Abstract
Patients with Turner syndrome (TS) have an increased risk of cardiovascular morbidity. 29 TS and 25 healthy control subjects (CS) were included in the study. We investigated body mass index, waist circumference, fasting glucose and insulin, homeostatic model assessment (HOMA) index, serum lipids, oral glucose tolerance test, 24-h ambulatory blood pressure (BP) monitoring, and carotid intima-media thickness (CIMT) and compared them with CS. 28 % (N = 7) of TS had insulin resistance (IR), and 36 % (N = 9) had IGT. Mean systolic BP and diastolic BP (DBP) dip were 7.24 ± 3.97 % and 11.84 ± 6.2 %, respectively. CIMT was greater in TS than in CS (p = 0.00). CIMT was correlated positively with fasting insulin, HOMA index, and insulin-sensitivity check index (r = 0.563, p = 0.015; r = 0.603, p = 0.008; and r = 0.623, p = 0.006, respectively) and negatively with fasting glucose-to-insulin ratio and DBP dipping (r = −0.534, p = 0.022; r = −0.534, p = 0.00, respectively) in the two groups combined. These results provide additional evidence for the presence of subclinical cardiovascular disease and its relation to hypertension in TS. They also indicate a significant relation between DBP dipping and increased arterial stiffness. It is also important to note that our findings show significant relationships between insulin sensitivity and cardiovascular changes and underline the importance of insulin resistance for predicting cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turner syndrome (TS), first described in 1938, is the most common sex-chromosome abnormality in female conceptions. TS is an important cause of short stature in girls and primary amenorrhea in young women and is usually caused by loss of part or all of an X-chromosome [25].
It has long been known that left-sided congenital cardiac abnormalities are more prevalent in women with TS, and recent studies have shown that these women have increased mortality, primarily as a result of cardiovascular complications [18, 21] as well as several risk factors for ischemic heart disease, including hypertension [6, 13], insulin resistance [5, 23], and hyperlipidemia [5, 27].
Autopsy studies in adolescents and young adults document that atherosclerosis begins in adolescence and that traditional risk factors are associated with its development [15].This emphasizes the importance of evaluating the development of macrovascular changes in children and adolescent patients with TS. Carotid intima-media thickness (IMT) is a structural feature of the common carotid artery (CCA). Increased IMT is a marker of early carotid atherosclerosis and an independent predictor of an adverse cardiovascular prognosis in the general population [20]. Based on this background, we designed a clinical study aimed at evaluating the metabolic and cardiovascular profile in a group of children with TS compared with a group of age- and sex-matched normal healthy subjects.
Subjects and Methods
From patients attending our endocrinology outpatient clinic, 29 patients age 9–17 year were included in this study. Twenty-five age- and sex-matched healthy female adolescents served as a control group (control subjects [CS]). The study was approved by the local Ethics Committee.
All subjects were examined by the same physician. Body weight (BW) and waist and hip circumferences were measured by standard methods and devices. Waist-to-hip ratio (WHR) was calculated for each subject. Body mass index (BMI) was calculated by the following equation: BW (kg)/height (m2).
Among the patients enrolled in the study, two patients had autoimmune hypothyroidism, which was under appropriate treatment with l-thyroxine at the time of the study (leading to normal circulating TSH, free tri-iodothyronine, and free thyroxine levels); 7 patients were under oral 17b-estradiol treatment; and 8 patients had short stature and were treated with rhGH; they showed normal IGF1 levels at the time of the study. No patients were affected by congenital cardiovascular disease or renal disease.
Biochemical Evaluation
Fasting blood samples were drawn in the morning, between 08:00 and 09:00 am, from all patients and CS. After an overnight fast, venous blood samples were taken for determination of serum glucose and lipid metabolism parameters. Serum levels of glucose, insulin, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were determined in our biochemistry laboratory using standard methods. In accordance with the values given by the International Diabetes Federation for adolescents, TG > 150 mg/dL was accepted as high, and HDL-C < 40 mg/dL was accepted as low [31]. Oral glucose tolerance test (OGTT) was performed for all subjects in the TS group. For this test, the subjects ingested an oral dose of 1.75 g/kg (maximum 75 g) of glucose. Venous blood samples were collected at 0, 30, 60, 90, and 120 min for determination of serum glucose and insulin levels. OGTT results were evaluated according to World Health Organization criteria [30].
The homeostasis model assessment of insulin resistance (HOMA-IR), quantitative insulin-sensitivity check index (QUICK-I), and fasting glucose-to-insulin ratio (FGIR) were derived as estimates of insulin resistance. FGIR was calculated as fasting insulin concentration (μU/mL)/fasting glucose concentration (mg/dL). HOMA-IR was calculated as fasting insulin concentration (μU/mL) × fasting glucose concentration (mmol/L)/22.5) [12]. Insulin resistance was defined as a HOMA-IR > 3.16 according to our previously published data [10]. Hyperinsulinism was defined from norms for pubertal stage: prepubertal > 15 mU/L and mid-puberty (stages 2 through 4) > 30 mU/L [4, 30]. QUICK- I was calculated as 1/[(log fasting insulin concentration (μU/mL) + log fasting glucose concentration (mg/dL)] [9].
BP Measurements
Clinic BP was measured three times at 1-min intervals, using a mercury sphygmomanometer after subjects had rested for at least 10 min. Clinical blood pressure (BP) was calculated as the mean of the three measurements. Hypertension was defined as clinical BP ≥ the 95th percentile for age, sex, and height [14]. The subjects underwent 24-h ambulatory BP (ABP) recording using a Spacelabs 90207 ambulatory monitor (Spacelabs Medical, Issaquah, WA).
Ambulatory hypertension was diagnosed when the average ambulatory systolic blood pressure (SBP) or diastolic blood pressure (DBP) for the period exceeded the 95th percentile BP on the basis of the subject’s sex and height according to normative values for ABP [26]. BP load was defined as the percentage of readings for a given period that exceeded the 95th percentile for that individual. Percent dipping was calculated for both average SBP and DBP using the following formula: [(daytime BP—nocturnal BP)/daytime BP] * 100. Each subject was categorized as a “dipper” (decrease in average SBP and DBP ≥ 10 % during sleep) or a “nondipper” (decrease < 10 %) [28].
Carotid Artery Ultrasonography
Longitudinal images of the common carotid artery were obtained by combined two dimensional–mode and color Doppler examinations. The IMT of the CCA far wall was measured using the electronic calipers of the machines as previously described [24]. On a longitudinal, echocardiographic image of the carotid artery, the posterior wall of the carotid artery was displayed as two bright white lines separated by a hypoechogenic space. The IMT was assessed at the far wall as the distance between the interface of the lumen and intima (first echogenic line) and the interface between the media and adventitia (second echogenic line). The mean carotid artery IMT was calculated for each child as the average of three consecutive measurements of the maximum far wall thickness obtained from the common carotid artery 10 mm below the carotid bulb.
Statistical Analysis
For statistical analysis, the SPSS (version 16.0; SPSS, Chicago, IL) package program was used. Categorical parameters are given as percent. Continuous variables (age, BMI, BW, height, lipid panel, blood glucose, SBP and DBP, and CIMT) are given with mean ± SD. These were evaluated with Kolmogrov–Smirnov test to determine if the data showed a normal distribution. The statistical analysis was performed using Mann–Whitney U-test for comparison between TS and CS. Correlations between CIMT and fasting insulin, HOMA-IR, QUICK-I, FGIR, and nocturnal dipping DBP were performed using Spearman correlation coefficient, and p < 0.005 was considered statistically significant.
Results
A total of 54 children consented to participate in the study (29 TS and 25CS). All of the study population comprised Turkish subjects. In all TS patients, the diagnosis was made in early childhood by the presence of short stature. The clinical diagnosis of TS was based on peripheral leucocyte karyotype analysis, and all subjects included in the study had 45,X (18:), 46,X,i (Xq) or 45,X/46,X,i (Xq) (N:9); 45,X/46,Xr (n:1); and 46,XXp- or 46,XXq- (n:1).
The characteristics of the study population are listed in Table 1. The groups were matched for age, sex and body size. Patients with TS had a shorter height but the same BMI as CS. Lipid profiles did not differ significantly between the groups. Mean fasting glucose, fasting insulin, QUICK-I, HOMA, and FGIR index were similar in TS and in CS, whereas 28 % (N = 7) of TS had IR, and 36 % (N = 9) had impaired glucose tolerance (IGT). The cardiovascular parameters of the study population are listed in Table 2.
Both the TS and CS group showed no significant differences in terms of casual SBP and DBP. In all CS, 24-h BP monitoring showed a normal profile with preserved circadian variation. In TS, although 8 subjects (27 %) were diagnosed to be hypertensive using casual BP readings, only 4 (13.7 %) had ABP measurements >95 % for height and sex.
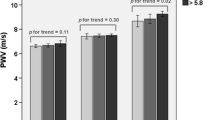
Mean SBP and DBP dip were 7.24 ± 3.97 % and 11.84 ± 6.2 %, respectively, with 7 study participants (24.1 %) who had impaired SBP dipping and 5 (17.2 %) who had impaired DBP dipping. Comparison of SPB and DBP nocturnal dippings are shown in Fig. 1. We also grouped the study population according to the degree of nocturnal dipping; no difference was found between the two groups for the anthropometric and metabolic variables.
CIMT was greater in TS than in CS (p = 0.00). Table 3 lists significant correlations between CIMT and other cardiovascular risk factors in children with TS. CIMT was correlated positively with fasting insulin, HOMA, QUICK-I (r = 0.563, p = 0.015; r = 0.603, p = 0.008; and r = 0.623, p = 0.006, respectively) and negatively with FGIR and nocturnal dipping DBP (r = −0.534, p = 0.022 and r = −0.534, respectively, p = 0.00) in the combined group. Correlation between CIMT and nocturnal dipping DBP are shown in Fig. 2.
Discussion
Children with TS have a broad range of later health problems, including an increased risk of cardiovascular morbidity and mortality [6]. Consistent with this finding, the results of our present study show that children with TS are characterized by a higher prevalence of insulin resistance, hypertension, IGT, and increased IMT compared with age- and sex-matched normal subjects. The increased insulin resistance, hypertension, IGT, and increased IMT in children with TS may be an early step in the development of atherosclerosis.
Glucose intolerance has been reported in both TS girls and women, and type 2 diabetes is four times more common (relative risk = 4.4) [6] in addition to increased mortality due to diabetes [22] and earlier onset than in the general population. Caprio et al. [2] found insulin resistance in girls with TS. Cicognani et al. [3] found IGT more frequently in girls, most markedly in young girls, with TS compared with CS. Other investigators documented IGT in 15 % of girls with TS [7, 29]. An early observation showed that many adults with TS developed type 2 diabetes or glucose intolerance [16]. In adolescents and adults, a large proportion of TS patients exhibited IGT or overt type 2 diabetes during OGTT [3, 8]. In agreement with other studies, we found impaired insulin sensitivity and abnormal glucose response to OGTT. The lack of any correlation between BMI or waist circumference and glucose tolerance is in agreement with previous studies that showed a higher frequency of type 2 diabetes mellitus and IGT in children with TS than in normal children independent of BMI [6].
In agreement with other reports, we showed a higher prevalence of hypertension in our patients. Furthermore, 24-h ambulatory BP monitoring showed a loss of nocturnal reduction in BP suggestive of a diagnosis of arterial hypertension in patients otherwise considered normotensive by single ambulatory BP evaluation. The exact mechanism of hypertension in TS has not been clearly identified: An increase in plasma renin activity has been found in 50 % of cases by some investigators, and normal vagosympathetic tone, explaining relative tachycardia, has recently been described [6]. Because hypertension is an important risk factor for cardiovascular complications, it is important that TS patients undergo 24-h ambulatory BP monitoring to detect the presence of hypertension that would be missed by a single BP measurement. In addition, using ambulatory BP monitoring (ABPM) allowed us to show blunted DBP dipping, which may represent one of the earliest abnormalities in BP patterns in this TS cohort.
Dipping of BP during the night is a normal physiological change that can be blunted by cardiovascular risk factors and the severity of hypertension. In healthy individuals, BP follows a circadian pattern. BP starts decreasing from late evening onward, reaches a nadir around midnight, and increases just after awakening in the morning. Dipping in BP has been described in three windows of sleep: BP starts decreasing in the vesperal window, reaches the plateau level in the basal window, and increases in the pre awakening window. This natural circadian variation may be altered by certain metabolic and cardiovascular changes. Decreased nocturnal dipping of BP can have profound impact in the overall management of hypertension [11].
Because increased IMT is considered a precursor of clinically detectable atherosclerosis and is associated with higher cardiovascular risk, we also evaluated this parameter in our patients compared with CS. Measuring carotid IMT with ultrasonography correlates well with pathological measurements and is reproducible [19]. Increased carotid IMT is significantly related to known cardiovascular risk factors and to carotid plaque, a more advanced atherosclerotic lesion [1]. Pirgon et al. [17] performed studies in children with type TS to investigate possible risks compared with age-matched CS. They showed a significant positive correlation between IMT and both LDL-C and SBP as well as a negative correlation with HDL-C. In the present study, the children with TS had significantly greater CCA IMT values than CS. CIMT was correlated positively with fasting insulin, HOMA, and QUICK-I and negatively with FGIR and nocturnal dipping DBP in the combined group.
Our study suffers from some limitations. First, the small number of patients, as well as their heterogeneity, did not allow us to draw definitive conclusions on morbidity. Second, we were unable to correlate the type of karyotype with the metabolic/cardiovascular profile because we included different karyotypes and therapeutic regimens.
In conclusion, our results indicate that children with TS are characterized by a higher frequency of insulin resistance, hypertension and increased IMT suggesting an increased cardiovascular risk. Our data underline the importance of monitoring BP in children and adolescents with TS. To provide early detection and management of risk factors for cardiovascular complications, ABPM should be considered in children and adolescents with TS. On the basis of these considerations, in childhood cardiovascular risk factors should be regularly checked in the follow-up of TS patients.
References
Bonithon-Kopp C, Touboul PJ, Berr C, Leroux C, Mainard F, Courbon D et al (1996) Relation of intima-media thickness to atherosclerotic plaques in the carotid arteries: the vascular aging (EVA) study. Arterioscler Thromb Vasc Biol 16(2):310–331
Caprio S, Boulware S, Diamond M, Sherwin RS, Carpenter TO, Rubin K et al (1991) Insulin resistance: an early metabolic defect of Turner’s syndrome. J Clin Endocrinol Metab 72(4):832–836
Cicognani A, Mazzanti L, Tassinari D, Pellacani A, Forabosco A, Landi L et al (1998) Differences in carbohydrate tolerance in Turner syndrome depending on age and karyotype. Eur J Pediatr 148(1):64–68
Goran MI, Gower BA (2001) Longitudinal study on pubertal insulin resistance. Diabetes 50(11):2444–2450
Gravholt CH, Naeraa RW, Nyholm B (1988) Glucose metabolism, lipid metabolism, and cardiovascular risk factors in adult Turner syndrome: the impact of sex hormone replacement. Diabetes Care 21(7):1062–1070
Gravholt CH, Juul S, Naeraa RW (1998) Morbidity in Turner syndrome. J Clin Epidemiol 51(2):147–158
Haeusler G, Frisch H (1992) Growth hormone treatment in Turner’s syndrome: short- and long-term effects on metabolic parameters. Clin Endocrinol 36(3):247–253
Holl RW, Kunze D, Etzrodt H, Teller W, Heinze E (1994) Turner syndrome: final height, glucose tolerance, bone density and psychosocial status in 25 adult patients. Eur J Pediatr 153(1):11–16
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85(7):2402–2410
Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C (2005) Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115(4):500–503
Mahabala C, Kamath P, Bhaskaran U, Pai ND, Pai AU (2013) Antihypertensive therapy: nocturnal dippers and nondippers. Do we treat them differently? Vasc Health Risk Manag 9:125–133
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Nathwani NC, Unwin R, Brook CG (2000) Blood pressure and Turner syndrome. Clin Endocrinol 52(3):363–370
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114(2 Suppl 4th Report):555–576
Newman WP, Freedman DS, Voors DW, Gard PD, Srinivasan SR, Cresanta JL et al (1986) Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. New Engl J Med 14(3):138–144
Nielsen J, Johansen K, Yde H (1969) The frequency of diabetes mellitus in patients with Turner’s syndrome and pure gonadal dysgenesis: blood glucose, plasma insulin and growth hormone level during an oral glucose tolerance test. Acta Endocrinol 62(2):251–269
Pirgon Ö, Atabek ME, Oran B, Güçlü R (2008) Atherogenic lipid profile and systolic blood pressure are associated with carotid artery intima-media thickness in children with Turner syndrome. J Clin Res Pediatr Endocrinol 1(2):62–71
Price WH, Clayton JF, Collyer S (1986) Mortality ratios, life expectancy, and causes of death in patients with Turner’s syndrome. J Epidemiol Commun Health 40(2):97–102
Riley WA, Barnes RW, Applegate WB, Dempsey R, Hartwell T, Davis VG et al (1992) Reproducibility of noninvasive ultrasonic measurement of carotid atherosclerosis: the asymptomatic carotid artery plaque study. Stroke 23(8):1062–1068
Salonen JT, Salonen R (1993) Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation 87(3 Suppl):II56–II65
Schoemaker MJ, Swerdlow AJ, Higgins CD (2008) Mortality in women with Turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab 93(12):4735–4742
Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH (2006) Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab 91(10):3897–3902
Stoppoloni G, Prisco F, Alfano C (1990) Characteristics of insulin resistance in Turner syndrome. Diabetes Metab 16(4):267–271
Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N et al (2007) Mannheim carotid intima-media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 23(1):75–80
Turner HH (1938) A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinology 28:566
Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M et al (2008) Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young and the Council for High Blood Pressure Research. Hypertension 52(3):433–451
Van PL, Bakalov VK, Bondy CA (2006) Monosomy for the X-chromosome is associated with an atherogenic lipid profile. J Clin Endocrinol Metab 91(8):2867–2870
Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F et al (1990) Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation 81(2):528–536
Wilson DM, Frane JW, Sherman B, Johanson AJ, Hintz RL, Rosenfeld RG (1988) Carbohydrate and lipid metabolism in Turner syndrome: effect of therapy with growth hormone, oxandrolone, and a combination of both. J Pediatr 112(2):210–217
World Health Organization (1999) WHO consultation: Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. WHO/NCD/NCS/99.2. WHO, Geneva, Switzerland
Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S et al (2007) International diabetes federation task force on epidemiology and prevention of diabetes. The metabolic syndrome in children and adolescents. Lancet 369(9579):2059–2061
Acknowledgments
We thank all of the patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akyürek, N., Atabek, M.E., Eklioglu, B.S. et al. Ambulatory Blood Pressure and Subclinical Cardiovascular Disease in Children With Turner Syndrome . Pediatr Cardiol 35, 57–62 (2014). https://doi.org/10.1007/s00246-013-0740-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-013-0740-2