Abstract
Obesity and left ventricular hypertrophy (LVH) have been identified as independent risk factors for cardiovascular events. The definition of LVH depends on the geometric algorithm used to calculate LV mass (LVM) by echocardiography and the method used to normalize LVM for body size. This study evaluates the effect of these methods on the prevalence of LVH in obese children. LVM for 109 obese and 109 age-matched non-obese children was calculated using M-mode or two-dimensional echocardiography (2DE). LVM was then normalized to height 2.7 as indexed LVM (LVMI), to body surface area (BSA), height, and lean body mass (LBM) as LVM Z-scores. LVH was defined as LVMI >95th ‰ using age-specific normal reference values or LVM Z-scores ≥2. The prevalence of LVH by LVMI and LVM Z-scores was compared. There was a correlation between LVM determined by M-mode and by 2DE (R 2 = 0.91), although M-mode LVM was greater than 2DE LVM. However, the difference between these values was greater in obese children than in non-obese children. Based on the method of normalization, the prevalence of LVH among obese children was 64 % using LVMI, 15 % using LVM Z-scores for height, 8 % using LVM Z-scores for BSA and 1 % using LVM Z-scores for LBM. Height-based normalization correlates with obesity and hypertension. The methods used to measure and normalize LVM have a profound influence on the diagnosis of LVH in obese children. Further study is needed to determine which method identifies children at risk for cardiovascular morbidity and mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
The presence of left ventricular (LV) hypertrophy (LVH) has been identified as a predictor of adverse cardiovascular events. In adults, LVH has been associated with complications such as myocardial infarction, cerebrovascular events, congestive heart failure, and death [1–5]. In fact, the importance of LVH as an independent risk factor for future cardiovascular events is second only to older age. In children, establishing the diagnosis of LVH requires that LV mass (LVM) be adjusted for the effects of body size [6]. Several body size parameters have been used. In this population, LVH has been associated with hypertension [7–10] and obesity [11–18] in studies using height as the body size parameter. Also in studies using body surface area (BSA) as the body size parameter, LV outflow tract obstruction has been associated with LVH [19].
Recent studies show that 34 % of American children 12–19 years of age are overweight or obese [20]. These adolescents are more likely to present with cardiovascular risk factors such as hypertension [21], abnormal lipid levels [22], prediabetes or diabetes [23], and increased C-reactive protein [24, 25]. With the increasing prevalence of childhood obesity, characterizing the relationship between LVM and obesity has become more important as LVH may be an early indicator of cardiovascular disease in this population.
Echocardiography is the most common modality used to estimate LVM in children [26]. There are several algorithms to calculate LVM, including the prolate ellipsoid formula using M-mode measurements and the area–length formula using two-dimensional echocardiographic (2DE) measurements [27–29]. Each approach makes geometric assumptions about the shape of the ventricle, and there is controversy over which method to use in children [30]. Because of the cubing function associated with the prolate ellipsoid formula, small measurement errors are magnified. In addition, the accuracy of M-mode measurements can be limited by oblique sampling, particularly in the setting of limited echocardiographic windows. In contrast, limited echocardiographic windows, especially in obese children, often involve foreshortening of the LV in 2DE apical views, thereby affecting the measurements in the area–length formula. Studies comparing methods to calculate LVM have been limited to non-obese children.
As stated previously, LVM must be adjusted for body size in order to determine LVH [30]. The most commonly used parameters for which LVM has been adjusted include height, BSA, and lean body mass (LBM), and the choice of method may affect which patients are diagnosed with LVH, thereby affecting management of children at risk [30–38]. Since in subjects with normal weight LVM strongly correlates with LBM, many suggest that LBM is the ideal parameter [39]. However, LBM is not readily measured, and both height and BSA have been used as surrogates for LBM [40]. Other studies have evaluated LVM normalization in overweight and mildly obese children. However, they have not involved children with moderate-to-severe obesity [41]. This limitation is especially important because of the increasing prevalence of severe obesity among children and adolescents [42]. Previous investigations that demonstrate high prevalence of LVH in childhood obesity have used the prolate ellipsoid formula using M-mode measurements and have focused primarily on the effect of height [43–48]. However, method of calculation of LVM and its normalization may affect this prevalence. As 2DE method of measurement of LVM results in lower estimates of LVM and BSA is disproportionately higher than height in obese children, LVM by 2DE method when adjusted for BSA may result in underestimation of the prevalence of LVH. This study was performed to determine whether the method used to calculate and normalize LVM significantly affects the prevalence of LVH in obese children. The hypothesis was that LVM adjusted for height would result in a higher prevalence of LVH compared to LVM adjusted for BSA.
Methods
Study Population
This retrospective analysis of clinical and echocardiographic data was approved by the Institutional Review Board. Several standard definitions were utilized for this study. Patients with a body mass index (BMI) ≥97.5th ‰ (Z-score ≥ 2) were considered as obese. Those with a BMI between 95th and 97.5th ‰ were classified as mildly obese, between 85th and 95th ‰ as overweight, and ≤15th ‰ as underweight. Hypertension was defined by the presence of a systolic and/or diastolic blood pressure (BP) ≥95th ‰ (Z-score ≥ 1.6). Two groups were identified among the children 3–17 years of age who had an echocardiogram performed at the Children’s Hospital at Montefiore from January 2009 to December 2010. The obese group consisted of patients with a BMI ≥97.5th ‰ undergoing evaluation for a heart murmur or hypertension. The control group consisted of patients who had a BMI between 15th and 85th ‰ and a BP <95th ‰, were evaluated for a heart murmur, and were matched for age, gender, and race to the obese patients. Patients were excluded if any of the following criteria were present: (1) congenital heart disease, (2) systemic disorders such as sickle cell disease, cancer, rheumatic heart disease, Kawasaki disease, systemic lupus erythematosus, pulmonary hypertension, solid organ transplant, Marfan syndrome, and genetic syndromes, (3) arrhythmias, and (4) treatment with antihypertensive medications.
Demographic and Clinical Data
Age, gender, and self-reported race data (when available) were collected for each patient. Height and weight were measured by trained personnel using a SCALE-TRONIX 5002 stand-on scale (height minimum 0.5 cm, weight minimum 0.1 kg) (SCALE-TRONIX, Carol Stream, IL), and BSA was calculated using the Haycock formula (BSA = 0.024265 × weight 0.5378 × height 0.3964) [49]. Z-scores for weight, height, and BMI were calculated using the Lambda Mu Sigma (LMS) tables published by the Centers for Disease Control [50]. LBM was estimated for each participant using validated sex-specific predictive equations (males: Ln(LBM) = −2.8990 + 0.8064 × Ln(height) + 0.5674 × Ln(weight) + 0.0000185 × weight2 − 0.0153 × BMI Z score2 + 0.0132 × age; females: Ln(LBM) = −3.8345 + 0.954 × Ln(height) + 0.6515 × Ln(weight) − 0.0102 × BMI Z score2) [51]. Systolic and diastolic blood pressures were measured in a sitting position using a DINAMAP (PROCARE 400 GE, Fairfield, CT), and all measurements were converted to Z-scores using the Fourth Report Criteria of the National High Blood Pressure Education Program Working Group in the USA [10].
Echocardiographic Data
All patients underwent an echocardiographic evaluation using either the IE33 (Philips Inc., Andover, MA, USA) or Sequoia 512C (Siemens Medical Solutions Inc. Mountain View, CA, USA) ultrasound systems. 2DE and M-mode measurements were obtained using a standard protocol [26] in subcostal, apical four-chamber, and/or parasternal short-axis views. All studies were stored digitally and re-analyzed by one author (J.M.). Additional author (P.H.) repeated all measurements and calculations in 20 % of the studies (n = 43) and these measurements used for interobserver variability analysis. The second reader was blinded to previous measurements and allowed to select the best image for measurement. Both readers were blinded to prior measurements and calculations. The following M-mode measurements were obtained: LV end-diastolic diameter (EDD), LV end-diastolic septal wall thickness (SWT), and LV end-diastolic posterior wall thickness (PWT). The following 2DE measurements were obtained: LV end-diastolic endocardial area, LV end-diastolic endocardial length, LV end-diastolic epicardial area, and LV end-diastolic epicardial length. Measurements were performed once and within a single cardiac cycle.
Echocardiographic Calculations and Indices
M-mode LVM was calculated using the prolate ellipsoid formula as recommended by the chamber quantification guidelines for adult echocardiography [27]. 2DE LVM was calculated by determining LV endocardial and epicardial end-diastolic volumes (EDV) using the area–length formula [26] and subtracting the endocardial EDV from the epicardial EDV and multiplying the difference (myocardial volume) by 1.05 g/mL, the myocardial density [52].
Considering that published normal values for LVM normalized to height and LBM only used M-mode-derived values, M-mode LVM values were normalized for body size using the following four methods: LVM index (LVMI) calculated as LVM divided by height 2.7 and LVM Z-scores based on height; BSA and LBM calculated from normal data published previously [6, 41, 53]. Patients were classified as having LVH based on two possible combinations: (1) LVMI >95th ‰ (based on age and gender specific reference values for normal children) [54] or (2) LVM Z-scores for height, BSA and LBM ≥2.
Statistical Analysis
Descriptive statistics (mean ± standard deviation) were generated for all demographic characteristics. All values were checked for normality. Means, confidence intervals, standard deviations, standard errors, ranges, P values, and equality of variances were calculated for all variables. Demographic information, anthropomorphic data, and all echocardiographic measurements and calculations and their corresponding Z-scores were compared between the obese and control group.
LVM calculations based on M-mode and 2DE measurements were compared by calculating the percentage differences (absolute difference between measurements divided by the mean value). Interobserver percentage differences were also calculated. Intraclass correlation coefficients with absolute agreement of single measures were used to test for the correlation between the two methods and the two observers. Bland–Altman limits of agreement were evaluated for LVM in the obese and control groups [55].
The agreements between the LVM Z-scores were assessed using intraclass correlation coefficient with absolute agreement of single value. The relationships between BMI Z-score and systolic BP Z-score and each of LVM Z-scores were evaluated among control and obese groups. These relations were investigated using linear regression.
Fishers exact, t tests, and analysis of variance were performed to compare the means. χ 2 test for significance was used to evaluate differences in the proportion of patients with LVH using different normalization methods. All tests were two-sided. Statistical significance was defined as P < 0.05. Statistical analyses were performed using SPSS version 22 (IBM, Armonk, NY).
Results
Study Population
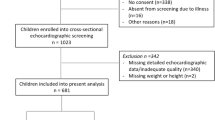
Twelve hundred and twenty-one patients between the ages of 3 and 17 years underwent echocardiographic evaluation for a murmur or for hypertension during the study period. The obese group consisted of 112 patients of whom 109 had the required echocardiographic views for LVM measurements. In this group, 40/109 patients had an abnormally high BP during the echocardiogram (N = 28) and/or were referred for cardiac evaluation of hypertension (N = 24). The control group consisted of 109 patients matched for age, gender, and race (Fig. 1). No patients in the control group had an abnormally high BP or a history of hypertension. Demographic and anthropometric data are compared between groups in Table 1.
M-mode Versus 2DE Measurements
Intraclass correlation coefficients and percentage differences of the mean between the M-mode and 2DE LVM for the obese and control groups are given in Table 2. The linear correlation and Bland–Altman scatter plots between the M-mode LVM and 2DE LVM for the obese and control groups are shown in Fig. 2a, b. Although M-mode LVM correlated well with 2DE LVM, M-mode LVM was higher than 2DE LVM in both groups. In addition, the percentage differences between M-mode LVM and 2DE LVM were higher for the obese group (12.7 ± 8.3 % in controls vs. 16.9 ± 11.8 % in the obese group, P = 0.003).
a Obese group: LVM linear correlation between M-mode and 2DE and Bland–Altman scatter plot of difference between methods against the average of the two (N = 109). b Control group: LVM linear correlation between M-mode and 2DE and Bland–Altman scatter plot of difference between methods against the average of the two (N = 109)
Interobserver Variability
Interobserver variability data are summarized in Table 3. There was no significant difference in the percentage differences for M-mode LVM and 2DE LVM for both the obese group and control group. There was a significant difference in the percentage differences for EDV between obese and control group (P = 0.001).
Echocardiography
Echocardiographic measurements and calculations, including mean Z-scores if reference values were available, were compared between the obese and control groups in Table 4. The mean Z-scores for all measurements and calculations were within normal limits for both groups. The mean M-mode EDD and 2DE EDV Z-scores were lower, and the mean M-mode SWT and PWT Z-scores were higher in the obese group. The mean M-mode LVM Z-scores based on LBM and height were significantly higher in the obese group, but the mean Z-scores based on BSA were not different. Agreement between different LVM Z-scores using intraclass correlation coefficient is given in Table 5. There were good agreements between all three Z-scores in the control group. Agreement between LVM Z-scores by BSA and height in the obese group was poor. In both groups, there was better agreement between LVM Z-scores by BSA and LBM compared to LVM Z-scores by height and LBM. Heteroscedasticity was seen in the control group with a persistent relationship between LVMI and height (r = −0.238, P = 0.006) and obese group with a persistent relationship between LVM Z-scores by height and height (r = 0.361, P < 0.001), but they were not seen in other normalizations in obese and control groups.
Correlations between normalized LVM and measured risk factors for LVH in our cohort are given in Table 6. There was a significant correlation between BMI Z-score and sys BP Z-score with LVMI and LVM Z-scores by height in obese group. The correlations were also significant for BMI Z-score with LVMI and LVM Z-score for height in control group.
Prevalence of LVH
The prevalence of LVH based on the LVMI and LVM Z-scores for the obese and control groups is given in Table 7. Based on LVMI and LVM Z-score by height, the prevalence of LVH in obese was significantly higher than control group. The prevalence of LVH by different methods in obese group was significantly different (range 1–64 %, P < 0.001).
Discussion
Despite the strong correlation between M-mode and 2-DE methods for calculating LVM in normal children, LVM derived from the prolate ellipsoid M-mode method is higher than LVM derived from the area–length 2DE method in children. Other studies have reported similar findings along with the fact that both methods overestimate LVM values calculated from three-dimensional echocardiography in normal children, but these have not been investigated in obese children [28, 29]. There is greater discordance between M-mode and 2DE LVM in obese children, suggesting diminished accuracy or increased variability in obese patients. The higher interobserver variability of EDV measurements suggests diminished accuracy of the 2DE method in obese patients, although the variability was similar for 2DE LVM in both groups. Another study demonstrated better agreement with three-dimensional echocardiography and MRI for 2DE measurements compared to M-mode measurements, but interobserver variability for 2DE was higher than for M-mode and three-dimensional echocardiography [56].
Echocardiographic measurements must be adjusted for body size in order to allow for comparisons among children with and without heart disease. Traditional approaches to normalization are based on ratiometric relationships (the cardiovascular parameter is divided by some measure of body size). In contrast, allometric normalization allows for a nonlinear relationship between the measurement and the body size parameter by using a body size variable raised to a scalar exponent, an approach which has become increasingly popular because it avoids assumptions about variance [57]. Recommended normalization methods have involved BSA, height, height to the power of 1.7 and 2.7, and LBM (fat-free) [37, 39]. The contribution of adipose tissue to BSA is significant, especially in obese children for whom normalization of the sizes and function of cardiovascular structures is important [39]. Alternatively, LVM, like cardiac output, is determined primarily by the demands of metabolically active tissue. Although height correlates very well with LBM, the correlation is not perfect, especially in obese children.
Currently available definitions for LVH include LVMI >95th ‰ and LVM Z-scores ≥2 (LVM ≥ 97.5th ‰). In an effort to replicate common practice using the two different approaches, all comparisons in this study utilized these definitions. Several studies involving children with kidney disease have shown that different normalization methods affect the estimated prevalence of LVH, varying by as much as 37 % (18 vs. 55 %) depending on the methodology used [58, 59]. In these studies, LVM was determined using the M-mode method only. The variability is even more accentuated when both M-mode and 2DE measurements are included. Our data, along with previously published reports [28, 29, 60], demonstrate a significant difference between LVM measured by 2DE and M-mode.
The marked discrepancy in the prevalence of LVH in obese children using the different methods (1–64 %) seen in our study has been seen previously in adults with hypertension and obesity [35]. In obese children, LVM normalized to BSA probably underestimates the prevalence of LVH, but it is also likely that normalizing to height overestimates the prevalence of LVH in this population. In fact, this study along with other previously published studies demonstrates that LVM values normalized to BSA were lower in normotensive obese subjects compared to non-overweight subjects, while the LVM values normalized to height were not significantly different [61]. This may be attributed to the finding that overweight subjects tend to have a higher absolute LVM, and the effect of body fat on LVM is thought to be less than the effect of fat-free mass. We found a better correlation between BSA- and LBM-based normalization compared to height-based normalization. In a recent study among overweight children using LBM as the gold standard for normalization, Foster et al. [41] found that BSA underestimates and height overestimates the prevalence of LVH. Also their study demonstrates better agreement between BSA- and LBM-based normalization. Of note, the median BMI Z-score of overweight children in the Foster study was 1.4, which is lower than mean Z-score of 2.4 in our cohort. This may explain the higher discrepancy in the prevalence of LVH. The BSA- and LBM-based Z-scores were not available in 22 and 28 % of our obese population, respectively. This practical problem is very important as there will not be any normal child with the BSA and LBM of the severely obese child who may be at highest risk of cardiovascular involvement. We found a correlation between height-based normalization and BMI and blood pressure in our population which implies that height may improve identification of abnormality in obese children. This is in concordance with previous studies done in the context of adult obesity whose proposed height is the best body size measure to be used in adult obesity [62–64].
One problem with normalized LVM values is the persistent dependence of the adjusted value on the parameter used to adjust the measurement, a phenomenon known as heteroscedasticity. This has been seen when LVM is adjusted for height to the power of 2.7 [53]. In our study, there is a persistent relationship between LVMI (adjusted for height) and height for the control group, but this heteroscedasticity is not seen in the obese group. The use of Z-scores is particularly useful, as it does not rely on a single mathematical relationship between LVM and height across the full range of pediatric body sizes. Although the use of Z-scores may be more appropriate, to replicate common practice, LVMI also was used to assess the effect of normalization to height. In our study, there is no persistent relationship between LVM Z-scores and BSA in both groups implying that Z-scores may avoid the heteroscedasticity problem.
Limitations
Several methodologies are available to assess LV geometric patterns using relative wall thickness or 2DE LVM-to-volume ratio, but this study was not powered to compare different geometric categories. LVM Z-scores were available only for patients with BSA <2.27 m2, limiting the sample size for our LVM Z-score comparison. In addition, the study focused strictly on obese patients and did not include mildly obese and overweight patients in the analyses, so the conclusions do not apply to the latter two groups. In addition, the control group was composed of patients seen in the outpatient clinic and may not fully represent a true normal population. Blood work to evaluate systemic inflammation or insulin resistance was not routinely performed in our obese population. In this retrospective study, each blood pressure measurement is obtained once for each patient by an oscillometric device, and we are not able to confirm the diagnosis of hypertension.
The prevalence of LVH in normal children using the M-mode measurements in our study is 9–10 %. Although a study using similar methodology in normal African-American adolescents reveals a prevalence of 12 %, this number is higher than the 2.3 % prevalence of LVH that one would expect in a normal population, which represents the prevalence corresponding to a Z-score ≥2. The high incidence of LVH in our normal population is likely related to several factors, including inconsistencies in published normal data, unknown confounding factors influencing LVM and the limitations of calculating or estimating LVM by echocardiography.
Conclusion
This study found that LVM values vary based on the method for performing the measurements and the formula for calculating the value. The methods used to determine and normalize LVM were found to have a significant effect on the prevalence of LVH in obese children. Using M-mode-based calculations and subsequent normalization based on height, the prevalence of LVH was 64 % in the obese patients in our study cohort, whereas 2DE-based calculations and normalization based on BSA resulted in no obese patients with LVH. Relationship between obesity and blood pressure with LVM normalized to height in obese group implies that using normalization to height may maximize population-attributable risk, which is the most important measure of incident disease for programs focused on prevention of obesity-related problems. Improving our understanding of how obesity affects the architecture of cardiac structures and function may help to improve identification of children at risk for obesity-related heart disease.
References
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322(22):1561–1566. doi:10.1056/NEJM199005313222203
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ (2003) The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 289(19):2560–2572. doi:10.1001/jama.289.19.2560
Mancini GB, Dahlof B, Diez J (2004) Surrogate markers for cardiovascular disease: structural markers. Circulation. doi:10.1161/01.CIR.0000133443.77237.2f
Ruilope LM, Schmieder RE (2008) Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 21(5):500–508. doi:10.1038/ajh.2008.16
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35(3):569–582
Sluysmans T, Colan SD (2005) Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol 99(2):445–457. doi:10.1152/japplphysiol.01144.2004
Richey PA, Disessa TG, Somes GW, Alpert BS, Jones DP (2010) Left ventricular geometry in children and adolescents with primary hypertension. Am J Hypertens 23(1):24–29. doi:10.1038/ajh.2009.164
Malcolm DD, Burns TL, Mahoney LT, Lauer RM (1993) Factors affecting left ventricular mass in childhood: the Muscatine Study. Pediatrics 92(5):703–709
Daniels SD, Meyer RA, Loggie JM (1990) Determinants of cardiac involvement in children and adolescents with essential hypertension. Circulation 82(4):1243–1248
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114(2 Suppl 4th Report):555–576
Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM (2013) Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol 62(15):1309–1319. doi:10.1016/j.jacc.2013.07.042
Mehta SK, Richards N, Lorber R, Rosenthal GL (2009) Abdominal obesity, waist circumference, body mass index, and echocardiographic measures in children and adolescents. Congenit Heart Dis 4(5):338–347. doi:10.1111/j.1747-0803.2009.00330.x
Dhuper S, Abdullah RA, Weichbrod L, Mahdi E, Cohen HW (2011) Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity 19(1):128–133. doi:10.1038/oby.2010.134
Van Putte-Katier N, Rooman RP, Haas L, Verhulst SL, Desager KN, Ramet J, Suys BE (2008) Early cardiac abnormalities in obese children: importance of obesity per se versus associated cardiovascular risk factors. Pediatr Res 64(2):205–209. doi:10.1203/PDR.0b013e318176182b
Chinali M, de Simone G, Roman MJ, Lee ET, Best LG, Howard BV, Devereux RB (2006) Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am Coll Cardiol 47(11):2267–2273. doi:10.1016/j.jacc.2006.03.004
Schuster I, Karpoff L, Perez-Martin A, Oudot C, Startun A, Rubini M, Obert P, Vinet A (2009) Cardiac function during exercise in obese prepubertal boys: effect of degree of obesity. Obesity 17(10):1878–1883. doi:10.1038/oby.2009.197
Sharpe JA, Naylor LH, Jones TW, Davis EA, O’Driscoll G, Ramsay JM, Green DJ (2006) Impact of obesity on diastolic function in subjects ≤16 years of age. Am J Cardiol 98(5):691–693. doi:10.1016/j.amjcard.2006.03.052
Di Bonito P, Capaldo B, Forziato C, Sanguigno E, Di Fraia T, Scilla C, Cavuto L, Saitta F, Sibilio G, Moio N (2008) Central adiposity and left ventricular mass in obese children. Nutr Metab Cardiovasc Dis NMCD 18(9):613–617. doi:10.1016/j.numecd.2007.09.002
Friedman KG, McElhinney DB, Rhodes J, Powell AJ, Colan SD, Lock JE, Brown DW (2012) Left ventricular diastolic function in children and young adults with congenital aortic valve disease. Am J Cardiol. doi:10.1016/j.amjcard.2012.09.026
Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA 307(5):483–490. doi:10.1001/jama.2012.40
Virdis A, Ghiadoni L, Masi S, Versari D, Daghini E, Giannarelli C, Salvetti A, Taddei S (2009) Obesity in the childhood: a link to adult hypertension. Curr Pharm Des 15(10):1063–1071
Lamb MM, Ogden CL, Carroll MD, Lacher DA, Flegal KM (2011) Association of body fat percentage with lipid concentrations in children and adolescents: United States, 1999–2004. Am J Clin Nutr 94(3):877–883. doi:10.3945/ajcn.111.015776
Lambert M, Delvin EE, Levy E, O’Loughlin J, Paradis G, Barnett T, McGrath JJ (2008) Prevalence of cardiometabolic risk factors by weight status in a population-based sample of Quebec children and adolescents. Can J Cardiol 24(7):575–583
Lande MB, Pearson TA, Vermilion RP, Auinger P, Fernandez ID (2008) Elevated blood pressure, race/ethnicity, and C-reactive protein levels in children and adolescents. Pediatrics 122(6):1252–1257. doi:10.1542/peds.2007-3162
Ford ES (2003) C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the national health and nutrition examination survey 1999–2000. Circulation 108(9):1053–1058. doi:10.1161/01.CIR.0000080913.81393.B8
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. doi:10.1016/j.echo.2010.03.019
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
Lu X, Xie M, Tomberlin D, Klas B, Nadvoretskiy V, Ayres N, Towbin J, Ge S (2008) How accurately, reproducibly, and efficiently can we measure left ventricular indices using M-mode, 2-dimensional, and 3-dimensional echocardiography in children? Am Heart J 155(5):946–953. doi:10.1016/j.ahj.2007.11.034
Poutanen T, Jokinen E (2007) Left ventricular mass in 169 healthy children and young adults assessed by three-dimensional echocardiography. Pediatr Cardiol 28(3):201–207. doi:10.1007/s00246-006-0101-5
Gidding SS (2010) Controversies in the assessment of left ventricular mass. Hypertension 56(1):26–28. doi:10.1161/HYPERTENSIONAHA.110.153346
Wong RC, Yip JW, Gupta A, Yang H, Ling LH (2008) Echocardiographic left ventricular mass in a multiethnic Southeast Asian population: proposed new gender and age-specific norms. Echocardiography 25(8):805–811. doi:10.1111/j.1540-8175.2008.00709.x
Rodriguez CJ, Diez-Roux AV, Moran A, Jin Z, Kronmal RA, Lima J, Homma S, Bluemke DA, Barr RG (2010) Left ventricular mass and ventricular remodeling among Hispanic subgroups compared with non-Hispanic blacks and whites: MESA (Multi-ethnic Study of Atherosclerosis). J Am Coll Cardiol 55(3):234–242. doi:10.1016/j.jacc.2009.08.046
Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA (2006) Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 186(6 Suppl 2):S357–S365. doi:10.2214/AJR.04.1868
Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP Jr (1999) Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson 1(1):7–21
Cuspidi C, Meani S, Negri F, Giudici V, Valerio C, Sala C, Zanchetti A, Mancia G (2009) Indexation of left ventricular mass to body surface area and height to allometric power of 2.7: is the difference limited to obese hypertensives? J Hum Hypertens 23(11):728–734. doi:10.1038/jhh.2009.16
Brumback LC, Kronmal R, Heckbert SR, Ni H, Hundley WG, Lima JA, Bluemke DA (2010) Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int J Cardiovasc Imaging 26(4):459–468. doi:10.1007/s10554-010-9584-5
Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, Claessens T, Gillebert TC, St John-Sutton M, Rietzschel ER (2010) Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension 56(1):91–98. doi:10.1161/HYPERTENSIONAHA.110.150250
Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report (2011) Pediatrics 128 Suppl 5:S213–256. doi:10.1542/peds.2009-2107C
Dewey FE, Rosenthal D, Murphy DJ Jr, Froelicher VF, Ashley EA (2008) Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation 117(17):2279–2287. doi:10.1161/CIRCULATIONAHA.107.736785
Daniels SR, Kimball TR, Morrison JA, Khoury P, Meyer RA (1995) Indexing left ventricular mass to account for differences in body size in children and adolescents without cardiovascular disease. Am J Cardiol 76(10):699–701
Foster BJ, Gao T, Mackie AS, Zemel BS, Ali H, Platt RW, Colan SD (2013) Limitations of expressing left ventricular mass relative to height and to body surface area in children. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 26(4):410–418. doi:10.1016/j.echo.2012.11.018
Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR, American Heart Association Atherosclerosis H, Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young CoNPA, Metabolism, Council on Clinical C (2013) Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation. doi:10.1161/CIR.0b013e3182a5cfb3
Di Salvo G, Pacileo G, Del Giudice EM, Natale F, Limongelli G, Verrengia M, Rea A, Fratta F, Castaldi B, D’Andrea A, Calabro P, Miele T, Coppola F, Russo MG, Caso P, Perrone L, Calabro R (2006) Abnormal myocardial deformation properties in obese, non-hypertensive children: an ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J 27(22):2689–2695. doi:10.1093/eurheartj/ehl163
McNiece KL, Gupta-Malhotra M, Samuels J, Bell C, Garcia K, Poffenbarger T, Sorof JM, Portman RJ (2007) Left ventricular hypertrophy in hypertensive adolescents: analysis of risk by 2004 National High Blood Pressure Education Program Working Group staging criteria. Hypertension 50(2):392–395. doi:10.1161/HYPERTENSIONAHA.107.092197
Peralta-Huertas J, Livingstone K, Banach A, Klentrou P, O’Leary D (2008) Differences in left ventricular mass between overweight and normal-weight preadolescent children. Appl Physiol Nutr Metabol 33(6):1172–1180. doi:10.1139/H08-082
Falkner B, Deloach S, Keith SW, Gidding SS (2012) High risk blood pressure and obesity increase the risk for left ventricular hypertrophy in African-American adolescents. J Pediatr. doi:10.1016/j.jpeds.2012.06.009
Crowley DI, Khoury PR, Urbina EM, Ippisch HM, Kimball TR (2011) Cardiovascular impact of the pediatric obesity epidemic: higher left ventricular mass is related to higher body mass index. J Pediatr. doi:10.1016/j.jpeds.2010.10.016
Hanevold C, Waller J, Daniels S, Portman R, Sorof J (2004) The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the international pediatric hypertension association. Pediatrics 113(2):328–333
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93(1):62–66
Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL (2002) 2000 CDC growth charts for the United States: methods and development. Vital and health statistics Series 11. Data Natl Health Surv 246:1–190
Foster BJ, Platt RW, Zemel BS (2012) Development and validation of a predictive equation for lean body mass in children and adolescents. Ann Hum Biol 39(3):171–182. doi:10.3109/03014460.2012.681800
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57(6):450–458
Foster BJ, Mackie AS, Mitsnefes M, Ali H, Mamber S, Colan SD (2008) A novel method of expressing left ventricular mass relative to body size in children. Circulation 117(21):2769–2775
Khoury PR, Mitsnefes M, Daniels SR, Kimball TR (2009) Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 22(6):709–714. doi:10.1016/j.echo.2009.03.003
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Chang SA, Kim HK, Lee SC, Kim EY, Hahm SH, Kwon OM, Park SW, Choe YH, Oh JK (2013) Assessment of left ventricular mass in hypertrophic cardiomyopathy by real-time three-dimensional echocardiography using single-beat capture image. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 26(4):436–442. doi:10.1016/j.echo.2012.12.015
Gutgesell HP, Rembold CM (1990) Growth of the human heart relative to body surface area. Am J Cardiol 65(9):662–668
Simpson JM, Savis A, Rawlins D, Qureshi S, Sinha MD (2010) Incidence of left ventricular hypertrophy in children with kidney disease: impact of method of indexation of left ventricular mass. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol 11(3):271–277. doi:10.1093/ejechocard/jep211
Borzych D, Bakkaloglu SA, Zaritsky J, Suarez A, Wong W, Ranchin B, Qi C, Szabo AJ, Coccia PA, Harambat J, Mitu F, Warady BA, Schaefer F (2011) Defining left ventricular hypertrophy in children on peritoneal dialysis. Clin J Am Soc Nephrol CJASN 6(8):1934–1943. doi:10.2215/CJN.11411210
Myerson SG, Bellenger NG, Pennell DJ (2002) Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension 39(3):750–755
Dai S, Harrist RB, Rosenthal GL, Labarthe DR (2009) Effects of body size and body fatness on left ventricular mass in children and adolescents: project HeartBeat! Am J Prev Med 37(1 Suppl):S97–S104. doi:10.1016/j.amepre.2009.04.011
de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, Lee ET, Devereux RB, Strong Heart Study I (2005) Normalization for body size and population-attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens 18(2 Pt 1):191–196. doi:10.1016/j.amjhyper.2004.08.032
Zong P, Zhang L, Shaban NM, Pena J, Jiang L, Taub CC (2014) Left heart chamber quantification in obese patients: how does larger body size affect echocardiographic measurements? J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 27(12):1267–1274. doi:10.1016/j.echo.2014.07.015
de Simone G, Galderisi M (2014) Allometric normalization of cardiac measures: producing better, but imperfect, accuracy. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 27(12):1275–1278. doi:10.1016/j.echo.2014.10.006
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Mahgerefteh, J., Linder, J., Silver, E.J. et al. The Prevalence of Left Ventricular Hypertrophy in Obese Children Varies Depending on the Method Utilized to Determine Left Ventricular Mass. Pediatr Cardiol 37, 993–1002 (2016). https://doi.org/10.1007/s00246-016-1380-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-016-1380-0