Abstract
Early results of the arterial switch operation (ASO) for transposition of the great arteries (TGA) are good, but there are few mid- and long-term data on postoperative arrhythmias, especially in Japan. In this study, clinical data on 624 1-year survivors who had an ASO between 1976 and 1995 were collected from six institutes in Japan up to October 2002. Sixty (9.6%) 1-year survivors had significant arrhythmias. Bradycardia occurred in 22 patients, including complete atrioventricular block (CAVB) in 12, sick sinus syndrome (SSS) in 6, and second-degree atrioventricular block in 4. Syncope developed in 2 with CAVB and 2 with SSS. Ten patients with bradycardia underwent permanent pacemaker implantation. Supraveutricular tachycardia (SVT) was seen in 25 patients, including paroxysmal supraventricular tachycardia in 16, atrial flutter in 7, and atrial fibrillation in 2. Six patients with SVT received antiarrhythmic medication. SVT was transient in 20 and persistent in 5. Ventricular arrhythmias occurred in 13 patients, including nonsustained ventricular tachycardia in 5, paroxysmal ventricular contractions with couplets in 5, ventricular flutter in 2, and sustained ventricular tachycardia in 1. Four patients with ventricular arrhythmias received antiarrhythmic medication. Of the study patients, 8 died 1 year or more after ASO. Death was directly related to arrhythmia in 1 patient and was due to nonsustained ventricular tachycardia with severe congestive heart failure. The presence of a ventricular septal defect (VSD) was a risk factor for postoperative arrhythmia. Patients with TGA and VSD had more arrhythmias than those with TGA and an intact ventricular septum (13.7 vs 8.7%, p < 0.05), and this was especially true for CAVB (3.9% vs 1.0%, p < 0.05). In 36 patients clearly documented time onset of postoperative arrhythmia arrhythmia developed in 18 (50%) after less than 1 year and in 15 (42%) after more than 5 years. In summary serious arrhythmias after ASO were uncommon, but postoperative arrhythmias, such as unpaced CAVB, SSS, and VT, were related to morbidity and mortality. VSD was a risk factor for postoperative arrhythmia, especially CAVB. Approximately half of the arrhythmias developed late. Lifelong monitoring with respect to arrhythmia is needed for patients after ASO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mustard or Senning procedures for transposition of the great arteries (TGA) are associated with a high incidence of cardiac rhythm abnormalities [3, 10]. The arterial switch operation (ASO), first reported by Jatene in 1975, has become the procedure of choice for patients with TGA and Taussig–Bing anomaly [7], and it has a low incidence of arrhythmias, at least in the short-term.
However, there are few reports that focus on postoperative arrhythmias during mid- and long-term follow-up. As follow-up lengthens, any advantage of anatomic correction with respect to arrhythmias should be confirmed.
To investigate the prevalence and risk factors of postoperative arrhythmias in mid- and long-term survivors after ASO, we organized a multicenter study group and collected data on patients who had survived at least 1 year after ASO from the following six centers in Japan: The Heart Institute of Japan, Tokyo Women’s Medical University; Fukuoka Children’s Hospital; National Cardiovascular Center; Shizuoka Children’s Hospital; Osaka University Hospital; and Chiba Cardiovascular Center.
Materials and Methods
Our study group included the six institutes listed previously, which were recruited to study the mid- and long-term impact of ASO on arrhythmias. A national funding organization provided financial support. We collected data on patients who underwent the operation before December 1995 and survived more than 1 year, as of October 2002. We constructed a data file on an electronic disk and distributed it to participating investigators for completion. Any arrhythmias were registered according to their first occurrence on either a standard electrocardiogram or a Holter recording. In cases with two or more arrhythmias, the most serious arrhythmia was registered as the final diagnosis.
We recorded the preoperative and postoperative characteristics, which were possibly related to the arrhythmias, Preoperative data included TGA type, coronary artery pattern, age at operation, previous balloon atrial septostomy, and pulmonary artery banding. Postoperative data included the presence of a high right ventricular pressure and aortic valve regurgitation, which were estimated by Doppler echocardiography.
JMP 5.01 software (SAS Institute, Cary NC, USA) was used for data analysis. Univariate analyses of continuous variables were performed with the Student t-test. Univariate comparisons for categorical variables were performed with the two-tailed chi-square test. Every univariate parameter that reached significance (p < 0.05) was tested in a multivariate logistic regression model.
Results
Population
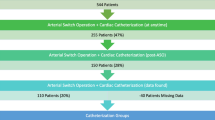
A total of 721 patients with TGA or TGA-like defects, such as Taussig–Bing anomaly, underwent an ASO before December 1995, with a 1-year actuarial survival of 88% (Fig. 1). A total of 624 patients fulfilled the criteria for our study. Preoperative characteristics of 1-year survivors are shown in Table 1. Age at operation was 0.7 ± 1.5 years (range, 0 days to 17.3 years; median, 2 months). Follow-up period was 9.9 ± 3.8 years (range, 1.1–24.8; median, 9.8). TGA with an intact ventricular septum (IVS) was present in 391 patients (62.7%), and the other 233 (37.3%) had a coexisting ventricular septal defect (VSD), including Taussig–Bing and other variants.
Late Deaths
Eight patients died 1 year or more after surgical repair. Causes of death are shown in Table 2. The age at death averaged 6.7 years (range, 1.2–8.3) and the follow-up duration averaged 4.5 years (range, 1.0–12.1). Cause of sudden death in 2 patients was unclear but was possibly due to arrhythmia or myocardial infarction. Only one death, in a patient with nonsustained ventricular tachycardia (VT) with severe congestive heart, was directly due to a postoperative arrhythmia. At catheterization, this patient had a left ventricular end diastolic volume that was 286% of normal and an ejection fraction of 30%. The patient had been treated with mexiletine, digoxin, angiotensin converting enzyme inhibitor, and diuretics but died 12 years after ASO.
Prevalence of Arrhythmias
Sixty patients (9.6%) had clinically significant postoperative arrhythmias. Their types of arrhythmias and clinical courses are shown in Fig. 2. Twenty-two patients had bradycardias, including sick sinus syndrome (SSS), second-degree atrioventricular block (2AVB), and complete atrioventricular block (CAVB). Two patients with SSS had syncope and underwent a permanent pacemaker implantation (PMI). Of the CAVB patients, 2 had syncope and 8 underwent PMI.
Arrhythmia types and follow-up. Paroxysmal supraventricular tachycardia (PSVT) was the most common but complete Atrioventricular Block CAVB) was the most persistent and serious. SSS sick sinus syndrome 2AVB, second-degree atrioventricular block; AFL, atrial flutter AF, atrial fibrillation PVCc, paroxysmal ventricular contraction with couplets; nsVT, nonrsustained ventricular tachycardia sVT, sustained ventricular tachycardia VF ventricular fibrillation
Twenty-seven patients had supraventricular tachycardias, including paroxysmal supraventricular tachycardia (PSVT), atrial flutter, and atrial fibrillation. PSVT was present in 16, making it the most frequent postoperative arrhythmia. Six patients were receiving medication, such as digoxin, verapamil, mexiletine, rismodan, and propranolol. Supraventricular tachycardia was transient in 20 and persistent in 5 patients.
Thirteen patients had ventricular arrhythmias, including ventricular tachycardia (VT), ventricular fibrillation, and paroxysmal ventricular contractions in couplets. Two, with persistent nonsustained VT, were treated with mexiletine. Sustained VT occurred persistently in 1 patient and was treated with verapamil. Ventricular fibrillation was present in 2 patients one received mexiletine and both had good control. None of the patients with paroxysmal ventricular contractions with couplets received antiarrhythmic medication.
Time of Onset of Postoperative Arrhythmias
Time of onset of postoperative arrhythmias is shown in Table 3. Eighteen patients (50%) developed arrhythmias within 1 year of ASO, and in 15 patients (42%) arrhythmias developed after more than 5 years. All CAVB and atrial flutter occurred within 1 year of the operation. Most of accelerated arrhythmias, such as PSVT, were late developments. The deceased patient with nonsustained VT was included in this group.
Risk Factors for Arrhythmias
Risk factors for postoperative arrhythmias of the 1-year survivors are shown in Table 4. By univariate analysis, arrhythmias were more frequent in patients with a coexisting VSD (TGA/VSD) than in those with no VSD (TGA/IVS) (p < 0.05), and the patients with arrhythmias had ASO at an older age (p < 0.00l). Multivariate analysis confirmed a significant influence of an older operation age (p < 0.05). There was no relation between the arrhythmias and other characteristics, such as coronary artery pattern, previous balloon atrial septostomy, pulmonary artery banding, postoperative pulmonary artery stenosis, and aortic valve regurgitation.
Discussion
We determined the prevalence of clinically important arrhythmias in 1-year survivors mid- and long term after ASO in Japan. The majority of patients had preserved sinus node function, whereas arrhythmias were present in 60 (9.6%). Our results agree with those reported by Vetter and Tanner [14], and the prognosis was almost as good [6, 9]. However, the prevalence of postoperative arrhythmia mid- and long-term was greater than at short-term. Approximately half of the arrhythmias in the SSS group and some types of supraventricular and ventricular arrhythmias developed more than 5 years after ASO. Not all these arrhythmias were symptomatic, but some could potentially cause catastrophic complications, such as syncope, stroke, or sudden death. In adults after ASD repair, for example, stroke is an important cause of morbidity and late death [8]. The evaluation of patients after ASO may be more complicated, and we should carefully investigate long-term survivors with respect to arrhythmias.
In our study, arrhythmia-based symptoms were recognized in five patients—syncope in four and death in one. All episodes of syncope were caused by CAVB or SSS, and many occurred early and persisted. Once CAVB and SSS, are confirmed not to be transient, they should be treated urgently by permanent pacemaker implantation according to the ACC/AHA guidelines [5]. In one patient, the cause of death was thought to be nonsustained VT with severe congestive heart failure. VT is considered one of the most likely causes of sudden death in patients with repaired CHD [13]. In several clinical trials of implantable cardioverter defibrillators (ICDs), patients with ejection fractions of 35% or less had a significant reduction in mortality with an ICD [1, 2, 11]. Any patient with documented VT and severe congestive heart failure should be considered to be at risk of sudden death and, if possible, treated not only by medicines but also by ICD implantation.
We also determined the risk factors for postoperative arrhythmias, which included a significantly older age at operation and the presence of a VSD. For TGA/VSD, the operation age was older and the arrhythmias, especially CAVB, were more frequent (Table 5). This is probably because the VSD plays a role in keeping the left ventricular pressure level [4], whereas the VSD and coexisting complexities, such as aortic arch anomalies, make the ASO more difficult [6, 9].
Rhodes et al. [12] compared the electrocardiographic rhythm in patients with TGA/IVS and TGA/VSD after ASO; no patients with TGA/IVS had either second atrioventricular block or CAVB during follow-up. In contrast, we observed CAVB in four patients with TGA/IVS; two had temporary CAVB and the other two had persistent CAVB accompanied by SSS. Probably, this is because not only atrioventricular conduction but also sinus node function was impaired through damage to the coronary blood supply, although we did not find any particular coronary pattern that was related to the arrhythmia. In the future, we should collect more detailed data on such patients, for example, by scintigraphy, contrast computed tomography, electrophysiologic studies, or coronary angiography.
The multicenter nature of this follow-up study is its main limitation, and there was some variation in data collection among the participating centers. Quantification of aortic valve incompetence and pulmonary artery stenosis, for example, is extremely observer dependent. Furthermore, the study is retrospective and therefore analysis of the results was rather simple. We could not collect complete data on the time of onset of postoperative arrhythmias. More detailed characteristics, such as catheterization and electrophysiologic studies, are not included, and timing of the Holter monitoring was variable. Nevertheless, this study includes a large number of patients and we believe it represents the overall prevalence and clinical significance of arrhythmias in patients mid-and long term after ASO.
Conclusion
The prevalence of serious arrhythmias after ASO was low, but un-controlled CAVB, SSS and VT carried a risk of morbidity and mortality. About half of the arrhythmias first occurred at mid and long-term and lifelong monitoring with respect to arrhythmias is essential for all patients after ASO.
References
A1-Khatib SM, Sanders GD, Mark DB, et al. (2005) Expert panel participating in a Duke Clinical Research Institute-sponsored conference. Implantable cardioverter defibrillators and cardiac resynchronization therapy in patients with left ventricular dysfunction: randomized trial evidence through 2004. Am Heart J 149:1020–1034
Bardy GH, Lee KL, Mark DB, et al. (2005) Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) investigators, amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352:225–2373
Beerman LB, Neches WH, Fricker FJ, et al. (1983) Arrhythmias in transposition of the great arteries after the Mustard operation. Am J Cardiol 51:1530–1535
Duncan BW, Poirier NC, Mee RB, et al. (2004) Selective timing for the arterial switch operation. Ann Thorac Surg 77:1691–1696
Gregoratos G, Cheitlin MD, Conill A, et al. (1998) ACC/AHA guidelines for implantation of cardiac pacemakers and arrhythmia devices; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Pacemaker Implantation). J Am J Coll Cardiol 3:1175–1209
Haas F, Wottke M, Poppert H, Meisner H (1999) Long-term survival and functional follow-up in patients after the arterial switch operation. Ann Thorac Sur 68:1692–1697
Jatene AD, Fontes VF, Paulista PP, et al. (1975) Successful anatomic correction of transposition of the great vessels: a preliminary report. Arq Bras Cardiol 28:461–464
Konstantinides S, Geibel A, Olschewski M, et al. (1995) A comparison of surgical and medical therapy for atrial septal defects in adults. N Engl J Med 333:469–473
Losay J, Touchot A, Serraf A, et al. (2001) Late outcome after arterial switch operation for transposition of the great arteries. Circulation 104:121–126
Martin RP, Radley-Smith R, Yacoub MH (1987) Arrhythmia before and after anatomic correction of transposition of the great arteries. J Am Coll Cardiol 10:200–204
Moss AJ, Zareba W, Hall WJ, et al. (2002) Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346:877–883
Rhodes LA, Wernovsky G, Keane JF, et al. (1955) Arrhythmias and intracardiac conduction after the arterial switch operation. J Thorac Cardiovasc Surg 109:303–310
Silka MJ, Hardy BG, Menashe VD, Morris CD (1998) A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol 32:245–251
Vetter VL, Tanner CS (1988) Electrophysiologic consequences of the arterial switch repair of d-transposition of the great arteries. J Am Coll Cardiol 12:229–237
Acknowledgments
This work was supported by a research grant for cardiovascular diseases (12C-11 Chair: Makoto Nakasawa MD) from the Ministry of Health, Labor and Welfare. We thank Drs. Peter M. Olley and Setsuko Olley for assistance in preparing the manuscript. We also thank Dr. Seiji Yamaguchi, Professor of Pediatrics, Shimane University School of Medicine, for comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashi, G., Kurosaki, K., Echigo, S. et al. Prevalence of Arrhythmias and Their Risk Factors Mid- and Long-Term After the Arterial Switch Operation. Pediatr Cardiol 27, 689–694 (2006). https://doi.org/10.1007/s00246-005-1226-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-005-1226-7