Abstract
This study aimed to investigate left ventricular myocardial deformation in children with Kawasaki disease during the acute phase of their illness. A total of 50 patients and 35 normal control subjects were assessed. Data were obtained from the patients during the acute and convalescent phases of Kawasaki disease. Analyses of myocardial deformation [strain (ε), strain rate (SR)] was performed using two-dimensional speckle-tracking imaging in three directions (longitudinal, circumferential, and radial) at the basal and mid levels of the left ventricular myocardium. Basal longitudinal ε (P < 0.001) and midlongitudinal ε (P < 0.0001) were lower during the acute phase of the disease than in the control subjects and associated with serum albumin level and left ventricular mass index (LVMI). Midlongitudinal SR (P < 0.0001) was lower during the acute phase of Kawasaki disease than in the control subjects and associated with LVMI. Decreased systolic SR was not detected in any direction. In conclusion, left ventricular longitudinal systolic ε was significantly decreased during the acute phase of Kawasaki disease. This may be a result of myocardial swelling from myocarditis during the acute phase of the disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Decreased myocardial contractility caused by myocarditis is common during the acute phase of Kawasaki disease [7, 8], and such decreased contractility is included in the criteria for the diagnosis of an incomplete attack of the disease [9]. Thus, evaluation of myocardial contractility is an important step in the initial diagnosis of Kawasaki disease.
Recently, myocardial deformation analyses, including strain (ε) and strain rate (SR) imaging, have been applied in clinical practice, especially for evaluation of systolic contractile function [2]. In pediatric clinical practice, myocardial deformation analysis has been used to diagnose various heart problems in children [12].
This study aimed to investigate left ventricular myocardial deformation in children with Kawasaki disease during the acute phase of the illness and to explore any relationships between such deformation and clinical, laboratory, and other echocardiographic variables.
Materials and Methods
Subjects
The study subjects consisted of 50 children registered at Konkuk University Hospital from December 2007 through February 2009 and at the Asan Medical Center from March 2009 through September 2009. All the subjects fulfilled the diagnostic criteria for Kawasaki disease, which required more than four principal criteria and a fever duration longer than 4 days [9]. In addition, all the subjects had undergone initial echocardiographic examination before intravenous immunoglobulin infusion therapy during the acute phase and were examined again echocardiographically during the convalescent phase 6–12 weeks after the onset of disease.
The control group comprised 35 children of comparable age who had been referred to the aforementioned two hospitals for evaluation of a cardiac murmur during the study period and for whom no evidence of cardiac disease was found by clinical and echocardiographic examinations. The institutional review boards of our two hospitals approved this study.
Demographic and laboratory data were surveyed by a review of medical records. Four patients were nonresponders to initial intravenous immunoglobulin. None of the patients had a familial history of Kawasaki disease, and one patient had recurrent disease.
Echocardiographic Examinations
The subjects were prepared for echocardiography by administration of a sedative or antipyretic, as required. All echocardiographic examinations were performed by one echocardiographer (J.J.Y.), who obtained images using a Vivid 7 Vantage machine (GE Vingmed, Horten, Norway) equipped with 7- and 5-MHz transducers. Still images (two-dimensional, M-mode, and Doppler images) from apical four-chamber and parasternal long- and short-axis views and moving images (≥3 cardiac cycles, 40–100 frames/s) were stored for analyses of myocardial deformation from apical four-chamber, two-chamber, and long-axis views and from basal and midlevel parasternal short-axis views on magneto-optical discs.
Still images were obtained during the phase of end expiration, but respiratory control was not possible during the acquisition of moving images. Measurements were performed offline using EchoPAC PC version 7.0.0 (GE Vingmed, Horten, Norway) by another author (H.S.C.) blinded to the patient or control status of the study subjects.
Left ventricular dimensions were measured from the standard M-mode using the parasternal long-axis view according to the recommendations of the American Society of Echocardiography [10]. Fractional shortening was calculated from these dimensions. Left ventricular mass was calculated using the formula derived by Devereux and Reichek [3], and the index was obtained by dividing the calculated mass by the body surface area. Doppler interrogation of the mitral valve was performed according to conventional guidelines [1]. The ratio of peak early diastolic velocity of mitral inflow (E) to mean early diastolic tissue velocity of six basal segments (Em) was calculated.
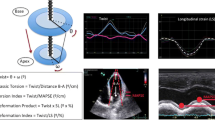
Analyses of Myocardial Deformation
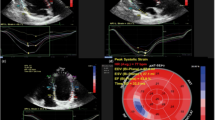
Analyses were performed from two-dimensional strain data using speckle-tracking imaging. Data were approved when the values for all six segments were considered acceptable by the software or when a value of any one segment (fewer than two segments) was rejected by the software but tracking of that segment was admissible (Fig. 1). For six patients, longitudinal analysis data from apical four-chamber images were abandoned because of failure to meet the preceding conditions.
Examples of two-dimensional strain analysis through speckle-tracking imaging. a Circumferential and radial analyses in the basal-level left ventricular myocardium. For all the segments, speckle tracking was approved by the software. b Longitudinal analysis of the left ventricular myocardium at the apical four-chamber view. In the basal lateral segment, speckle tracking was rejected by the software, but data from this analysis were approved by the authors because tracking of that segment was determined to be acceptable
All three directional (longitudinal, circumferential, and radial) data of myocardial deformation were measured in basal and mid levels, and the intrinsically negative measured data were converted to absolute values. In the measurement of basal-level data, the value of longitudinal Em was recorded for calculation of the aforementioned E/Em ratio.
In analyzing deformation, strain (ε), systolic strain rate (SR), and early diastolic SR were calculated by averaging the measured values from six segments (anteroseptal, anterior, lateral, posterior, inferior, and septal segments) in the respective three directions at two levels of the myocardial wall.
Reproducibility Test of Myocardial Deformation Analyses
For 15 randomly selected subjects, two authors (Y.B.K and H.S.C.) analyzed the moving images of midventricular segments to determine inter- and intraobserver variability. The percentage precision [5] of the measurements is shown in Table 1.
Statistical Analysis
All the data are presented as means ± standard deviations. All the statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC), and statistical significance was reflected by a P value less than 0.01. Chi-square analysis was used to determine whether the gender distribution differed between the patients and the control subjects. Unpaired Student’s t tests were used to compare numeric variables between patients and control subjects, and paired t tests were used to compare echocardiographic data obtained during the acute and convalescent phases of disease. Correlations between myocardial deformation data that showed a significant difference between patients and control subjects were assessed together with clinical, laboratory, and echocardiographic variables using Pearson’s correlation analysis.
Results
The demographic and anthropometric data of the patient and control groups did not differ significantly (Table 2). The clinical and laboratory data obtained during the acute phase of Kawasaki disease are shown in Table 3. Table 4 presents two-dimensional, M-mode, and flow Doppler echocardiographic measurements, and the results of left ventricular myocardial deformation measurements appear in Table 5.
Comparison of Data Between Groups
When we compared measurements obtained during the acute and convalescent phases in the Kawasaki disease group, we found that heart rate (P < 0.0001), left anterior descending coronary diameter (LAD; P < 0.0001), right coronary diameter (RCA; P < 0.001), left ventricular mass (P < 0.0001), left ventricular mass index (P < 0.0001), left atrial minimal dimension (P < 0.0001); E-wave peak velocity (P < 0.01), mitral inflow A-wave peak velocity (P < 0.001), E/Em ratio (P < 0.001), and mid circumferential SR (systolic: P < 0.01; early diastolic: P < 0.01), were significantly higher, whereas fractional shortening (P < 0.01), basal longitudinal ε (P < 0.001), and midlongitudinal (P < 0.001) ε were significantly lower during the acute phase.
When measurements obtained during the acute phase of Kawasaki disease patients were compared with those of control subjects, we found that heart rate (P < 0.0001), coronary diameters (left main coronary artery [LMCA]: P < 0.01; LAD: P < 0.01; RCA: P < 0.01), left ventricular mass index (P < 0.0001), E/Em ratio (P < 0.0001), and Tei index (P < 0.01) were significantly higher, whereas fractional shortening (P < 0.01), basal longitudinal ε (P < 0.001), basal circumferential ε (P < 0.01), midlongitudinal ε (P < 0.0001), and midlongitudinal early diastolic SR (P < 0.01) were significantly lower in the patients.
Analyses of Correlation Between Left Ventricular Deformation and Other Variables During the Acute Phase of Kawasaki Disease
We attempted to correlate clinical, laboratory, and echocardiographic variables and four myocardial deformation data during the acute phase of Kawasaki disease, which showed significant difference between the patients and the control subjects. Basal longitudinal ε was related to white blood cell (WBC) count (R = −0.54; P < 0.001), serum albumin level (R = 0.41; P < 0.01), and left ventricular mass index (R = −0.44; P < 0.01). Basal circumferential ε was related to fractional shortening (R = 0.46; P < 0.001). Midlongitudinal ε was related to albumin level (R = 0.52; P < 0.001) and left ventricular mass index (R = −0.43; P < 0.01). Midlongitudinal early diastolic SR was related to age (R = −0.39; P < 0.01), left ventricular end systolic dimension (R = −0.44; P < 0.01), and left ventricular mass index (R = 0.40; P < 0.01).
Analysis of the correlation between echocardiographic variables and deformation data also was performed for the data of the convalescent phase and for the control subjects, but no significant relationship was observed.
Discussion
Significantly decreased longitudinal ε was observed in both basal and mid myocardium during the acute phase of Kawasaki disease. Serum albumin level and left ventricular mass index were significantly related to it. We previously reported that left ventricular mass index was increased and associated with serum albumin level during the acute phase of Kawasaki disease [14]. In that report, we hypothetically concluded that elevation of the left ventricular mass index during the acute phase of Kawasaki disease is due to myocardial swelling from increased vascular permeability.
Furthermore, it was reported by Terai et al. [11] that increased vascular leakage can cause hypoalbuminemia. Therefore, it is presumed that decreased longitudinal ε in this study was the result of myocardial wall motion disturbance from the elevation of the left ventricular mass index. Also, decreased longitudinal ε should be regarded as a myocardial functional disturbance in the Kawasaki myocarditis, because the diameters of coronary arteries were not related to it in this study and because elevation of the left ventricular mass index is an index of geometric change in myocarditis.
In this study, we used speckle tracking methods instead of the spectral Doppler method to measure Em value. The Em value measured through the speckle tracking method usually is lower than the value measured through spectral Doppler method [4]. Therefore, the E/Em ratio in our study appeared to be higher than the usual value, even among the control subjects.
The higher E/Em ratio and the increased left atrial minimal dimension observed during the acute phase of Kawasaki disease relative to controls may reflect increased left ventricular end diastolic pressure during the acute phase of Kawasaki disease [7]. Decreased midlongitudinal early diastolic SR during the acute phase of the disease also may reflect left ventricular diastolic dysfunction. Although it showed growth impact (association with age) and was not significantly related to other diastolic function indices, it was related to the left ventricular end systolic dimension and the left ventricular mass index. Additionally, in our previous report [14], we observed a significant relationship between the left ventricular mass index and several other diastolic function indices during the acute phase of Kawasaki disease.
Systolic SR usually has been regarded as the most significant index of left ventricular contractility among the indices obtained from myocardial deformation analyses [6, 13]. But we could not identify decreased systolic SR in any direction in basal and mid level myocardium.
Decreased left ventricular fractional shortening has been shown to result from the decreased systolic myocardial contractility, caused by myocarditis, during the acute phase of Kawasaki disease [8]. We observed the same finding during the acute phase of the disease. Therefore, the analysis of systolic SR seems not to be a better method than other conventional echocardiographic methods for detecting decreased myocardial contractility during the acute phase of Kawasaki disease.
This study had two limitations. First, the data set used for analysis was not complete. For six patients, longitudinal deformation analyses had to be excluded due to failure of imaging approval. Second, parts of the results for the reproducibility tests were unsatisfactory.
Conclusion
Left ventricular longitudinal systolic ε was significantly decreased during the acute phase of Kawasaki disease and associated with serum albumin level and left ventricular mass index. This may be a result of myocardial swelling from myocarditis during the acute phase of Kawasaki disease.
References
Appleton CP, Jensen JL, Hatle LK, Oh JK (1997) Doppler evaluation of left and right ventricular diastolic function: a technical guide for obtaining optimal flow velocity recordings. J Am Soc Echocardiogr 10:271–291
Dandel M, Hetzer R (2009) Echocardiographic strain and strain rate imaging: clinical applications. Int J Cardiol 132:11–24
Devereux R, Reichek N (1977) Echocardiographic determination of left ventricular mass in man: anatomic validation of the method. Circulation 55:613–618
Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N (2009) Correlation of tissue Doppler and two-dimensional speckle myocardial velocities and comparison of derived ratios with invasively measured left ventricular filling pressures. J Am Soc Echocardiogr 22:284–289
Galderisi M, Benjamin EJ, Evans JC, D’Agostino RB, Fuller DL, Lehman B, Wolf PA, Levy D (1992) Intra- and interobserver reproducibility of Doppler-assessed indexes of left ventricular diastolic function in a population-based study (the Framingham Heart Study). Am J Cardiol 70:1341–1346
Greenberg NL, Firstenberg MS, Castro PL, Main M, Travaglini A, Odabashian JA, Drinko JK, Rodriguez LL, Thomas JD, Garcia MJ (2002) Doppler-derived myocardial systolic strain rate is a strong index of left ventricular contractility. Circulation 105:99–105
Hiraish S, Yashiro K, Oguchi K, Kusano S, Ishii K, Nakazawa K (1981) Clinical course of cardiovascular involvement in the mucocutaneous lymph node syndrome. Am J Cardiol 47:323–330
Newburger JW, Sanders SP, Burns JC, Parness IA, Beiser AS, Colan SD (1989) Left ventricular contractility and function in Kawasaki syndrome. Circulation 79:1237–1246
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 114:1708–1733
Sahn DJ, DeMaria A, Kisslo J, Weyman A (1978) Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58:1072–1083
Terai M, Honda T, Yasukawa K, Higashi K, Hamada H, Kohno Y (2003) Prognostic impact of vascular leakage in acute Kawasaki disease. Circulation 108:325–330
Weidemann F, Eyskens B, Sutherland GR (2002) New ultrasound methods to quantify regional myocardial function in children with heart disease. Pediatr Cardiol 23:292–306
Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, De Scheerder I, Bijnens B, Rademakers FE (2002) Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol 283:H792–H799
Yu JJ, Kwak BO, Jeon YH, Park YM, Lee R, Chung S, Bae SH, Pyun BY (2009) Elevation of the index of left ventricular mass during the acute and subacute phase of Kawasaki disease, and its association with indexes of diastolic function. Cardiol Young 19:64–69
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J.J., Choi, H.S., Kim, Y.B. et al. Analyses of Left Ventricular Myocardial Deformation by Speckle-Tracking Imaging During the Acute Phase of Kawasaki Disease. Pediatr Cardiol 31, 807–812 (2010). https://doi.org/10.1007/s00246-010-9708-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-010-9708-7