Abstract
Pediatric patients with hypertrophic cardiomyopathy (HCM) and restrictive physiology (RP) with poor outcomes have been identified, but data on their course are limited. Our goal was to delineate the clinical features and course of children with HCM and RP. An institutional review of 119 patients identified between 1985 and 2010 with the diagnosis of HCM was performed. The diagnosis of RP was based on >1 echocardiogram along with at least one of the following: left atrial enlargement without evidence of left ventricle dilation, E/E′ ratio ≥ 10, and E/A ratio ≥ 3. Outcomes analysis was performed using Cox or Poisson regression when appropriate. RP was present in 50 (42%) patients. In patients without RP, 10-year freedom-from-death or aborted sudden cardiac death (aSCD), and death or heart transplant (HT), were 93.6 and 98.5%, respectively. In patients with RP, 10-year freedom-from-death or aSCD, and death or HT, were 59.0 and 71.2%, respectively. RP conferred a 3.5-fold increase in incidence rate of hospitalization (P = 0.01), a 3.8-fold increase in hazard of death or aSCD (P = 0.02), and a 5.7-fold increase in hazard of death or HT (P = 0.04). Assessment for RP is of paramount importance in children with HCM because those without RP have a good prognosis, and those with RP account for the majority of poor outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prevalence of hypertrophic cardiomyopathy (HCM) in the general population is approximately 1/500 individuals. HCM affects approximately 600,000 people in the United States and is the leading cause of death in young athletes in the United States [18, 21]. Compared with those diagnosed as adults, children and adolescents diagnosed with HCM appear to have more rapidly progressive hypertrophy and different modes of death [22, 23, 35]. A recent study in children with HCM found heart failure deaths to be at least as common as sudden deaths [10]. Risk factors for poor outcomes in children with HCM have included extreme left-ventricular hypertrophy (LVH) and abnormal blood-pressure (BP) response to exercise [10]. Abnormal diastology has also been shown to be an independent predictor of death or heart transplant (HT) [25]. A subgroup of pediatric patients with HCM and restrictive physiology (RP) with worse outcomes has been identified, but data on their clinical course are limited [6]. The purpose of this study was to delineate the clinical features and course of children with HCM and RP.
Methods
Study Patients
A retrospective review of patients identified between January 1, 1985, and January 1, 2010, with the diagnosis of HCM―including patients reported in a previous study [10] and patients enrolled from our institution in the National Institutes of Health-supported PCMR (Pediatric Cardiomyopathy Registry) database [9]―was performed through our institutional database. Criteria for inclusion in the study were age ≤18 years old at the time of diagnosis and echocardiographic evidence of HCM. HCM was defined as either concentric left-ventricular hypertrophy or asymmetric septal hypertrophy (defined as an interventricular septum [IVS] thickness or left-ventricular free wall (LVFW) thickness z-score ≥ 2) on echocardiogram without a possible secondary cause. Patients initially thought to have HCM and ultimately found to have another source of myocardial hypertrophy and those seen at our institution once and followed-up elsewhere were excluded. Demographic data, including age, sex, weight, history of any arrhythmia, history of ventricular arrhythmia, symptoms at the time of diagnosis of HCM, comorbid conditions, and associated cardiac anomalies, were recorded. Clinical end points included hospitalization for a cardiac cause, aborted sudden cardiac death (aSCD) since presentation, HT, and death. This study was approved by the Baylor College of Medicine Institutional Review Board. Individual consent was waived.
Management Algorithm
Patients were treated according to a previously published management algorithm [10]. Briefly, on diagnosis with HCM, patients were managed with beta blockade medication and exercise restriction. Additional medication, myectomy, and cardiac catheterization were reserved for patients with left-ventricular outflow tract obstruction (LVOTO) and refractory symptoms. Cardiac catheterization was also performed on patients with evidence of pulmonary hypertension or RP on echocardiogram and refractory symptoms. Patients with evidence of ventricular tachycardia, unexplained syncope, and/or aSCD underwent internal cardiac defibrillator (ICD) placement. Criteria used to list patients for HT included severe systolic or diastolic dysfunction with intractable heart failure symptoms and/or significant pulmonary hypertension.
Echocardiography
The relation between the E/A ratio and invasive hemodynamic parameters has been well established [1, 2, 7, 8, 33]. Preload dependence and psuedonormalization of the E/A ratio are well described phenomena and underscore the need for further measurements of diastolic function, including left atrial size and E/E′ ratio [4, 8, 27].
All echocardiogram reports of patients included in the study were reviewed. All studies were read by a pediatric echocardiographer at our institution. The left-ventricular shortening fraction (LVSF), peak resting gradient across the left-ventricular outflow tract (LVOT), tissue Doppler indices (TDIs), transmitral E/transmitral A (E/A) ratio, transmitral E/lateral mitral E′ (E/E′) ratio, transmitral E/septal E′ (septal E/E′) ratio, IVS z-score, LVFW z-score, and the presence and severity of left atrial enlargement (LAE) were recorded when available for the initial echocardiogram, the final echocardiogram, and all echocardiograms that demonstrated RP. If the E/A, mitral E/E′, and/or septal E/E′ ratios were not available in the echocardiogram report, the echocardiogram images were reviewed when available. In the absence of E/A, mitral E/E′, and septal E/E′ ratios, LAE was used as a surrogate for RP. Assessment of left atrial size was based on imaging from the parasternal long, parasternal short-axis, and four-chamber views. Due to the retrospective nature and prolonged study period, estimation of left atrial size was largely qualitative; therefore, only patients with multiple studies with consistent readings of LAE were included in the LAE group. To test the reliability of echocardiogram reports of left atrial size, 50 echocardiogram images available for review were graded by a single blinded observer for assessment of left atrial size. On review of available studies, LAE was based on left atrium–to–aortic root ratio ≥1.5:1, a threshold initially used to assess the presence or absence of a patent ductus arteriosus and used later by Russo and Webber in a pediatric study of restrictive cardiomyopathy [5, 14, 17, 30]. LAE was then further qualitatively categorized as mild, moderate, or severe, with the category of “severe enlargement” reserved for those cases that were the size of the left ventricle. The final echocardiogram was defined as the last echocardiogram before death, HT, or the end of the study. The diagnosis of RP was based on >1 echocardiogram with one or more of the following criteria: LAE without mitral regurgitation greater than mild or evidence of LV dilation (left-ventricular end diastolic dimension [LVEDD] z-score ≥ 2), mitral E/E′ ratio ≥ 10, and E/A ratio ≥ 3.
Catheterization
All available catheterization reports of patients who were included in the study were reviewed. All available hemodynamic information―including pulmonary capillary wedge pressure (PCWP), left atrial pressure (LAP), left-ventricular end diastolic pressure (LVEDP), systolic pulmonary arterial (PA) pressure, and pulmonary vascular resistance index (PVRI)―was recorded. Patients were classified as having hemodynamic parameters consistent with RP if LVEDP was ≥15. If an LVEDP was unavailable, PCWP or direct LAP was substituted.
Exercise Testing
Exercise testing was performed on a Marquette pediatric treadmill (Marquette Electronics, Milwaukee, WI) using a standard or modified Bruce protocol. Blood-pressure measurements were recorded with a manual sphygmomanometer in an upper extremity during testing. An abnormal BP response to exercise was defined as either a hypotensive response or a minimal increase in the systolic BP to increased workload compared with baseline (<20 mmHg). Continuous electrocardiograms were recorded in all cases. Peak oxygen consumption was measured when possible.
Statistical Analysis
Patient characteristics are expressed as number (%) for categorical variables, means ± SDs, or median with interquartile range (IQR) for continuous variables. The following clinical end points were studied: hospitalization, aSCD, HT, and death. Continuous variables assumed to follow a normal distribution were compared using paired Student t and Mann–Whitney U tests when variables were not assumed to follow a normal distribution. Categorical variables were compared using Chi-square test. Univariate time-dependent analysis was performed with log-rank analysis of Kaplan–Meier curves. Multivariable time-dependent analysis was performed using Cox or Poisson regression when appropriate. Covariates entered into the multivariate analysis included sex, age at presentation, symptoms at presentation, IVS z-score, family history of HCM, and presence of RP. In secondary analyses of individual measure of RP, only one measure (LA size, mitral E/E′ > 10, or septal E/E′ > 13) was entered into the model because these were found to be highly correlated. Results of Cox regression analysis are expressed as hazard ratios (HRs) with 95% CIs, and results of Poisson regression are expressed as incidence rate ratios (IRRs). Receiver operator characteristic (ROC) curves were used to determine optimal cut-off points for mitral and septal E/E′ ratios. To rate the degree of agreement between a single blinded observer and the final echocardiogram report when assessing LAE, Cohen’s kappa coefficient was used, and P ≤ 0.05 was deemed statistically significant. All data analysis was performed using SPSS software version 18.0 (SPSS, Chicago, IL).
Results
A total of 402 patients were diagnosed with possible hypertrophic cardiomyopathy between January 1, 1985, and January 1, 2010. There were 48 patients excluded due to follow-up at other institutions: 42 were one-time referrals for a second opinion; 5 were followed-up at other institutions after the initial diagnosis; and 1 was diagnosed within the last year of the study. There were 27 patients excluded due to diseases that cause increased afterload on the heart (systemic hypertension, aortic coarctation, or aortic valve stenosis). There were 113 patients excluded for possible metabolic or syndromic causes of secondary cardiac hypertrophy. There were 74 patients who were initially thought to have possible HCM but who were ultimately found to have resolution of their hypertrophy or a different cardiomyopathy. There was no echocardiogram report or insufficient information in the echocardiogram report in 31 patients. Some patients met >1 exclusion criteria. A total of 119 patients met inclusion criteria for isolated HCM and were followed-up for at least 1 year at our institution, or died, or underwent HT within 1 year of diagnosis (Fig. 1). The median follow-up time for the entire cohort was 4.4 years (IQR 2.1–6.6), with a maximum follow-up time of 18.9 years. Table 1 lists the characteristics of patients at diagnosis.
There were 26 patients (22%) hospitalized for a cardiac indication at least once during the follow-up period. The median and maximum numbers of hospitalizations were 1 and 3, respectively. Myectomy was performed in 4 patients; 9 patients had aSCD (3 at the time of diagnosis with HCM and 6 during the study period); 6 patients were listed for HT; 3 patients underwent HT; and 7 patients died. The cause of death was not available for 4 patients who died at home, 1 of whom died while listed for HT. Death occurred in 2 patients after devastating cerebrovascular accidents, and 1 patient died in the hospital after a cardiac arrest due to ventricular tachycardia. Neither age at presentation with HCM or diagnosis of HCM at age < 1 year were associated with death, transplant, or aSCD. Of 15 patients who presented with HCM at age < 1 year, 2 died at 7 and 95 days after presentation, respectively.
Symptoms at Presentation
Symptoms at the time of presentation with HCM were present in 31 (26%) patients and included ≥ 1 of the following: aSCD (n = 3 [2.5%]), syncope (n = 5 [4.2%]), respiratory distress (n = 7 [5.9%]), chest pain (n = 11 [9.2%]), dizziness (n = 5 [4.2%]), exercise intolerance (n = 4 [3.4%]) and palpitations/tachycardia (n = 5 [4.2%]). Symptoms at the time of presentation were associated with death or aSCD and death or HT regardless of the presence or absence of RP. Table 2 lists the results of time dependent multivariable analysis.
No Evidence of Restrictive Physiology
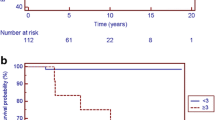
There was no evidence of RP in 69 (58%) patients. In 1 case, a patient with no evidence of RP who presented with severe systolic dysfunction, died 3 months after presentation. No patients without evidence of RP were listed for HT. aSCD occurred in 3 patients without evidence of RP. One case occurred on presentation, and the remaining 2 cases occurred at 1 and 1.9 years after diagnosis with HCM, respectively. In patients without evidence of RP, freedom from death or aSCD was 93.6% (Fig. 2) and freedom from death or HT was 98.5% (Fig. 3) at 10 years after diagnosis of HCM. RP had a negative predictive value of 98% for predicting death or HT. The absence of RP predicted a markedly lower likelihood of death or HT. In patients with no evidence of RP, the mean IVS z-score was 4.3 ± 3.4 on initial echocardiogram and 4.7 ± 4.9 on final echocardiogram (P = 0.3).
Restrictive Physiology
RP was diagnosed in 50 (42%) patients during the entire time course of the study. The initial echocardiogram was available in 38 (76%) of the patients diagnosed with RP. RP was present on the initial echocardiogram in 18 (47%) patients. The remaining 20 patients (53%) developed RP at a median of 2.3 years (IQR 0.74–3.3) after diagnosis with HCM. The method for diagnosis of RP is depicted in Fig. 1. In 38 of the 43 patients with LAE, the severity of LAE was specified. Of these, 4 (11%) had severe LAE, 15 (39%) had moderate LAE, and 19 (50%) had mild LAE. Of the 19 patients with mild LAE, 8 had no other evidence of RP. These 8 patients did not experience death, HT, or aSCD during the follow-up period. Of the 11 patients with mild LAE and other evidence of RP, 2 experienced aSCD and 1 died during the follow-up period. The kappa statistic to measure agreement between the echocardiogram report of LAE and a single blinded observer was 0.69.
Patients with RP were statistically more likely to be female, have a history of ventricular arrhythmia, and have an abnormal BP response to exercise compared with patients with no evidence of RP (Table 1). Their final echocardiograms had statistically greater IVS and LVFW z-scores compared with patients having no evidence of RP (Table 3). In patients with RP, the mean IVS z-score was 5.2 ± 3.0 on initial echocardiogram and 6.8 ± 4.4 on final echocardiogram (P = 0.03).
Of the 50 patients diagnosed with RP, 6 were listed for HT at a median of 5.2 years (IQR 3.7–8.5) after diagnosis with HCM; 3 underwent HT at 2.4, 3.3, and 8.4 years, respectively, and 2 patients were waiting on the HT list. Death occurred in 6 (12%) patients at a median of 0.15 years (IQR 0.06–4.7) after diagnosis of HCM, 1 of whom was on the HT list. At 10 years after diagnosis of HCM, freedom from death or aSCD was 59.0% (Fig. 2) and freedom from death or HT was 71.2% in patients with RP (Fig. 3). RP had a positive predictive value of 17% for predicting death or HT.
Cox regression analysis demonstrated that RP was associated with hospitalization, death or aSCD, and death or HT. LAE on echocardiogram was also associated with hospitalization, death or aSCD, and death or HT. Compared with patients who developed evidence of RP, patients who presented with RP were more likely to experience death or aSCD and experience death or HT (Table 2).
Mitral E/E′ ratios were available for review in 79 patients (66%), and septal E/E′ ratios were available in 71 patients (60%). Multivariable Cox regression analysis demonstrated that when analyzed as a continuous variable, mitral E/E′ ratio was associated with death or transplant and death or aSCD. Although the septal E/E′ ratio was associated with death or transplant, the association with death or aSCD did not reach statistical significance. When analyzed as binary variables, mitral E/E′ ≥ 10 and septal E/E′ ≥ 13 were associated with death or aSCD (Table 2).
Catheterization Data
Cardiac catheterization was performed in 17 (14%) patients. The indication for cardiac catheterization included one or more of the following: ICD placement (5 patients), LVOTO (5 patients), RP on echocardiogram (3 patients), heart failure symptoms with preserved systolic function (2 patients), family history of RP (2 patients), electrophysiology study (2 patients), transplant evaluation (1 patient), and dual-chamber pacemaker placement for atrioventricular block (1 patient). Hemodynamic parameters consistent with RP were present in 11 of 17 (65%) patients. Of these, 5 (45%) were listed for HT at a median of 6.0 years (IQR 4.3–8.7); 2 (18%) underwent HT at 8.4 and 2.4 years, respectively; and 3 (27%) died at 8 days, 73 days, and 4.8 years after diagnosis with HCM, respectively. One additional patient was listed for and underwent HT 3.3 years after diagnosis. That patient had electrocardiographic evidence of ischemia on rapid atrial pacing but did not have hemodynamic parameters listed in the catheterization report, although severe diastolic dysfunction was described. There were 6 patients with hemodynamic parameters consistent with RP not listed for transplant. Of these, 2 patients died at 2 weeks and 5 months after diagnosis, respectively. One patient was lost to follow-up at 1.5 years after catheterization, and 3 patients were transitioned to other institutions on reaching adulthood at 1.9, 1.9, and 5.6 years after catheterization, respectively.
Correlation Between Echocardiogram and Catheterization Data
Of the 17 patients who underwent catheterization, 13 had evidence of RP by echocardiogram, and 4 did not. Of the 11 patients with hemodynamic parameters consistent with RP, 10 had previous echocardiographic evidence of RP. The patients with no evidence of RP by echocardiogram had lower PCWP and PVR compared with those having evidence of RP (Table 4). Twelve patients had LAE by echocardiogram, and 5 did not. The patients with no evidence of LAE by echocardiogram had lower PCWP and PVR compared with those having evidence of LAE (Table 4).
Discussion
Our study demonstrates that in children with HCM, evidence of RP is independently associated with poor outcomes. Evidence of RP was independently associated with hospitalization, death or aSCD, and death or HT. Conversely, patients with HCM and no evidence of RP have an excellent prognosis. Previously identified risk factors for sudden death in patients with HCM have included younger age at diagnosis, family history of HCM, syncope, massive LVH, abnormal BP response to exercise, and previous history of ventricular arrhythmias [10, 12, 13, 18, 20, 22, 29, 31, 32]. The current study found that patients with RP have a greater incidence of ventricular arrhythmias, a greater incidence of abnormal BP response, and greater IVS and LVFW z-scores. This suggests that previously identified risk factors may be surrogates for this high-risk subgroup of patients.
Echocardiographic Indicators of RP
In the past, assessment of risk for death in HCM has focused on classic adult risk factors [18]. More recently attention has focused on echocardiographic measures of diastolic dysfunction, primarily in adults [3, 11, 24]. However, in 2004, McMahon et al. published a series of 80 children with HCM and age-matched controls. In that series, children with HCM were found to have lower early diastolic tissue Doppler velocities than age- and sex-matched controls. More importantly, greater septal E/E′ ratio predicted children at risk for death, ventricular tachycardia, or cardiac arrest [25]. The current study expands on the previous study by demonstrating cut-off points for mitral and septal E/E′ ratios and correlates with catheterization data. In a 2007 series of 96 Greek adult patients with HCM, Efthmiadis et al. [11] reported a powerful effect of septal E/E′ ratio in adults with HCM. In that series, no patient with a septal E/E′ ratio <15 had an adverse clinical outcome, whereas our cohort demonstrated cut-off points of 10 for mitral E/E′ and 13 for septal E/E′. In 2009, Biagini et al. published a series of 239 adult patients with HCM and found that RP, as assessed by mitral valve inflow, was associated with both SCD and heart failure-related death [3]. In our study, there was no difference in the average mitral E/A ratio between patients with and without RP. In fact, those with LAE had lower E/A ratios than those without LAE (Table 4). All patients with E/A ratio ≥ 3 had additional echocardiographic evidence of RP, whereas many patients with RP had an E/A ratio < 3. This is most likely due to pseudonormalization of the E/A ratio in the RP group. A study by Menon et al. demonstrated the association between left atrial size and other measures of abnormal diastology in children with HCM [26]. They also found that left atrial size correlates with symptoms and decreased exercise capacity. Only recently have normative values for left atrial volume in children been published [34]. Left atrial size in adults correlates with decreased exercise capacity and symptomatic heart failure [16, 28]. These studies suggest that those with worse diastolic dysfunction are at greater risk. Our study is the first to demonstrate that the worst end of the diastolic dysfunction spectrum, RP, is associated with ventricular tachycardia, abnormal BP response to exercise, and degree of septal hypertrophy; all commonly associated risk factors in HCM. Furthermore, our study demonstrates the importance of LAE in risk stratification of children with HCM. In those patients who underwent cardiac catheterization, LAE by echocardiogram was associated with greater PCWP and PVR, which are other markers of RP. Because normative values for left atrial volume have now been defined, further study of the impact of left atrial volume on long-term outcomes in children with HCM is warranted.
Progression of Disease
In primarily adult patients with HCM reported from nontertiary referral centers, life expectancy is relatively normal, and the disease is generally not progressive [20]. Our study also demonstrates an excellent prognosis in children with HCM who do not have RP, even though our center is a tertiary referral center. In adults, LV thickness is largely stable and does not progress [12, 19, 32]. In our cohort, children with RP demonstrated progressive IVS hypertrophy. Furthermore, approximately half of the patients with RP developed it during the follow-up period. Our data suggest that children and adolescents who present with symptoms or signs of RP are at highest risk; however, at least some of the remainder are still at risk of developing RP and falling into the greater-risk subgroup. Identifying these patients is of utmost importance.
Despite the increased risk in patients with RP, they continue to have a relatively low rate of death and/or HT. International Society for Heart and Lung Transplantation registry data indicate that during the current era, the 10-year pediatric heart transplant survival is likely >60%, which is comparable with the 71% 10-year freedom from death and/or transplant in patients with HCM and RP in our study [15]. Patients with consistent evidence of RP on echocardiogram and symptoms should undergo hemodynamic assessment by cardiac catheterization. Referral of these patients for HT should be made on a careful case-by-case basis.
Study Limitations
A main limitation to this study is its retrospective nature. Echocardiogram readers could not be blinded to the presence of disease. However, long-term outcomes were not known at the time of observation. During the study period, left atrial size in our center was measured subjectively. Therefore, at least two echocardiograms with evidence of RP were necessary for inclusion in the RP group. In addition, the echocardiogram reports had satisfactory agreement with a single blinded observer in measuring LAE. Although currently the most commonly used and validated measure of assessing for LAE involves measuring left atrial volumes, this was not possible in the majority of our patients because a two-chamber view had not consistently been obtained. Because the study period spanned 26 years, the frequency with which mitral inflow velocities and TDIs were measured increased throughout the study period. Those patients who did not have mitral E/A or E/E′ ratios measured, and who had normal left atrial size on echocardiogram, were placed in the category of patients without RP. There is evidence that LAE in patients with HCM correlates with other measures of diastolic dysfunction [26]. This may bias the study toward overstating the importance of RP. Because the presence of RP is one component of the evaluation for HT in patients with HCM at our center, the use of HT as an end point may cause some selection bias. However, the number of patient actually listed for HT was relatively low. Finally, because our institution is a teritary center, there may have been a referral bias, skewing our patients toward those with more severe disease. However, the excellent long-term outcomes in the patients without evidence of RP suggest this is not the case.
References
Appleton CP, Hatle LK, Popp RL (1988) Demonstration of restrictive ventricular physiology by Doppler echocardiography. J Am Coll Cardiol 11(4):757–768
Appleton CP, Hatle LK, Popp RL (1988) Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 12(2):426–440
Biagini E, Spirito P, Rocchi G, Ferlito M, Rosmini S, Lai F et al (2009) Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol 104(12):1727–1731
Border WL, Michelfelder EC, Glascock BJ, Witt SA, Spicer RL, Beekman RH III et al (2003) Color M-mode and Doppler tissue evaluation of diastolic function in children: Simultaneous correlation with invasive indices. J Am Soc Echocardiogr 16(9):988–994
Brown OR, Harrison DC, Popp RL (1974) An improved method for echographic detection of left atrial enlargement. Circulation 50(1):58–64
Calvet D, Touze E, Varenne O, Sablayrolles JL, Weber S, Mas JL (2010) Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation 121(14):1623–1629
Choong CY, Abascal VM, Thomas JD, Guerrero JL, McGlew S, Weyman AE (1988) Combined influence of ventricular loading and relaxation on the transmitral flow velocity profile in dogs measured by Doppler echocardiography. Circulation 78(3):672–683
Choong CY, Herrmann HC, Weyman AE, Fifer MA (1987) Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol 10(4):800–808
Colan SD, Lipshultz SE, Lowe AM, Sleeper LA, Messere J, Cox GF et al (2007) Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation 115(6):773–781
Decker JA, Rossano JW, Smith EO, Cannon B, Clunie SK, Gates C et al (2009) Risk factors and mode of death in isolated hypertrophic cardiomyopathy in children. J Am Coll Cardiol 54(3):250–254
Efthimiadis GK, Giannakoulas G, Parcharidou DG, Karvounis HI, Mochlas ST, Styliadis IH et al (2007) Clinical significance of tissue Doppler imaging in patients with hypertrophic cardiomyopathy. Circ J 71(6):897–903
Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ (2001) Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet 357(9254):420–424
Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A et al (2000) Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol 36(7):2212–2218
Iyer P, Evans N (1996) Re-evaluation of the left atrial to aortic root ratio as a marker of patent ductus arteriosus. Arch Dis Child Fetal Neonatal 70(2):F112–F117
Kirk R, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Dobbels F et al (2010) The Registry of the International Society for Heart and Lung Transplantation: thirteenth official pediatric heart transplantation report―2010. J Heart Lung Transplant 29(10):1119–1128
Kjaergaard J, Johnson BD, Pellikka PA, Cha SS, Oh JK, Ommen SR (2005) Left atrial index is a predictor of exercise capacity in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr 18(12):1373–1380
Lester LA, Vitullo D, Sodt P, Hutcheon N, Arcilla R (1979) An evaluation of the left atrial/aortic root ratio in children with ventricular septal defect. Circulation 60(2):364–372
Maron BJ (2010) Contemporary insights and strategies for risk stratification and prevention of sudden death in hypertrophic cardiomyopathy. Circulation 121(3):445–456
Maron BJ (2002) Hypertrophic cardiomyopathy: a systematic review. JAMA 287(10):1308–1320
Maron BJ, Casey SA, Poliac LC, Gohman TE, Almquist AK, Aeppli DM (1999) Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. JAMA 281(7):650–655
Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE (1995) Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 92(4):785–789
Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE et al (2000) Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 102(8):858–864
Maron BJ, Spirito P, Wesley Y, Arce J (1986) Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N Engl J Med 315(10):610–614
Matsumura Y, Elliott PM, Virdee MS, Sorajja P, Doi Y, McKenna WJ (2002) Left ventricular diastolic function assessed using Doppler tissue imaging in patients with hypertrophic cardiomyopathy: relation to symptoms and exercise capacity. Heart 87(3):247–251
McMahon CJ, Nagueh SF, Pignatelli RH, Denfield SW, Dreyer WJ, Price JF et al (2004) Characterization of left ventricular diastolic function by tissue Doppler imaging and clinical status in children with hypertrophic cardiomyopathy. Circulation 109(14):1756–1762
Menon SC, Ackerman MJ, Cetta F, O’Leary PW, Eidem BW (2008) Significance of left atrial volume in patients <20 years of age with hypertrophic cardiomyopathy. Am J Cardiol 102(10):1390–1393
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA et al (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133
Nistri S, Olivotto I, Betocchi S, Losi MA, Valsecchi G, Pinamonti B et al (2006) Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian Registry for Hypertrophic Cardiomyopathy). Am J Cardiol 98(7):960–965
Olivotto I, Maron BJ, Montereggi A, Mazzuoli F, Dolara A, Cecchi F (1999) Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J Am Coll Cardiol 33(7):2044–2051
Russo LM, Webber SA (2005) Idiopathic restrictive cardiomyopathy in children. Heart 91(9):1199–1202
Sadoul N, de Chillou C, Aliot E, McKenna WJ (1999) Evaluation of the risk of sudden death in hypertrophic cardiomyopathy. Arch Mal Coeur Vaiss 92(Spec no 1):65–73
Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ (2000) Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 342(24):1778–1785
Stoddard MF, Pearson AC, Kern MJ, Ratcliff J, Mrosek DG, Labovitz AJ (1989) Left ventricular diastolic function: comparison of pulsed Doppler echocardiographic and hemodynamic indexes in subjects with and without coronary artery disease. J Am Coll Cardiol 13(2):327–336
Taggart NW, Cetta F, O’Leary PW, Seward JB, Eidem BW (2010) Left atrial volume in children without heart disease and in those with ventricular septal defect or patent ductus arteriosus or hypertrophic cardiomyopathy. Am J Cardiol 106(10):1500–1504
Varnava AM, Elliott PM, Mahon N, Davies MJ, McKenna WJ (2001) Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol 88(3):275–279
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maskatia, S.A., Decker, J.A., Spinner, J.A. et al. Restrictive Physiology is Associated With Poor Outcomes in Children With Hypertrophic Cardiomyopathy. Pediatr Cardiol 33, 141–149 (2012). https://doi.org/10.1007/s00246-011-0106-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-011-0106-6