Abstract
An eight-month-old boy with findings of persistent left pulmonary basal infiltrate was diagnosed with congenital unilateral pulmonary vein atresia by bronchoscopy. Cardiac catheterization documented slow left pulmonary venous return to atretic pulmonary veins. Conservative treatment was chosen because the child was asymptomatic and corrective surgery or percutaneous intervention was not technically possible. After a 3-year follow-up, the child still has no documented pulmonary hypertension. Early diagnosis of unilateral pulmonary vein atresia is important to anticipate potential threatening complications like pulmonary hypertension and hemoptysis. Surgical treatment of this entity might be drastic and complex and should be weighed against a conservative alternative and careful follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Unilateral pulmonary vein atresia is a rare isolated congenital anomaly [1, 2]. Clinical presentation has been usually described in children with recurrent unilateral pneumonia, hemoptysis, and chest X-ray findings of pulmonary hypoplasia and ipsilateral diffuse interstitial infiltrate [1, 2, 4, 8]. It might be complicated by pulmonary hypertension and systemico-pulmonary collateral vessel bleeding, for which pneumonectomy is then suggested [1, 7, 8]. In this context, early diagnosis and close follow-up can decrease the morbidity and might delay pneumonectomy [1, 5].
Case Report
An 8-month-old boy was referred with persistent pulmonary infiltrate 3 months after a bronchiolitis-like syndrome treated with two courses of antibiotics. On clinical examination, the child was in good health with no respiratory distress symptoms. Breath sounds were slightly decreased on the left basal field.
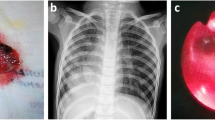
Chest X-ray showed reduced volume of the left lung with mediastinal shift toward the left, a left basal infiltrate, and minimal pleural effusion (Fig. 1), confirmed by the thoracic computed tomography (CT) scan. Fiber-optic bronchoscopy revealed submucosal varices of the left bronchial tree, predominantly on the left superior lobe and lingula, suggesting an obstruction of the left pulmonary venous drainage (Fig. 2). These varices had a particular streaked appearance and bled on contact with the bronchoscope. The bronchoalveolar lavage was normal. Cardiac ultrasound showed normal intracardiac anatomy, but pulmonary veins were not demonstrated on the left side. There were no signs of pulmonary hypertension or of right ventricular volume overload suggestive of abnormal venous return to the right-sided heart. Cardiac magnetic resonance imaging (MRI) could not identify the left pulmonary veins, with absent pulmonary venous return on that side neither to the left atrium nor to the systemic veins, thus evoking unilateral pulmonary vein hypoplasia or atresia. The ventilation-perfusion scanning was highly abnormal with 95% of the perfusion to the right side.
Cardiac catheterization confirmed the diagnosis of unilateral pulmonary vein atresia. Angiography of the main pulmonary artery showed a slightly smaller left pulmonary artery with preferential flow toward the right. The pulmonary venous return was normal on the right side but highly abnormal on the left, with slow capillary transit through very small tortuous pulmonary veins (<2 mm), joining the systemic venous return via veno-venous collaterals to the azygos vein (Fig. 3A and Fig. 3B). This small pulmonary-to-systemic drainage was considered of no clinical relevance, as no step-up in oxyhemoglobin saturation from the innominate vein (73%) to the pulmonary artery (74%) was documented. The mean pulmonary artery pressure was normal (15 mm Hg) with elevation of the left capillary wedge pressure (13 mm Hg) compared to the right (7 mm Hg). No aorto-pulmonary collateral vessels were seen on the aortography.
A Postero-anterior view after selective angiogram of the left pulmonary artery showing the connection between the pulmonary veins (white arrows) and the azygos system. B Postero-anterior view demonstrating the small atretic pulmonary veins (white arrows) and the absence of their connection onto the left atrium (black arrow)
Because pulmonary venous connection to the atria was determined technically impossible and the child had neither clinical symptoms nor complications, no intervention was undertaken.
After a 3-year follow-up, the child still has no symptoms, no hemoptysis, and no signs of pulmonary hypertension on echocardiography.
Discussion
Unilateral pulmonary vein atresia is a rare, isolated congenital anomaly that carries a high morbidity and mortality rate [1, 2]. Diagnosis is usually made in childhood, but it is sometimes not made until adulthood [4]. The majority of cases are thought to be congenital, resulting from late failure of incorporation of the common pulmonary vein into the left atrium [10], with subsequent obstruction of part of its branches and persistence of only minimal connection between the pulmonary and systemic venous return [1, 3]. This congenital hypothesis is strengthened by the association of other congenital cardiac anomalies in up to 50% of cases [1, 2], most often interventricular septal defect or patent ductus arteriosus. Some acquired cases are described as secondary to inflammatory processes leading to obliteration of the vessel lumen [8].
Clinical presentation consists of recurrent pulmonary infections, wheezing, hemoptysis, and exercise intolerance [1, 2, 7, 8]. The chest X-ray is suggestive when ispilateral lung hypoplasia, mediastinal shift to the affected side, and interstitial infiltrate often more pronounced in the ipsilteral lower lobe are present [1,4], but rarely do these findings lead to the diagnosis in infants or small children, especially when respiratory symptoms are absent.
Hemoptysis is the major complication, related to the high pressure and consequent risk of rupture of dilated bronchial veins. The association of bronchial varicosities with obstruction to the pulmonary venous return in the setting of unilateral pulmonary vein atresia has rarely been described [12]. These can be explained by increased pulmonary venous pressure leading to communications between the pulmonary and bronchial veins and represent a mechanism of drainage of the pulmonary venous return into the systemic venous system. As a matter of fact, isolated bronchial varices should also be considered in the differential diagnosis of this anomaly. In our case, strongly abnormal bronchoscopic findings of submucosal varices documented only on one side of the bronchial tree suggested the diagnosis of obstruction to the left pulmonary venous return. These bronchial varices might be absent when other collateral channels are present. This was not the case in our patient, who had only tiny collaterals between the atretic pulmonary veins and the azygos system.
The isotopic scan is strongly suggestive when unilateral greatly diminished perfusion is associated with grossly normal ventilation distribution. Definite diagnosis of this rare anomaly can only be made by cardiac catheterization. The angiography in the pulmonary trunk will mostly reveal preferential flow to the normal side, with a smaller pulmonary artery on the affected side. Selective pulmonary artery wedge injection reveals the unilateral atretic pulmonary venous system and no contrast media returning to the left atrium, but small tortuous anastomotic vessels, often communicating with the azygos system [1]. Their hemodynamic implication can be assessed by step-up oxyhemoglobin saturation in the pulmonary artery. The pulmonary wedge pressure is usually elevated to some degree on the affected side, due to pulmonary venous congestion, which can progress to pulmonary arterial hypertension. The presence of systemico-pulmonary collateral vessels must be assessed by aortography.
Management depends on the severity and extent of the anomaly. When localized stenosis is present, percutaneous balloon dilation with stent implantation at the junction of the pulmonary venous confluence and left atrium junction has been tried [9, 10], but the long-term prognosis of this procedure still has to be evaluated. When the size of the veins is accessible and their localization is close to the atria [7], surgical anastomosis of the pulmonary veins to the left atrium can be attempted. The outcome is often mitigated with a restenosis rate of up to 18% [11]. The sutureless repair technique introduced in 1996 provides better midterm results than any other technique, with freedom from mortality and recurrence improving from 65% to 90% [6]. In the case of atresia, there is no corrective option and treatment is conservative.
In our patient, the pulmonary veins were atretic, leading to no surgical or interventional corrective option. Because the child has been asymptomatic and has had no complications, the chosen treatment was conservative. A close follow-up will be essential to detect the occurrence of complications, such as hemoptysis or pulmonary hypertension. The authors recommend regular echocardiographic follow-up to detect signs of pulmonary hypertension, every 6 months soon after the diagnosis and at least every year for the first few years. Cardiac MRI, less invasive than cardiac catheterization, is now a reliable tool to detect systemico-pulmonary collateral vessels.
Pneumonectomy is indicated once symptoms or complications are present. Nevertheless, pneumonectomy is burdened with increased morbidity in small growing children, like scoliosis, kyphosis, or unilateral hypoplastic chest wall, and should therefore be delayed as much as possible to avoid these complications, all being more pronounced when the child is younger. Another complication worth mentioning is postpneumonectomy syndrome, related to extrinsic compression of the main or a lobe bronchus resulting from excessive mediastinal shift and lung herniation.
Conclusion
Congenital unilateral pulmonary vein atresia is rare and seldom diagnosed in infancy. This diagnosis should be considered in children with recurrent unilateral pneumonia and ispilateral radiographic findings of lung hypoplasia with interstitial infiltrate. Bronchoscopic findings of unilateral submucosal bronchial varices should be clearly evocative of unilateral obstruction to the pulmonary venous return. Cardiac catheterization is the cornerstone to establish a definite diagnosis allowing one to elucidate whether the pulmonary veins are atretic or stenotic and is also helpful in providing assessment and eventual interventional management of the potential complications. In symptomatic patients, pneumonectomy is indicated as an attempt to reduce mortality, essentially related to hemoptysis and pulmonary hypertension. If asymptomatic, the conservative alternative with a careful follow-up should be weighed against a potentially drastic and complicated surgical treatment, especially in small children.
References
Beerman LB, Oh KS, Park SC, et al. (1983) Unilateral pulmonary vein atresia: clinical and radiographic spectrum. Pediatr Cardiol 4:105–112
Cullen S, Deasy PF, Tempany E, Duff DF (1990) Isolated pulmonary vein atresia. Br Heart J 63:350–354
Edwards JE (1960) Congenital stenosis of pulmonary veins. Pathologic and developmental considerations. Lab Invest 9:46–66
vHeyneman LE, Nolan RL, Harrison JK, McAdams HP (2001) Congenital unilateral pulmonary vein atresia: radiologic findings in three adult patients. Am J Roentgenol 177:681–685
Kingston HM, Patel RG, Watson GH (1983) Unilateral absence or extreme hypoplasia of pulmonary veins. Br Heart J 49:148–153
Lacour-Gayet F (2006) Surgery for pulmonary venous obstruction after repair of total anomalous pulmonary venous return. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 45–50. Review
Nasrallah AT, Mullins CE, Singer D, Harrison G, McNamara DG (1975) Unilateral pulmonary vein atresia: diagnosis and treatment. Am J Cardiol 36:969–973
Pourmoghadam KK, Moore JW, Khan M, et al. (2003) Congenital unilateral pulmonary venous atresia: definitive diagnosis and treatment. Pediatr Cardiol 24:73–79
Sacco O, Fregonese B, Fregonese L, Gambini C, Pongiglione G, Rossi GA (2002) Recurrent unilateral bacterial pneumonias and interstitial fibrosis associated with pulmonary vein atresia: successful treatment with endovascular stent implantation. Pediatr Pulmonol 34:324–328
Ussia GP, Marasini M, Rimini A, Pongiglione G (2004) Atresia of right pulmonary veins with intact atrial septum and major aorto-pulmonary collateral treated with percutaneous stent implantation and embolization. J Intervent Cardiol 17:183–187
van Son JA, Danielson GK, Puga FJ, Edwards WD, Driscoll DJ (1995) Repair of congenital and acquired pulmonary vein stenosis. Ann Thorac Surg 60:144–150
Wiebe S, Maclusky I, Manson D, Holowka S, Yoo SJ. (2003) Hemoptysis: a rare cause can be related to a bronchial varix due to pulmonary venous obstruction. Pediatr Radiol 33:884–886
Acknowledgment
The authors would like to thank Constance Barazzone, MD, for her support in the follow-up of this patient.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tissot, C., Corbelli, R., Aggoun, Y. et al. Bronchoscopic Diagnosis of Asymptomatic Unilateral Pulmonary Vein Atresia in an Infant. Pediatr Cardiol 29, 976–979 (2008). https://doi.org/10.1007/s00246-007-9143-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-007-9143-6