Abstract
Postoperative arrhythmia (POA) is the most common complication encountered after cardiopulmonary bypass (CPB). The preventive effect of magnesium in POA has been confirmed by metaanalyses in adults, but less is known in pediatric patients. A metaanalysis of published trials was conducted to examine the efficacy of magnesium supplementation in POA prevention among pediatric patients undergoing CPB. Relevant trials were identified from electronic databases (Medline, Embase, Web of Science, and Cochrane library). Pooled relative risk (RR) and 95 % confidence intervals (CI) were calculated using Mantel–Haenszel random-effects models, and heterogeneity was determined qualitatively according to I 2 and chi-squared statistical analyses. Among 121 potentially relevant studies, five randomized controlled trials met the inclusion criteria, resulting in a pooled total of 348 participants. Compared with placebo, magnesium supplementation decreased the incidence of arrhythmia after CPB in pediatric patients by 66 % (RR, 0.34; 95 % CI, 0.18–0.65; P = 0.001), with no heterogeneity between trials (heterogeneity P = 0.68; I 2 = 0 %). Magnesium supplementation significantly reduces the incidence of postoperative arrhythmias in pediatric patients undergoing CPB. Although the findings encourage the use of magnesium as an alternative to postoperative arrhythmias after CPB in pediatric patients, higher-quality randomized clinical trials are necessary before the findings can be generalized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Magnesium (Mg) is widely recognized as an adjunct for the treatment of dysrhythmia after cardiac surgery [39]. Although Mg deficiency is uncommon in healthy individuals, hypomagnesemia occurs frequently among patients after cardiopulmonary bypass (CPB) [24, 35]. Hypomagnesemia and its role in postoperative arrhythmia after CPB have been well studied in adults [1, 11, 18, 20, 37], but disparities occur among studies relating to the effect of CPB on Mg concentrations in pediatric patients.
Previous studies on the effectiveness of Mg in children found no correlation between serum and ionized Mg and the development of postoperative arrhythmias [19, 30, 34]. However, recent clinical trials have provided evidence that Mg prophylaxis after CPB lowers the incidence of postoperative arrhythmias in pediatric patients [28, 41]. Although the numbers of vulnerable pediatric patients undergoing CPB are limited, we undertook this metaanalysis of randomized controlled trials (RCTs) to examine whether Mg supplementation is effective in preventing postoperative cardiac arrhythmias in pediatric patients.
Methods
Search Strategy and Selection Criteria
We searched the PubMed, Embase, Web of Science, and Cochrane databases for studies published until November 2012. The search terms included “magnesium,” “cardiac surgery,” “postoperative arrhythmias,” and “children.” The literature search was limited to clinical trials in English publications. In addition, we searched the reference lists of included studies and related publications. The results then were hand searched for eligible trials. Only full-text articles were included when they met the inclusion criteria.
The inclusion criteria specified pediatric patients undergoing CPB surgery, randomized allocation trials, Mg supplementation with an inactive placebo group, age range from day 1 to 18 years, and incidence of arrhythmias measured as trial outcomes. The exclusion criteria ruled out studies with no established direct comparison of Mg and placebo, unavailable information regarding duration of follow-up evaluation, and small sample size (n < 10) that could affect the generalizability of the findings.
Data Extraction and Quality Assessment
Two investigators (S.G. and H.Y.) independently extracted the following information from the studies: name of first author, year of publication, country of origin, study design (randomized or not), type of controls, data collection method (prospective or not), type of blinding, duration of Mg dosing in follow-up regimen, timing of Mg initiation (postoperative or not), participants’ characteristics, number of patients, ages, procedure-related characteristics such as the types of congenital heart diseases (CHD) and the surgery performed, CPB time, aortic cross-clamp time, pre- and postoperative Mg level, and incidence and types of postoperative arrhythmias in each group. Disagreements during data extraction were resolved after discussion with the third investigator (E.Y.).
To assess the quality of the included trials, we used the Cochrane risk-of-bias tool [21], which addresses five items: sequence generation, allocation concealment, blinding/masking, incomplete outcome data, and selective outcome reporting. This tool shows empirical evidence for whether a study bias influences the estimates of an intervention’s effectiveness in randomized trials [27].
Statistical Analysis
Data were analyzed using RevMan version 5.2 (Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2012). The incidence of postoperative arrhythmias was assessed in terms of dichotomous variables and expressed as relative risk (RR) with 95 % confidence intervals (CI) for the included studies. Pooled estimates of efficacy were calculated through the Mantel–Haenszel random-effects model [13] to generalize the findings to studies not included in the metaanalysis [7]. To assess heterogeneity, we used the I 2 test, which describes the percentage of total variation across studies due to heterogeneity rather than chance. Calculation of I 2 can be performed as follows:
where Q represents Cochrane’s heterogeneity statistics and df represents degrees of freedom. Negative values of I 2 equal zero, and I 2 ranges between 0 % (no observed heterogeneity) and 100 %. Higher values show increased heterogeneity. Studies with an I 2 statistic of 25–50 % are considered to have low heterogeneity. Those with an I 2 statistic of 50–75 % are considered to have moderate heterogeneity, and those with an I 2 statistic exceeding 75 % are considered to have a high degree of heterogeneity [22].
To detect publication bias, a funnel plot analysis was done using the Egger test. An asymmetric plot suggests possible publication bias [17].
Results
Description of Included Studies
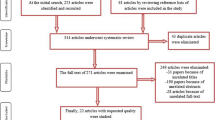
We identified 121 articles potentially relevant for retrieval after review of PubMed (n = 65), Embase (n = 29), Cochrane library (n = 8), and Web of Science (n = 19). After 99 nonrandomized trials had been excluded, 22 titles and abstracts were reviewed by two independent investigators. Duplicate studies and trials with unmet inclusion criteria were removed from the study. After the reference lists had been searched, one more randomized trial was included (Fig. 1).
A total of 348 pediatric patients were enrolled from the five studies included in this analysis. The efficacy of intravenous placebo or Mg supplementation for pediatric patients in terms of preventing postoperative arrhythmias was examined in these trials.
The baseline characteristics of the included studies are shown in Table 1. Two of the five trials were performed in the United States, and one each was conducted in Germany, China, and India. The sample size ranged from 28 to 131 individuals. The follow-up time ranged from stopping of CPB to 24 h after surgery. Four of the RCTs were prospective studies, but one did not mention how the outcomes were measured.
Table 2 shows the procedure-related characteristics, incidence, and types of postoperative arrhythmias in the study population. The included studies reported no significant surgical or CHD type differences between the treatment and control groups.
The CPB procedure was carried out for various types of CHD including atrial septal defect (ASD), ventricular septal defect (VSD), tetralogy of Fallot (TOF), transposition of great arteries (TGA), and the like. The CPB and aortic cross-clamp times did not differ significantly between the treatment and control groups. Also, the preoperative ionized Mg levels were similar between the two groups. However, the postoperative ionized Mg levels were significantly elevated in the treatment group but decreased in the control group below normal values in all the included studies except one [14], in which it decreased to normal preoperative values. The Mg normal range estimation was based on Munoz et al. [30]. Junctional ectopic tachycardia (JET) was the most frequently reported arrhythmia in four of the included studies.
Risk of Bias Within Studies
Few methodologically sound studies have adequate sample sizes to determine the efficacy of Mg supplementation in preventing arrhythmias after CPB in children. As outlined in Table 3, all the included studies were RCTs, but the method of randomization was detailed in only two trials [14, 41]. Allocation concealment was implemented in two trials [14, 28], and blinding was performed in four trials [14, 16, 28, 41]. Incomplete outcome data [14] and selective outcome reporting data [28] were shown in one trial. None of the methodologic quality domains could be assessed in one study [25]. Inclusion and exclusion criteria were clearly indicated in all the trials. The participants of all the included studies were pediatric patients ranging in age from birth to 18 years.
Effect of Magnesium Supplementation on Postoperative Arrhythmias
Pooling all five trials, 348 patients were examined. After cessation of CPB, arrhythmias developed for 11 of the 193 pediatric patients in the treatment group and 31 of the 155 patients in the control group. The analysis identified that Mg supplementation for pediatric patients undergoing CPB significantly reduced the incidence of arrhythmias after surgery by 66 % (RR, 0.34; 95 % CI, 0.18–0.65; P = 0.001; heterogeneity P = 0.68, I 2 = 0 %). The forest plot showing the incidence of postoperative arrhythmia after Mg supplementation in pediatric patients undergoing CPB is depicted in Fig. 2.
Publication Bias
A funnel plot evaluating the preventable effects of Mg supplementation on pediatric patients with CPB showed moderate publication bias (Fig. 3).
Discussion
We conducted a metaanalysis to evaluate the efficacy of Mg supplementation in the preventing postoperative arrhythmias after CPB in pediatric patients. Our finding suggests that Mg supplementation is effective in preventing postoperative arrhythmias in the pediatric population. We combined the effect sizes of five RCTs through a random-effects model and found that intravenous Mg significantly reduced the incidence of postoperative arrhythmias by 66 %. To the best of our knowledge, no previous metaanalyses have been conducted to evaluate the efficacy of Mg supplementation in pediatric populations.
To investigate the efficacy of intravenous Mg in preventing postoperative atrial fibrillation (POAF) after coronary artery bypass graft surgery in adult populations, Gu et al. [20] conducted a metaanalysis using seven double-blind, placebo-controlled, randomized trials. This metaanalysis concluded that intravenous Mg reduced the incidence of POAF by 36 % (RR, 0.64; 95 % CI, 0.50–0.83; P = 0.001), with no heterogeneity between trials (heterogeneity P = 0.8; I 2 = 0 %). Previous findings in adult populations had determined that intravenous Mg supplementation was associated with a significant risk reduction in postoperative atrial fibrillation (RR, 0.64; 95 % CI, 0.47–0.87; P = 0.004), with low heterogeneity between trials (heterogeneity P = 0.37; I 2 = 6.6 %) [2]. The result from our analysis shows higher benefits of Mg supplementation in pediatric populations.
Postoperative arrhythmias are widely accepted as complications of cardiac surgery in both adults and children [3, 10, 18, 26, 38]. In the study of Hoffman et al. [23], 29 % of the patients experienced one or more types of postoperative arrhythmias in a pediatric cardiac intensive care unit (CICU). Early postoperative arrhythmia is a predictor for late complications such as ventricular dysfunction, late arrhythmias, and late mortality [5, 12, 15]. Such complications caution that prevention of arrhythmias is important for postoperative courses and outcome improvement.
There are several types of postoperative arrhythmias such as supraventricular tachycardia, atrial flutter, atrial fibrillation, and JET [32]. The most frequently reported arrhythmia in this metaanalysis was JET. Studies show that JET is almost exclusively encountered during the immediate postoperative period in the pediatric population, and the risk factors for the development of postoperative JET include young age, low weight, longer CPB and aortic cross-clamp times, and hypothermic circulatory arrest [6, 9, 31].
A recently published nested case-control study that included an infant patient cohort undergoing open cardiac surgery identified that longer CPB and aortic cross-clamp times were the risk factors for JET found via univariate analysis. Also, the five congenital heart lesions including TOF, aortic arch repair with VSD closure, dextro-TGA with VSD closure, ASVD, and anomalous pulmonary venous return had higher individual incidences of JET that accounted for 65 % of the patients with JET. Furthermore, the multivariate analysis indicated only a longer aortic cross-clamp time, and patients who underwent complete repair of TOF remained at greater risk for the development of JET [43]. This suggests that the incidence of postoperative arrhythmias in the pediatric population is intricately tied to particular anatomic lesions and procedures for surgical corrections.
Nevertheless, procedure-related characteristics such as the types of CHD included, the time for CPB and aortic cross-clamping, and the preoperative Mg levels were fairly balanced between the treatment and control groups in the study population, except that the postoperative Mg levels were significantly elevated in the treatment arm, whereas the control group had hypomagnesemia in most of the included trials. Perioperative hypomagnesemia is common and possibly has a clinically relevant occurrence among pediatric patients undergoing repair of congenital heart lesions that might have resulted from various factors including the large volume of CPB prime solution compared with the circulating blood volume of pediatric patients, hemodilution, blood transfusion causing chelation of Mg, continuous hemofiltration, and administration of large doses of calcium and diuretics [28, 30]. Amidst these circumstances, we tried to determine how improving electrolyte imbalance could affect postoperative arrhythmias in pediatric patients, focusing on Mg deficiency.
Several studies have indicated causes for the development of arrhythmias after cardiac surgery in adults and children, including electrolyte disturbances such as hypomagnesemia. Magnesium is an important intracellular component, with 67 % of total-body Mg present in bone, 31 % contained intracellularly (especially in muscle cells), and 1–2 % located in extracellular fluid compartments. As a key cofactor for Na+-K+-adenosine triphosphatase (ATPase), Mg is a critical transporter that maintains the cell membrane potential. As such, Mg functions in the regulation of some cell membrane K+ channels and calcium antagonists. Therefore, hypomagnesemia often is related to hypokalemia [36].
In addition to its involvement in the myocardium, Mg depletion in skeletal muscle is well documented during CPB in adults [34]. Under CPB procedures with high stress, a large amount of Mg might be released from intracellular compartments [8]. Furthermore, related studies [4, 29, 33] have reported that CPB results in decreased Mg concentration levels in pediatric patients. This decrease may be attributable to underlying factors that affect the greater volume of prime solution compared with the circulating blood volume in pediatric patients, such as hemodilution, blood transfusions that increase chelation of Mg, continuous use of higher doses of calcium and diuretics, malnutrition, shifts in intracellular elimination induced by the extracorporeal circulation, and a decrease in body temperature during surgery.
Total serum hypomagnesemia has been reported in up to 94 % of adult patients undergoing CPB [16] and 34 % of pediatric cases [30]. Hoshino et al. [24] reported that Mg concentration levels decreased significantly after CPB in pediatric patients. However, this stage of hypomagnesemia did not increase the incidence of dysrhythmia due to the return of Mg levels to presurgical values 48 h after surgery. This Mg level was compared with the preoperative level within 24 h after CPB.
Fox et al. [19] used Plegisol, a cardioplegic solution, to detect whether Mg supplementation was useful for prevention of dysrhythmias among patients ranging in age from 1 day to 21 years. They found that Plegisol was not effective in preventing postoperative arrhythmias because no statistical differences in Mg levels were detected between patients who experienced arrhythmias and those who did not (P = 0.22).
Mildh et al. [29] found that Mg levels were within normal values in groups that experienced JET compared with those that did not. However, this was a retrospective study with children, resulting in deficient standardization of anesthesia, cardioplegia, CPB, and postoperative care.
Study Limitation
A potential limitation of this metaanalysis was the sample size of the included studies. Although postoperative arrhythmias occur frequently after cardiac operations in children [23, 40, 42], the availability of methodologically sound clinical trials with a large sample are lacking. Thus, we accepted studies with modest sample size. The inadequate randomization, lack of allocation concealment, and highly selected participants [2] could have been a potential cause for overestimating the effect of Mg.
A recent metaanalysis that included 20 RCTs totaling 3,696 adult patients showed that the effect of Mg in reducing postoperative supraventricular arrhythmias was significant only with the lower-quality studies but not when examined using higher-quality studies [11]. The different intravenous Mg doses supplied among the studies may have resulted in clinical heterogeneity, although no statistical heterogeneity was observed.
One study included in this analysis [16], for which the applicability of findings from cohorts analyzed a decade ago could be debated, may not be relevant currently with respect to the incidence of postoperative arrhythmia. We believe this to be unlikely because the surgical repair for the majority of cardiac lesions included in this analysis has remained insignificant regarding adjustments in surgical techniques during the last decade [43]. Moreover, an analysis not considering this study also showed a similar incidence of postoperative arrhythmia in the pediatric population (RR, 0.36; 95 % CI, 0.19–0.70; P = 0.003), with no heterogeneity between trials (heterogeneity P = 0.62, I 2 = 0 %). Also, because this study was the first prospective RCT conducted in the pediatric population to examine the effect of Mg treatment on the incidence of postoperative arrhythmias, we believe the inclusion of this trial remains valuable.
We excluded non-English-language studies and studies with a sample smaller than 10 patients, which could have potentiated bias in effect size. However, the included studies had participants comparable with patients observed in routine cardiac surgical practice.
Conclusions
This metaanalysis of five randomized placebo-controlled clinical trials identified that intravenous Mg supplementation added to the surgical regimen reduces the incidence of postoperative arrhythmias in children by 66 %. Based on the findings, we recommend the use of intravenous Mg as an alternative to prevent arrhythmias after CPB in pediatric patients. However, high-quality RCT studies with large samples of pediatric patients still are needed before the findings of this study can be generalized.
References
Aglio LS, Stanford GG, Maddi R et al (1991) Hypomagnesemia is common following cardiac surgery. J Cardiothorac Anesth 5:201–208
Alghamdi AA, Al-Radi OO, Latter DA (2005) Intravenous magnesium for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and meta-analysis. J Card Surg 20:293–299
Andrews TC, Reimold SC (1991) Prevention of supraventricular arrhythmias after coronary artery bypass surgery: a meta-analysis of randomized control trials. Circulation (Suppl) 84:236–244
Atallah MM, Saber HI, Mageed NA et al (2011) Feasibility of adding magnesium to intrathecal fentanyl in pediatric cardiac surgery. Egypt J Anaesth 27:173–180
Azzam FJ, Fiore AC (1998) Postoperative junctional ectopic tachycardia. Can J Anaesth 45:898–902
Batra AS, Chun DS, Johnson TR et al (2006) A prospective analysis of the incidence and risk factors associated with junctional ectopic tachycardia following surgery for congenital heart disease. Pediatr Cardiol 27:51–55
Borenstein M, Hedges LV, Higgins JT et al (2009) Introduction to meta-analysis, 1st edn. Wiley, Sussex, pp 77–186
Chen-Yuan Lu, Tan Ping-Heng et al (2003) Body weight-related ionized hypomangesemia in pediatric patients undergoing cardiopulmonary bypass for surgical repair of congenital cardiac defects. J Clin Anesth 15:189–193
Collins KK, Van Hare GF, Kertesz NJ (2009) Pediatric non-postoperative junctional ectopic tachycardia medical management and interventional therapies. J Am Coll Cardiol 53:690–697
Creswell LL, Schuessler RB (1993) Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 56:539–549
De Oliveria GS, Knautz JS, Sherwani S et al (2012) Systemic magnesium to reduce postoperative arrhythmias after coronary artery bypass graft surgery: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 26:643–650
Deal BJ, Mavroudis C, Backer CL (2008) The role of concomitant arrhythmia surgery in patients undergoing repair of congenital heart disease. Pacing Clin Electrophysiol 31(Suppl 1):S13–S16
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Dittrich S, Germanakis DahnertI et al (2003) Randomized trial on the influence of continuous magnesium infusion on arrhythmias following cardiopulmonary bypass surgery for congenital heart disease. Intensive Care Med 29:1141–1144
Dodge-Khatami A, Miller OI et al (2002) Surgical substrates of postoperative junctional ectopic tachycardia in congenital heart defects. J Thorac Cardiovasc Surg 123:624–630
Dorman BH, Sade RM, Burnette S et al (2000) Magnesium supplementation in the prevention of arrhythmias in pediatric patients undergoing surgery for congenital heart defects. Am Heart J 139:522–528
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
England MR, Gordon G, Salem M et al (1992) Magnesium administration and dysrhythmias after cardiac surgery. JAMA 268:2395–2402
Fox ML, Burrows FA, Reid RW et al (1997) The influence of cardiopulmonary bypass on ionized magnesium in neonates, infants, and children undergoing repair of congenital heart lesions. Anesth Analg 84:497–500
Gu WJ, Wu ZJ, Wang PF et al (2012) Intravenous magnesium prevents atrial fibrillation after coronary artery bypass grafting: a meta-analysis of 7 double-blind, placebo-controlled, randomized clinical trials. Trials 13:41
Helfand M, Balshem H (2010) Principles for developing guidance: AHRQ and the effective health care program. J Clin Epidemiol 63:484–490
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Hoffman TM, Wernovsky G, Wieand TS, Cohen MI, Jennings AC, Vetter VL, Godinez RI, Gaynor JW, Spray TL, Rhodes LA (2002) The incidence of arrhythmias in a pediatric cardiac intensive care unit. Pediatr Cardiol 23:598–604
Hoshino K, Ogawa K, Hishitani T et al (2003) Influence of heart surgery on magnesium concentrations in pediatric patients. Pediatr Int 45:39–44
Jian W, Su L, Yiwu L (2003) The effects of magnesium prime solution on magnesium levels and potassium loss in open heart surgery. Pediatr Anesth 96:1617–1620
Krongard E (1984) Postoperative arrhythmias in patients with congenital heart disease. Chest 85:107–113
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. doi:10.1136/bmj.b2700
Manrique AM, Arroyo M, Lin Y et al (2010) Magnesium supplementation during cardiopulmonary bypass to prevent junctional ectopic tachycardia after pediatric cardiac surgery: a randomized controlled study. J Thorac Cardiovasc Surg 139:162–169
Mildh L, Hiippala A, Rautiainen P et al (2011) Junctional ectopic tachycarida after surgery for congenital heart disease: incidence, risk factors, and outcome. Eur J Cardiothorac Surg 39:75–80
Munoz R, Laussen PC, Palacio G et al (2000) Whole-blood ionized magnesium: age-related differences in normal values and clinical implications of ionized hypomagnesemia in patients undergoing surgery for congenital cardiac disease. J Thorac Cardiovasc Surg 119:891–898
Rekawek J, Kansy A, Miszczak-Knecht M et al (2007) Risk factors for cardiac arrhythmias in children with congenital heart disease after surgical intervention in the early postoperative period. J Thorac Cardiovasc Surg 133:900–904
Rhodes LA, Wernovsky G, Keane JF et al (1995) Arrhythmias and intracardiac conduction after the arterial switch operation. J Thorac Cardiovasc Surg 109:303–310
Satur CMR (1997) Magnesium and cardiac surgery. Ann R Coll Surg Engl 79:349–354
Satur CM, Stubington SR, Jennings A et al (1995) Magnesium flux during and after open heart operations in children. Ann Thorac Surg 59:921–927
Scheinman MM, Sullivan RW, Hyatt KH (1969) Magnesium metabolism in patients undergoing cardiopulmonary bypass. Circulation 34:I235–I241
Skippen PW, Sanatani S, Gow RM et al (2009) Diagnosis of postoperative arrhythmias following paediatric cardiac surgery. Anaesth Intensive Care 37:705–719
Storm W, Zimmerman JJ (1997) Magnesium deficiency and cardiogenic shock after cardiopulmonary bypass. Ann Thorac Surg 64:572–577
Tam KS, Miller JM (1991) Unexplained sustained ventricular tachyarrhythmias after cardiac operations. J Thorac Cardiovasc Surg 102:883–889
Turnier E, Osborn JJ, Gerbode F et al (1972) Magnesium and open heart surgery. J Thorac Cardiovasc Surg 64:694–705
Valsangiacomo E, Schmid ER, Schupbach RW, Schmidlin D, Molinari L, Waldvogel K, Bauersfeld U (2002) Early postoperative arrhythmias after cardiac operation in children. Ann Thorac Surg 74:792–796
Verma YS, Chauhan S, Gharde P et al (2010) Role of magnesium in the prevention of postoperative arrhythmias in neonates and infants undergoing arterial switch operation. Interact Cardiovasc Thorac Surg 11:573–576
Yildirim SV, Tokel K, Sayqili B et al (2008) The incidence and risk factors of arrhythmias in the early period after cardiac surgery in pediatric patients. Turk J Pediatr 50:549–553
Zampi JD, Hirsch JC, Gurney JG et al (2012) Junctional ectopic tachycardia after infant heart surgery: incidence and outcomes. Pediatr Cardiol 33:1362–1369
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H.Y., Ghimire, S. & Kim, E.Y. Magnesium Supplementation Reduces Postoperative Arrhythmias After Cardiopulmonary Bypass in Pediatrics: A Metaanalysis of Randomized Controlled Trials. Pediatr Cardiol 34, 1396–1403 (2013). https://doi.org/10.1007/s00246-013-0658-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-013-0658-8