CA showed complete occlusion of LCA in two patients and a sub-occlusion in one patient. In the last case, it was not possible to cross the stenosis with a coronary guide-wire.
Comparing the segments with LGE by MRI with regional longitudinal strain a value of less than −14.8% allowed the prediction of segmental myocardial fibrosis with a sensibility of 92.5%, a specificity of 93.7%; a positive predicted value of 73.5%; and a negative predicted value of 98.5%. The area under the receiver operating characteristic curve was 0.86.
Discussion
Anomalous origin of the left coronary artery from the pulmonary artery is a rare and life-threatening malformation due to an embryological defect during heart development. Surgical correction by direct aortic re-implantation of the anomalous coronary artery is mandatory as soon as possible after diagnosis.
Standard echo data confirmed that most of the patients recover systo-diastolic LV function after successful LCA re-implantation, but the evidence of residual myocardial fibro-elastosis and mild residual mitral valve regurgitation is relatively common, although well tolerated by patients [13]. The reasons of functional recovery are partially unknown: some Authors have speculated the presence of hibernated myocardium [14, 15], others hypothesized that the growth of healthy myocytes was able to reduce perceptually the scar extension [14], others the proliferation of stem cells after recovery of coronary flow [16]. Latus et al. [8], using MRI, recently demonstrated that the scar often appears after surgery. Even after successful coronary re-implantation with subsequent recovery of LV function, the possibility of persistent or recurrent myocardial damage and subclinical ischemia was still present [17]. Similar data were described by Secinaro et al., [7] who showed that sub-endocardial scarring occurred in five of the six patients despite normal LV function, and Fratz et al. [14] who described to a little extent (0–11% of LV mass) of myocardial scarring in 71% of the patients after ALCAPA repair, but this was not related to the LV dimension, function, or exercise capacity.
Other studies reported a reduced longitudinal strain values in ALCAPA patients; however, the data were reported as a global LV value [13, 18]. In our study, S-SR imaging demonstrated the presence of residual sub-endocardial dysfunction in LCA territory of these patients, while the sub-endocardial segments perfused by RCA were preserved. These data are not surprising, because previous studies indicated the high incidence of residual ischemia in ALCAPA patients induced by stress test [7, 13, 14]. These Authors advocated many possible causes for these findings, mostly surgical variables such as myocardial protection during cardioplegia, LCA stenosis after surgery or long ischemic time. Our data showed that the damage was isolated to LCA territories, and the sub-endocardial hypo-contractility was present in all patients. The sub-endocardium may be the most susceptible area to ischemia due to a competitive flow between collateral and the “inverted” flow in LCA after surgery. This hypothesis is supported by experimental studies performed on pigs by Nordgaard et al. [19].
Schmitt et al. studied the prevalence of wall motion abnormalities by stress MRI [17]. In their study, the prevalence of scar as well as of wall motion abnormalities in one or more LV segments was high (67%), with inducible perfusion deficit found in 3 over 12 patients. However, the perfusion deficit was not apparently related to LCA stenosis. On the other hand, cardio-pulmonary exercise test did not show any relevant impairment of exercise performance. Accordingly, none of the patients enrolled in our Institution showed inducible ischemia by exercise test or SPECT. The false-negative results obtained by standard evaluation may be due to the adequacy of hetero-coronary collaterals or to the intrinsic limits of the test commonly used [20]. On the other hand, these patients may be at higher risk (unquantifiable at the moment) of events or clinical worsening in the long-term follow-up.
Our data showed that the 30% of patients showed a silent LCA ostial occlusion or sub-occlusion. The prevalence of late LCA ostial lesions is unknown, because often these patients have a normal LV function by echo, and the anatomical imaging is not routinely assessed. Secinaro et al. [7] studied six patients with evidence of inducible ischemia 10 years after coronary re-implantation and found out that the LCA was occluded in three of them. However, all patients were asymptomatic, and no standard echo parameter was able to identify systolic or diastolic dysfunction.
On the contrary, longitudinal strain was more specific in these patients, confirming the value of this parameter in the identification of regional dysfunction. The sub-endocardial layer is, in fact, the terminal layer for coronary perfusion, so it is the most susceptible to the ischemia despite the adequate hetero-coronary collaterals, as well as may be sensible to the competitive flow between collaterals and LCA flow. On the contrary, radial and circumferential strain are preserved in these patients. The compensatory effect of external myocardial layers is the reason of the preserved EF in these patients.
Our C-MRI data showed that fibrosis of one or more segments was evident in all the patients studied and not related to age at surgery. In particular, postero-lateral papillary muscle was constantly involved. However, a more extended fibrosis was evident in patient with LCA occlusion. More interestingly, the evidence of a dilated RCA (>5 mm) was evident in all except in patients with LCA ostial stenosis/occlusion.
Study Limitations
This study is limited by the small number of patients enrolled; however, the pathology studied is extremely rare and is difficult to enroll a large number of patients in a single center. Second, the study was cross sectional, and S-SR values were not available before surgery.
Conclusions
After LCA re-implantation, ALCAPA patients showed a residual sub-endocardial damage in LCA territories, due to the low perfusion before, during or after surgical re-implantation. Radial function was generally preserved in patients with normal ejection fraction. Despite a normal systolic and diastolic function, the prevalence of residual coronary lesions was high (30%). For these reasons, an anatomic imaging of proximal coronaries arteries should be warranted to all asymptomatic patients in adolescence. Speckle tracking may play a role in the identification of ischemic or necrotic segments also in this group of patients, improving the diagnostic capability of echocardiography. This tool could be particularly useful in infants in order to limit the use of exams requiring sedation, radionuclide infusion, or exposure to radiations.Author: Kindly check whether the processed reference 15 is correct or not.Yes. I tested successfully the reference on pubmedAuthor: Figure [1] was received; however, no citation was provided in the manuscript. Please check and provide it.Fig. 1 was cited in the results, together with Table 2
Abbreviations
- ALCAPA:
-
Anomalous origin of left coronary artery from the pulmonary artery
- C-MRI:
-
Cardiac magnetic resonance imaging
- IVRT:
-
Isovolumetric relaxation time
- LCA:
-
Left coronary artery
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle
- RCA:
-
Right coronary artery
- RV:
-
Right ventricle
- S:
-
Strain
- SR:
-
Strain rate
- TDI:
-
Tissue Doppler imaging
References
Cowies RA, Berdon WE (2007) Bland—White—Garland syndrome of anomalous left coronary artery arising from the pulmonary artery [ALPACA]: a historical review. Pediatr Radiol 37:890–895
Dodge-Khatami A, Mavroudis C, Backer CL (2002) Anomalous origin of the left coronary artery from the pulmonary artery: collective review of surgical therapy. Ann Thorac Surg 74:946–955
Wesselhoeft H, Fawcett JS, Johnson AL (1968) Anomalous origin of the left coronary artery from the pulmonary trunk: its clinical spectrum, pathology, and pathophysiology, based on a review of 140 cases with seven further cases. Circulation 38:403–425
Shivalkar B, Borgers M, Daemen W, Gewillig M, Flameng W (1994) ALCAPA syndrome: an example of chronic myocardial hypoperfusion? J Am Coll Cardiol 23:772–778
Smith A, Arnold R, Anderson RH, Qureshi SA, Gertis LM, Mckay R. (1989) Anomalous origin of the left coronary artery from the pulmonary trunk: anatomic findings in relation to pathophysiology and surgical repair. J Thorac Cardiovasc Surg 98:16–24.
Singh TP, Dicarli MF, Sullivan NM, Leonen MF, Morrow WR (1998) Myocardial flow reserve in long term survivors of repair of anomalous left coronary artery from pulmonary artery. J Am Coll Cardiol 31:437–443
Secinaro A, Ntsinjana H, Tann O et al (2001) Cardiovascular magnetic resonance findings in repaired anomalous left coronary artery to pulmonary artery connection (ALCAPA). J Cardiovasc Magn Reson 13:27
Latus H, Gummel K, Rupp S et al. (2014) Cardiovascular magnetic resonance assessment of ventricular function and myocardial scarring before and early after repair of anomalous left coronary artery from the pulmonary artery. J Carvasc Magn Reson 16:3
Di Salvo G, Pacileo G, Rea A et al (2005) Quantitative evaluation of regional myocardial function using strain and strain rate imaging: normal values in pediatric age. Ital Heart J 6:420–426
Di Salvo G, Pacileo G, Gala S et al (2006) Strain rate imaging: data acquisition and post processing. Minerva Cardioangiol 54:451–459
Langeland S, D’hooge J, Wouters PF et al (2005) Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation 112:2157–2162
Castaldi B, Santoro G, Di Salvo G et al (2013) Impact of the Amplatzer atrial septal occlude device on left ventricular function in pediatric patients. Pediatr Cardiol 34(7):1645–1651
Cabrera AG, Chen DW, Pignatelli RH et al (2015) Outcomes of anomalous left coronary artery from pulmonary artery repair: beyond normal function. Ann Thorac Surg 99(4):1342–1347
Fratz S, Hager A, Schreiber C, Schwaiger M, Hess J, Stern HC (2011) Long-term myocardial scarring after operation for anomalous left coronary artery from the pulmonary artery. Ann Thorac Surg 92(5):1761–1765
Shivalkar B, Borgers M, Daenen W, Gewillig M, Flameng W. (2009) ALCAPA syndrome: an example of chronic myocardial hypoperfusion? J Am Coll Cardiol 23(3): 772–778
Bergmann O, Bhardwaj RD, Bernard S et al (2009) Evidence for cardiomyocyte renewal in humans. Science 324:98–102
Schmitt B, Bauer S, Kutty S, Nordmeyer S, Nasseri B, Berger F, Alexi-Meskishvili V (2014) Myocardial perfusion, scarring, and function in anomalous left coronary artery from the pulmonary artery syndrome: a long-term analysis using magnetic resonance imaging. Ann Thorac Surg 98(4):1425–1436
Di Salvo G, Eyskens B, Claus P et al (2004) Late post-repair ventricular function in patients with origin of the left main coronary artery from the pulmonary trunk. Am J Cardiol 93:506–508
Nordgaard H, Swillens A, Nordhaug D et al (2010) Impact of competitive flow on wall shear stress in coronary surgery: computational fluid dynamics of a LIMA-LAD model. Cardiovasc Res 88(3):512–519
Neglia D, Rovai D, Caselli C et al. (2015) Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging 8(3):e002179
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Castaldi, B., Vida, V., Reffo, E. et al. Speckle Tracking in ALCAPA Patients After Surgical Repair as Predictor of Residual Coronary Disease. Pediatr Cardiol 38, 794–800 (2017). https://doi.org/10.1007/s00246-017-1583-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1583-z