Abstract
Sinus venosus defect (SVD) is an uncommon type of interatrial communication in which cardiac magnetic resonance (CMR) is increasingly used as an alternative imaging modality. The goal of this study was to determine the accuracy of CMR in patients with SVD compared with surgical findings. The diagnostic studies and operative reports of all patients who had CMR followed by surgical repair of SVD (n = 16) from 1996 to 2005 were reviewed and discrepancies were recorded. CMR studies included assessment of anatomy (evaluated by a combination of gradient echo cine, spin echo, and gadolinium-enhanced three-dimensional magnetic resonance angiography), ventricular volumes and function, and flow measurements. The median age at CMR was 14 years (range, 0.4–42). Compared with operative findings, there were no major discrepancies with CMR. The SVD was clearly imaged in all patients and 36 anomalously draining pulmonary veins were identified. The median pulmonary-to-systemic flow ratio was 2.4 (range, l.3–4.6). Patients had an average of 1.7 previous diagnostic tests (range, 1–3; 19 transthoracic echo, 5 catheterizations, and 3 transesophageal echo). Before CMR, SVD was diagnosed in 1 patient, suspected in 7, and not suspected in 8. Additional unsuspected findings identified by CMR included malposition of septum primum (n = 2), left superior vena cava to coronary sinus (n = 2), and aortic arch anomalies (n = 2). CMR accurately depicts SVD anatomy and associated anomalous pulmonary venous drainage, provides quantitative information on the hemodynamic burden, and reveals additional cardiovascular abnormalities. This experience indicates that CMR provides the information necessary for surgical planning of SVD repair.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sinus venosus defect (SVD) is an uncommon cause of left-to-right interatrial shunting associated with anomalous pulmonary venous drainage [3, 9]. Van Praagh et al. [12] described the morphologic basis of the defect as deficiency of the common wall between the right superior vena cava (SVC) and the right upper pulmonary vein or the wall between the right atrium and the right lower and middle pulmonary veins. The latter, also termed SVD of the right atrial type, is a rare defect that can be associated with malposition of septum primum and anomalous drainage of the right pulmonary veins to the right atrium despite normal connection to the posterior wall of the atria between the left and right superior vena cavae or their remnants.
Surgical repair of SVD is usually recommended because the combination of a sizeable interatrial communication and anomalous pulmonary venous drainage often results in a large left-to-right shunt and dilatation of the right heart. Preoperative planning requires accurate assessment of the interatrial communication, delineation of the anomalously draining pulmonary veins and their drainage site(s), and evaluation of the hemodynamic burden imposed by the shunt. Several diagnostic techniques have been used in patients with SVD, including transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and cardiac catheterization [5, 6, 8]. However each of these techniques has limitations. Cardiac magnetic resonance imaging (CMR) is increasingly utilized as a noninvasive test in the evaluation of many congenital heart defects, including anomalies of the atrial septum and pulmonary veins [2, 4, 7]. Although the use of CMR in patients with SVD has been reported in the context of other anomalies, its diagnostic accuracy in the preoperative evaluation of this lesion has not been evaluated in detail. Therefore,this study was designed to assess the diagnostic accuracy of CMR in patients with SVD by comparing these findings with those at surgery.
Materials and Methods
Patients
We searched the electronic records of all CMR scans performed at Children’s Hospital Boston from June 1996 through July 2005 for the diagnosis of SVD. Of the 23 patients with SVD, 16 patients had a preoperative CMR examination followed by surgical repair at our institution and they comprised the study group. Of the remaining 7 patients, 4 had undergone surgical repair prior to CMR evaluation, and 3 had not undergone surgical correction at the time of this analysis. The Children’s Hospital Committee on Clinical Investigations approved the review of medical records and CMR studies.
CMR
All studies were performed with commercially available 1.5T MRI scanners (GE Medical Systems, Milwaukee, WI, USA or Philips Medical Systems, Best, The Netherlands). The following radiofrequency coils were used depending on patient size: torso phased array (n = 10), cardiac phased array (n = 4), body (n = l), and head (n = l). General anesthesia or intravenous sodium pentobarbital (Nembutal) 4–6 mg/kg were used in 2 patients who were unable to cooperate. There were no anesthetic or other complications related to the CMR studies.
CMR imaging techniques utilized for evaluation of SVD changed during the course of the study as newer techniques became available. ECG-triggered, breath-hold gradient echo cine MR was used for assessment of SVD and pulmonary venous anatomy and for quantitative evaluation of biventricular volumes and function. A segmented k-space fast spoiled gradient echo recall sequence (n = 7) was used before 2002 [echo time (TE), 3.6–7.0 msec; repetition time (TR), 11–18 msec; flip angle (FA), 15°; matrix, 128 (phase) × 256 (frequency); number of excitations (NEX), 1; slice thickness (ST), 5–7 mm]. A segmented k-space steady-state free precession (SSFP) gradient echo sequence (n = 9) was used for cine MR after January 2002 (TE, 1.5–1.6 msec; TR, 3.6–3.9 msec; FA, 45°; matrix, 192 × 256; NEX, 1; ST, 6–7 mm). ECG-triggered Tl-weighted fast spin echo with double inversion recovery (FSE-DIR) (TE, 40 msec; TR, 1237 msec; FA, 90°; matrix, 256 × 256; NEX, 1; ST, 4 mm) was performed in 8 patients. Gadolinium (Gd)-enhanced three-dimensional (3D) magnetic resonance angiography (MRA) (n = 15) was performed with post processing of maximum intensity projection images (TE, 1.2–2 msec; TR, 5.2–6.4 msec; FA, 45°; matrix, 192 × 256; NEX, 0.5–l; ST, 1.2–4 mm). Flow velocity was measured in the ascending aorta and main pulmonary artery for calculation of the pulmonary-to-systemic flow ratio in all patients using an ECG-triggered, breath-through velocity-encoded phase contrast sequence (CinePC) with 24 or 25 reconstructed frames per cardiac cycle (TE, 3.6–10 msec; TR, 10–32 msec; FA, 15°; matrix, 128 × 256; NEX, 2–3; ST, 6 mm).
Data Analysis
The surgical records of the patients were reviewed and the following information was abstracted: size and location of SVD, pulmonary vein anatomy, and associated anomalies. The following CMR data were recorded: size and location of SVD; pulmonary vein anatomy, including number of anomalously draining pulmonary veins and their site of drainage; associated anomalies; right ventricular size and function; pulmonary-to-systemic flow ratio. The CMR findings in the report were compared with the operative note for discrepancies, which were classified as none, minor (when no significant clinical impact was expected), or major (when the discrepancy was judged to have a potentially significant impact on patient treatment or outcome). The CMR studies were also reviewed off-line for measurements of the SVD size. Measurements were performed with electronic calipers in two orthogonal planes using an Advantage Windows workstation version 4.2 (GE Medical Systems).
Results
Patient characteristics are summarized in Table 1. At the time of CMR evaluation, the median age of the patients was 14 years. The patients had an average of 1.7 (range, 1–3) other diagnostic imaging studies performed prior to CMR (Table 2). All patients had undergone at least one transthoracic echocardiogram, and only one patient had a conclusive diagnosis of SVD. That patient was referred for delineation of pulmonary venous anatomy. Invasive testing included five cardiac catheterizations and three transesophageal echocardiograms. One patient had CMR performed at another institution, which did not fully demonstrate the anatomy of the pulmonary veins. In general, patients were referred for CMR in cases in which previous diagnostic studies did not provide conclusive information and to further delineate pulmonary venous anatomy for surgical planning.
Compared with operative findings, there were no major discrepancies with CMR. The SVD was correctly identified in all cases (Table 2). Of these, 14 patients had a superior type of SVD (Fig. 1), and 2 had a right atrial type of SVD, associated with malposition of septum primum (Fig. 2). All patients had right ventricular dilatation, with quantitative assessment performed in the most recent 10 patients. The right ventricular end diastolic volumes ranged from 104–185 ml/m2 (normal values, 75.2 ± 13.8 in females, 86.2 ± 14.1 in males) [1]. The median pulmonary-to-systemic blood flow ratio was 2.4 (ranges, 1.3 – 4.6).
ECG-triggered, breath-hold steady-state free precession cine MR showing sinus venosus defect of the superior vena cava (SVC) type. (A) Axial plane image showing the defect (asterisk) in the tissue that separates the posterior wall of the SVC and the anterior wall of the right upper pulmonary vein (RUPV). The arrow indicates the left atrial orifice of the right upper pulmonary vein, which serves as a communication between the left atrium (LA) and the SVC. (B) Sagittal plane: The arrows indicate the sinus venosus defect between the RUPV and the SVC. Ao, aorta; RA, right atrium
A total of 36 anomalously draining pulmonary veins were identified. The anomalous pulmonary venous drainage involved the right lung in all patients (Table 2). One patient had an anomalous connection of the left upper lobe pulmonary vein to the left innominate vein in addition to a right upper lobe pulmonary vein draining to the junction of the SVC and right atrium (Fig. 3). CMR identified small accessory anomalously draining pulmonary veins in six patients, none of which were described in the surgical reports. These veins drained either to the cephalic aspect of the SVC or to the azygos vein. One of these patients had repeat CMR 17 months after surgical repair, and the small accessory right upper lobe pulmonary vein branch connecting to the SVC was again identified. However, the indexed right ventricular end diastolic volume was within normal limits, and flow quantification did not indicate any significant residual shunt.
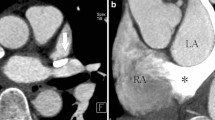
ECG-triggered, breath-hold steady-state free precession cine MR showing sinus venosus defect of the right atrial type. (A) Axial plane image showing the defect between the right lower pulmonary vein (RLPV) and the atrial septum (arrow). (B) Sagittal plane image at the level of the defect showing the absence of septum between the RLPV and the right atrium (asterisk), just above the entrance of the inferior vena cava (IVC) to the right atrium. RPA, right pulmonary artery
Additional cardiac anomalies were identified in three patients. Two patients had a left SVC draining to the coronary sinus, one of whom also had a right aortic arch with an aberrant origin of the left subclavian artery. A third patient had a left aortic arch with an aberrant origin of the right subclavain artery.
Discussion
The results of this study demonstrate that CMR is an excellent noninvasive imaging modality in the evaluation of SVD. The findings at surgical repair confirmed that CMR accurately delineated the anatomy of the SVD and pulmonary veins in all patients. Additional information included assessment of the hemodynamic burden from the defect, including measurements of right ventricular volumes and function and the pulmonary-to-systemic flow ratio. Moreover, CMR detected additional, previously unsuspected abnormalities in three patients.
Several diagnostic techniques have been used in patients with SVD. In young patients and in those with good acoustic windows, TTE provides the information necessary for surgical planning in the majority of cases [10]. However, in older patients and in those with restricted acoustic windows, TTE is limited in its ability to delineate the SVD and associated anomalous pulmonary venous drainage. Kronzon et al. [8] reported that TTE identified the SVD in only 25% of adult patients and did not delineate the abnormal pulmonary venous drainage in any. TEE provides an excellent alternative to TTE in patients with suboptimal transthoracic acoustic windows [6]. The proximity of the ultrasound probe to the atrial structures usually allows for excellent visualization of the atrial and the sinus venosus septa as well as the cardiac ends of the pulmonary veins. Disadvantages of TEE include its invasive nature, which requires anesthesia or sedation, and limited ability to image extracardiac structures. Especially relevant to SVD, TEE is limited in its ability to image the cranial aspect of the SVC where accessory right upper lobe pulmonary veins sometimes connect. Pascoe et al. [11] reported that TEE identified 37 anomalously connecting pulmonary veins in 25 patients with SVD. However, 10 additional anomalous right pulmonary veins, mostly from the right upper lobe to the cranial aspect of the SVC, were found at surgery. [11].
Cardiac catheterization can be used in the preoperative evaluation of patients with suspected SVD; however, its invasive nature and exposure to ionizing radiation are significant drawbacks. Moreover, accurate delineation of the SVD and pulmonary venous anatomy depends on angiographic visualization of the relevant anatomy. Freed et al. [5] reported that of the five patients with atrial septal defects that were missed by catheterization, four had SVD. Multidetector computed tomography is another imaging modality that can potentially illustrate the anatomy of SVD and associated anomalous pulmonary venous drainage. However, this technique has not been evaluated in SVD, and exposure to ionizing radiation and its limited ability to evaluate function and blood flow are significant weaknesses.
CMR overcomes many of the previously mentioned limitations and is capable of providing the anatomic and functional information necessary for surgical planning. Most patients older than 7 or 8 years do not require sedation or anesthesia. CMR is noninvasive, and is not associated with ionizing radiation exposure. Furthermore, steady-state free precession cine MR and spin echo sequences provide clear images of the SVD, and gadolinium-enhanced 3D magnetic resonance angiography is ideally suited for imaging of the pulmonary veins [7]. The results of this study support the use of CMR for preoperative evaluation of patients with suspected SVD, in those with unexplained enlargement of right heart chambers, and when the findings on TTE are inconclusive or incomplete.
References
Alfakih K, Plein S, Thiele H, et al. (2003) Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 17:323–329
Beerbaum P, Korperich H, Esdorn H, et al. (2003) Atrial septal defects in pediatric patients: noninvasive sizing with cardiovascular MR imaging. Radiology 228:361–369
Edwards J (1953) Symposium on anomalous pulmonary venous connections (drainage): pathologic and developmental considerations in anomalous pulmonary venous connection. Pro Staff Mtgs Mayo Clinic 28:441–452
Ferrari VA, Scott CH, Holland GA, Axel L, Sutton MS (2001) Ultrafast three dimensional contrast-enhanced magnetic resonance angiography and imaging in the diagnosis of partial anomalous pulmonary venous drainage. J Am Coll Cardiol 37:1120–1128
Freed MD, Nadas AS, Norwood WI, Castaneda AR (1984) Is routine preoperative cardiac catheterization necessary before repair of secundum and sinus venosus atrial septal defects. J Am Coll Cardiol 4:333–336
Gnanapragasam JP, Houston AB, Northridge DB, Jamieson MP, Pollock JC (1991) Transoesophageal echocardiographic assessment of primum, secundum and sinus venosus atrial septal defects. Int J Cardiol 31(2):167–174
Greil GF, Powell AJ, Gildein HP, Geva T (2002) Gadolinium-enhanced three-dimensional magnetic resonance angiography of pulmonary and systemic venous anomalies. J Am Coll Cardiol 39:335–341
Kronzon I, Tunick PA, Freedberg RS, et al. (1991) Transesophageal echocardiography is superior to transthoracic echocardiography in the diagnosis of sinus venosus atrial septal defect. J Am Coll Cardiol:17:537–542
Moller JH, Nakib A, Andersona RC, Edwards JE (1967) Congenital cardiac disease associated with polysplenia. A developmental complex of bilateral “left-sidedness” Circulation 36:789–799
Muhler EG, Engelhardt W, von Bernuth G (1992) Detection of sinus venosus atrial septal defect by two-dimensional echocardiography. Eur Heart J 13:453–456
Pascoe RD, Oh JK, Warnes CA, et al. (1996) Diagnosis of sinus venosus atrial septal defect with transesophageal echocardiography. Circulation 94:1049–1055
Van Praagh S, Carrera ME, Sanders SP, Mayers JE, Van Praagh R (1994) Sinus venosus defects: unroofing of the right pulmonary veins—anatomic and echocardiographic findings and surgical treatment. Am Heart J 128:365–379
Acknowledgment
Dr. Valente was supported by a grant from the Children’s Miracle Network.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valente, A.M., Sena, L., Powell, A.J. et al. Cardiac Magnetic Resonance Imaging Evaluation of Sinus Venosus Defects. Pediatr Cardiol 28, 51–56 (2007). https://doi.org/10.1007/s00246-006-1477-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-006-1477-y