Abstract
Our purpose was to investigate the effects of body size on the sizes of mitral (MV) and aortic valve (AV) areas by three-dimensional (3-D) and two-dimensional (2-D) echocardiography and to create the normal values for 3-D echocardiography. A total of 168 healthy subjects aged 2–27 years were studied by digitized 3-DE, 2-DE, and Doppler echocardiography.
3-D echocardiography was performed by using rotational acquisition of planes at 18° intervals from a parasternal view with electrocardiogram gating and without respiratory gating. The annular levels of MV and AV were identified from short-axis cut planes and their areas were measured by planimetry. The diameters of mitral annulus, left ventricular outflow tract (LVOT), and aortic annulus were measured by 2-DE from the apical and parasternal long-axis views. Flow indices were measured by Doppler from MV inflow and the flow in LVOT and in the ascending aorta. Both MV and AV annular areas increased linearly in relation to body size. In the total study group the estimated areas for MV were 5.2 ± 0.9 cm2/m2by 3-DE, 3.7 ± 0.5 cm2/m2 by 2-DE, and 2.0 ± 0.4 cm2/m2 by continuity equation. The respective values for AV were 2.7 ± 0.5, 2.1 ± 0.3, and 1.8 ± 0.4 cm2/m2. MV velocity time integral (VTI)/ascending aorta VTI increased from 0.80 (0.26) to 0.95 (0.23) with increased body surface area (BSA), whereas MV VTI / LVOT VTI was 1.2 (0.2) in all BSA groups. MV and AV annulus areas increase linearly in relation to body size. 3-DE gives greater estimates for the areas than 2-DE and Doppler equation methods. The data obtained from 168 healthy subjects may serve as a reference for clinical use in patients with various cardiac abnormalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Knowledge of normal dimensions of aortic (AV) and mitral valve (MV) areas is important in the management of children with congenital heart disease. By means of two-dimensional (2D) and Doppler echocardiography, valve areas can be measured by planimetry or estimated indirectly by the continuity equation. Planimetry has not been widely used with children because of difficulties in measuring small orifices of congenitally malformed valves. The pressure half-time method is also used for calculation of stenotic mitral valve areas in adults, but the method is affected by the size of the subject and by changing hemodynamic conditions [3]. 2DE and Doppler echocardiography have been shown to provide a reasonable estimate of mitral and aortic annulus diameter in adults [12, 28]. In children, measurements must be standardized according to normal growth. Data on aortic [8, 25, 27] and mitral [18] valve annulus based on 2DE and aortic valve area measurements based on Doppler echocardiography [6, 13] have been reported for children.
Three-dimensional echocardiography (3DE) is a new noninvasive imaging technique that has been shown to be accurate in determining cardiac volume and mass [24]. Evaluation of valvular heart disease can also be improved by optimal cross-sectional planes obtained by 3DE [17, 24]. Studies show that 3DE is feasible in the assessment of mitral stenosis [2, 4, 14, 30]. Visualization of the valve leaflets by 3DE allows accurate localization and identification of pathology, facilitating the preoperative planning of MV repair [9, 19]. The feasibility of 3DE in reconstruction of the aortic valve and determination of stenotic AV area have also been shown [11, 16, 17, 20]. To our knowledge, there are no published data on aortic and mitral valve areas in healthy subjects in relation to body size assessed by 3DE.
Methods
The study was carried out at Kuopio University Hospital, Finland. Written informed consent was obtained from the parents or subjects. The study was approved by the ethics committee of the hospital.
Study Population
A total of 168 healthy children and young adults (85 males and 83 females) aged 2–27 years (mean, 11.1 ± 5.1; median, 10.5) were enrolled in the study. Weight and height of all subjects were recorded and body surface area (BSA) was calculated [7]. No subject had heart disease, as judged by medical history, clinical examination, and echocardiography. For the analysis, the subjects were divided into five groups according to BSA: 0.5–0.75, 0.75–1.0, 1.0–1.25, 1.25–1.5, and more than 1.5 m2. The clinical details of the subjects are shown in Table 1.
Echocardiographic Examination
Transthoracic echocaiographic examination was performed with the patient lying supine or in the left lateral semirecumbent position. Sedation was not used. Examinations were carried out by a single observer using the GE Vingmed System FiVe ultrasound scanner (Horten, Norway) and saved in digital form on the hard disk of the ultrasound scanner. The transducer frequencies used were 2.2, 3.5, or 5 MHz. Standard parasternal, apical, and subcostal views were used. 3DE was performed by using rotational image acquisition from a parasternal long-axis view, with electrocardiograph gating and without respiratory gating. The subjects were breathing normally during the scanning of 3D cineloops. The normal transducer was fixed to a lightweight, computer-controlled motored carriage that was easy to hold manually. An optimal acoustic window was first selected by 2D scanning. Pressing a button in the motored carriage initiated 3D data acquisition. The transducer rotated in a semicircle of 180° around the central axis of the imaging plane resulting in a conical data set. Ten complete cardiac cycles were collected during the rotation. Scanning of a plane took place if the pulse rate was within ± 20 bpm of the average. Thus, automatic selection of scanning times by RR intervals allowed adequate registration of the cardiac cross sections. Three-dimensional cineloops (10 views; median, 54 frames/heart cycle; range, 25–86) were digitally stored and transferred to a detached computer for analysis. Together with the first loading of the raw data set, a postprocessing was performed by the computer in which it converted the recorded 2DE sequences into a volumetric data set. The 3D echocardiographic method used has been described in detail [21, 22].
Echocardiographic Analysis
The echocardiographic data were analyzed by a single observer (TP). In addition, 30 patients were examined by another observer (TT) for the purpose of interobserver analysis.
3 DE
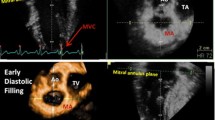
The postprocessed data sets were used for displaying the desired cross section of mitral and aortic valves (anyplane mode). Planimetry was performed in optimal short-axis views of mitral and aortic valves at the level of valve annulus (Fig. 1). The mitral annular area was measured in early diastole and the aortic annular area in early systole, when the valves were opened maximally. The means of three planimetered areas were calculated.
2 DE
The diameter of the mitral valve annulus was measured from the apical four-chamber view. The diameters of the left ventricular outflow tract (LVOT) and aortic valve annulus were measured from the parasternal long-axis view. LVOT diameter was obtained perpendicularly to the long axis of the outflow tract, approximately 0.5 cm below the aortic annulus. Valve and LVOT areas were calculated assuming circular annular geometry (π (d/2)2).
Doppler Echocardiography
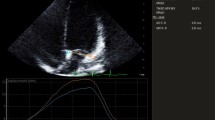
Transmitral flow velocity patterns were recorded from the apical four-chamber view, with small sample volume being positioned between the tips of the mitral valve leaflets. LVOT flow velocity was recorded from the apical five-chamber view. The sample volume was placed in the outflow tract at the same level where the LVOT diameter was measured. Ascending aortic flow velocity was obtained from the suprasternal notch. Measurements obtained on three consecutive heart cycles were averaged. MV and AV areas were calculated using the continuity equation (MV area = LVOT velocity time interval (VTI) × LVOT area (2DE) / MV VTI; AV area = LVOT VTI × LVOT area (2DE)/ ascending aorta VTI) [29].
Statistical Analysis
Interobserver and intraobserver variability of 3DE was determined. Bland–Altman analysis was used to identify possible bias (mean difference between the two methods) and the limits of agreement (2SD of the difference). Interobserver analysis was calculated between the analysis made by observer 1 and that by observer 2. Intraobserver analysis was calculated between analyses from two examinations made by observer 1. Differences between means were assessed with Student’s paired or unpaired t-test or one-way analysis of variance. In case of nonnormality, the nonparametric Mann–Whitney/Wilcoxon test was used. Valve area estimates obtained by different methods were compared by Pearson’s correlation coefficient. The data are given as mean (SD).
Results
All 3DE examinations were carried out successfully, and manual tracing of images was possible. The acquisition time for a 3DE data set was 10–15 seconds, depending on the frame rate used and the heart rate. Image transfer to the detached computer took 2 or 3 minutes. The time required for image analysis ranged between 5 and 10 minutes.
The clinical details of the subjects are presented in Table 1. All subjects were in good clinical condition with normal exercise tolerance. Seven children had asthma without symptoms at the time of examination. Three of the children with asthma had medication with inhaled corticosteroids.
Reproducibility of MV and AV Measurements
Interobserver Variability
The bias ± 2SD between the two observers for MV and AV area estimates by 3DE was −0.44 ± 2.59 and −0.27 ± 1.10 cm2, respectively. Correlation coefficients for 3DE MV and AV estimates between two observers were 0.63 and 0.75 (p < 0.001), respectively. The bias ± 2SD for 2DE MV and AV area measurements was −0.14 ± 1.41 and −0.13 ± 0.39 cm2, respectively, and the respective correlations were r = 0.82 and 0.87 (p < 0.001).
Intraobserver Variability
The bias ± 2 SD for 3DE MV area was 0.31 ± 1.56 cm2, and that for 3DE AV area was 0.09 ± 0.64 cm2 with the respective correlations being 0.79 and 0.89 (p < 0.001). The agreement analysis between MV and AV measurements by 2DE resulted in a bias of 0.21 ± 1.05 and 0.06 ± 0.50 cm2, respectively, and the respective correlations were r = 0.82 and 0.87 (p < 0.001).
2 DE and 3 DE Echocardiography
Mitral Valve Measurements
The mean MV area for the whole study group was 5.2 ± 0.9 cm2/m2 by 3DE and 3.7 ± 0.5 cm2/m2 by 2DE (Table 2). MV area measurements by 3DE and 2DE correlated well (r = 0.82, p < 0.001), but the values measured with 3DE were consistently larger than those obtained by 2DE. Figure 2 presents MV areas measured with 3DE and 2DE in relation to BSA. Correlation coefficients of MV area estimates with BSA and height were 0.85 and 0.82 for 3DE and 0.91 and 0.87 for 2DE (p < 0.001 for all). Table 3 shows the regression equations for MV estimates calculated in relation to BSA. No significant differences between males and females were found in MV area measurements by 3DE and 2DE. The agreement analysis between 3DE and 2DE for MV area estimates resulted in a bias of 1.84 ± 2.52 cm2 (p < 0.001) (Fig. 3).
Aortic Valve Measurements
The mean AV area for the study group was 2.7 ± 0.5 cm2/m2 by 3DE and 2.1 ± 0.3 cm2/m2 by 2DE (Table 2). A good correlation was found between 3DE and 2DE estimates of AV area (r = 0.88, p < 0.001). Figure 2 presents AV areas measured with 3DE and 2DE in relation to BSA. Both 3DE and 2DE AV measurements correlated closely with BSA and height (r = 0.85 and 0.84, r = 0.92 and 0.90, respectively; p < 0.001). Regression equations for AV areas and BSA are shown in table 3. 3DE and 2DE AV area estimates/BSA tended to be greater in males than in females, but statistical significance was reached only in the 1.0–1.25 and >1.5 m2 BSA groups (p < 0.05). In agreement analysis between 3DE and 2DE, the bias was 0.74 ± 1.93 cm2 (p < 0.001) (Fig. 3).
LVOT Measurements
2DE estimates for LVOT area are shown in Table 2. There were significant correlations between LVOT and BSA and height (r = 0.89, r = 0.87, respectively; p < 0.001). LVOT area estimates/BSA tended to be greater in males than in females, and statistical significance was reached in the 0.75–1.5 m2 BSA groups (p < 0.05 for all).
Doppler Echocardiography
Mitral Valve Measurement
Continuity equation based on LVOT data resulted in the lowest estimates for MV area (2.0 ± 0.4 cm2/m2). In agreement analysis between 3DE and Doppler measurements, the bias and limits of agreement were 3.9 ± 3.0 cm2 (p < 0.001). However, MV area estimates by continuity equation correlated with 3DE and 2DE estimates (r = 0.79 and 0.80, respectively, p < 0.001). There were also significant correlations between MV area estimates by continuity equation and BSA and height (r = 0.81 and 0.77, respectively; p < 0.001). Regression equations for MV and AV areas calculated by continuity equation and BSA are shown in Table 3.
Aortic Valve Measurement
AV area estimates calculated by continuity equation were lower (1.8 ± 0.4 cm2/m2) than those measured by 3DE and 2DE, but the estimates correlated well (r = 0.76 and 0.83, respectively; p < 0.001). In agreement analysis between 3DE and Doppler measurements, the bias for AV estimates was 1.1 ± 1.4 cm2 (p < 0.001). There were significant correlations between AV area estimates by continuity equation and BSA and height (0.81 and 0.79, respectively; p < 0.001).
Table 4 summarizes the flow measurements from MV, LVOT, and ascending aorta (Ao asc). MV VTI/Ao asc VTI increased from 0.80 (0.26) to 0.95 (0.23) with increased BSA, whereas MV VTI/LVOT VTI was 1.2 (0.2) in all BSA groups. Both MV VTI and LVOT VTI increased with increasing BSA and with decreasing heart rate (r = 0.49 and −0.64, and 0.56 and −0.52, respectively; p < 0.001 for all).
Discussion
Current reference values for MV and AV size are based on 2DE and Doppler measurements. Both of these methods present valvular area according to mathematical estimates assuming circular geometry of the valve. However, the normal mitral valve apparatus has a consistent saddle-shaped configuration [5, 14, 18] and LVOT is frequently elliptical [1]. 3DE allows direct visualization of valves and measurement of the valve area at the region of interest (valve annulus, smallest orifice area). Thus, our hypothesis is that 3DE provides a more reliable estimation of the valvular areas than the older methods. This study presents reference data on mitral and aortic annular areas obtained with 3DE and 2DE in 168 healthy children and young adults. 3DE estimates were greater than estimates calculated from 2DE dimensions. Our results showed a linear increase with increasing body size in mitral and aortic valve dimensions.
Mitral Valve Measurements
The estimates of MV area obtained in this study were 3.7 ± 0.5 cm2/m2 by 2DE and 5.2 ± 0.9 cm2/m2 by 3DE. In a postmortem study of children, the mitral valve diameter was 2.3 cm/m2, which was greater than the 2DE diameter (2.0 cm/m2) in our study [26]. The area calculated from postmortem MV diameter using circular geometry was only slightly smaller than the 3DE estimate in our study [26]. In a previous study of 103 children (aged 1 day to 15 years), mitral annular lateral and anteroposterior diameters were estimated to be 3.2 and 2.4 cm/m2, suggesting an elliptical shape of the mitral valve [18]. If the mathematical formula of ellipse ((π × diameter1 × diameter2)/4) was used, the previously mentioned mitral valve diameters would have resulted in a valve annular area estimate of 6.0 cm2/m2. The mean MV area of 4.7 cm2/m2 was obtained by the 2DE method in healthy children (a diastolic cross-sectional valve area at the base of the left ventricle, near the tips of MV leaflets) [23]. Mitral annular area estimates of 4.9–5.4 cm2/m2 (the size changed during the cardiac cycle) were obtained by 3DE in healthy adults [14]. The previously reported dimensions of MV annulus in children have a logarithmic relationship to BSA [18]. In contrast, our results by 3DE, 2DE, and continuity equation show an obvious linear relationship of MV area to increasing BSA. The 3DE estimates of MV annulus in our study are in agreement with the 2DE values reported in children [18] and also with recently reported 3DE estimates of MV annulus in healthy adults [14]. The slightly smaller postmortem MV area measurements compared with 3DE may be due to rigor mortis, which is known to cause a shrinking effect, especially on atrioventricular valves [12, 26, 31]. An additional factor causing overestimation maybe distention of the annulus in the blood-filled beating heart compared to autopsy values [31]. However, comparing the results from earlier studies is difficult due to the various methods used and because measurements have been made at different levels of the mitral valve (annulus, orifice).
Aortic Valve Measurements
Our study supports the finding that AV area is lineally related to BSA [13]. Our AV area estimates by Doppler echocardiography are slightly greater than those previously reported in children or adults. AV areas assessed by continuity equation with Doppler echocardiography were 1.33 cm2/m2 in children and 1.57 cm2/m2 in adults [6, 13]. However, it has been demonstrated that LVOT is frequently elliptical and that calculating its area from diameter alone results in an underestimation of the cross-sectional area [1]. In the current study, the continuity equation seemed to underestimate aortic valve area when compared to 3DE or 2DE.
The estimates of AV area obtained in our study were 2.1 ± 0.3 cm2/m2 by 2DE and 2.7 ± 0.5 cm2/m2 by 3DE. In a postmortem study, the aortic valve diameter was 1.4 cm/m2, in agreement with our 2DE estimate (1.5 ± 0.3 cm/m2) and less than our 3DE estimate, as was seen in the results of the MV [26]. Also, aortic annular dimensions by 2DE reported in children are similar to 2DE estimates in this study [8, 25, 27].
Three-Dimensional Echocardiography in MV and AV Measurements
3DE is a new noninvasive imaging technique that has been shown to be accurate in calculating cardiac volumes and mass and that also has improved evaluation of valvular disease [24, 30]. 3DE overcomes the limitations of image plane positioning inherent in 2DE and it offers a more precise approach to measure MV and AV areas. However, only a few studies have reported MV orifice areas in patients assessed by transfhoracic 3DE [2] and transesophageal 3DE [4, 14]. Two of these studies found good correlations between MV orifice area estimates obtained by 3DE, 2DE, and the Doppler pressure half-time method in adults with mitral stenosis [2, 4]. One study reported mitral annular areas measured by 3DE in healthy adults [14].
According to the literature, one of the most likely practical applications for 3DE is for interventions in valvular disease [15]. Aortic valve orifice areas have been measured with transesophageal 3DE in patients with aortic stenosis [11, 16, 20]. Transesophageal 3DE was found to be an accurate technique to measure AV area when compared with transesophageal 2DE and invasive techniques [11, 20]. Similar estimates for AV orifice area were found with transesophageal 2DE and 3DE [11]. In our transthoracic study, 3DE estimates for AV area were slightly greater than 2DE estimates.
Study Limitations
Dynamic 3D reconstruction is limited by the quality of the original 2D echo cross-sectional images, which can be adversely affected by minimal patient movements, breathing, or cardiac arrhythmia. Respiratory gating was ignored in this study in order to shorten the image acquisition time so that examination of small children without sedation was possible. The harmful effect of filled lungs was avoided by using a left semirecumbent position and by finding an optimal acoustic parasternal window near the mitral and aortic valves. The quality of 3DE data sets collected was suitable for analysis even in young adults. Ten image planes were chosen also for the purpose of short image acquisition time. Increasing the number of image planes would probably have increased the accuracy of the measurements and would have been necessary for the reconstruction 3D images of the valves and precise examination of valve morphology.
Boundary tracing is a well-known source of error common in all imaging systems. Although valve annular area measurements with optimal image quality are simple, one possible source of error is overestimation of valve areas with angulation or parallel shift of image planes when using 3DE and especially when using 2DE [16]. Therefore, considerable experience and operator skills are necessary for correct application of 3DE methods. These sources of error are possible reasons for greater inter- and intraobserver variability in 3DE measurements compared to 2DE measurements in our study.
The timing of measurement may affect the estimates of annular size. Studies have reported cyclic variation in mitral annular shape and area [10, 14]. The dimension of the mitral annulus is maximal and most eccentric at the time of normal maximal atrial volume. At mitral opening, the verticular and longitudinal motions cause major changes in dimensions [10]. The mitral annular movement and changes in valve area were also seen while analyzing 3DE data. We made mitral annular measurements in early diastole, when the valve was maximally opened.
Despite these limitations, the presented data on mitral and aortic valves in this large group of healthy children and young adults provide a framework for further use of 3DE in children with various heart diseases. In the near future, real-time 3DE echocardiography will become part of routine echocardiographic examination and 3DE measurements will be available for clinical decision making. Introduction of a new imaging method requires reference values based on new methodology, different from those based on 2DE or Doppler measurements.
References
Baumgartner H, Kratzer H, Helmreich G, Kuehn P (1990) Determination of aortic valve area by Doppler echocardiography using the continuity equation: a critical evaluation. Cardiology 77:101–111
Binder TM, Rosenhek R, Porenta G, Maurer G, Baumgartner H (2000) Improved assessment of mitral valve stenosis by volumetric real-time three-dimensional echocardiography. J Am Coll Cardiol 36:1355–1361
Braverman AC, Thomas JD, Lee RT (1991) Doppler echocardiographic estimation of mitral valve area during changing hemodynamic conditions. Am J Cardiol 68:1485–1490
Chen Q, Nosir YF, Vletter WB, et al (1997) Accurate assessment of mitral valve area in patients with mitral stenosis by three-dimensional echocardiography. J Am Soc Echocardiogr 10:133–140
Cheng TO, Xie MX, Wang XF, Li ZA, Hu G (1997) Evaluation of mitral valve prolapse by four-dimensional echocardiography. Am Heart J 133:120–129
Davidson WR Jr, Pasquale MJ, Fanelli C (1991) A Doppler echocardiographic examination of the normal aortic valve and left ventricular outflow tract. Am J Cardiol 67:541–549
DuBois D, DuBois EF (1916) A formula to estimate approximate surface area if height and weight be known. Arch Intern Med 17:863–871
el Habbal M, Somerville J (1989) Size of the normal aortic root in normal subjects and in those with left ventricular outflow obstruction. Am J Cardiol 63:322–326
Fabricius AM, Walther T, Falk V, Mohr FW (2004) Three-dimensional echocardiography for planning of mitral valve surgery: current applicability? Ann Thorac Surg 78:575–578
Fyrenius A, Engvall J, Janerot-Sjoberg B (2001) Major and minor axes of the normal mitral annulus. J Heart Valve Dis 10:146–152
Ge S, Warner JG Jr, Abraham TP, et al (1998) Three-dimensional surface area of the aortic valve orifice by three-dimensional echocardiography: clinical validation of a novel index for assessment of aortic stenosis. Am Heart J 136:1042–1050
Gutgesell HP, Bricker JT, Colvin EV, Latson LA, Hawkins EP (1984) Atrioventricular valve anular diameter: two-dimensional echocardiographic–autopsy correlation. Am J Cardiol 53:1652–1655
Gutgesell HP, French M (1991) Echocardiographic determination of aortic and pulmonary valve areas in subjects with normal hearts. Am J Cardiol 68:773–776
Kaplan SR, Bashein G, Sheehan FH, et al (2000) Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am Heart J 139:378–387
Kasprzak JD (2002) Clinical application of 3-dimensional echocardiography. Przegl Lek 59:650–654
Kasprzak JD, Nosir YF, Dall’Agata A, et al (1998) Quantification of the aortic valve area in three-dimensional echocardiographic data sets: analysis of orifice overestimation resulting from suboptimal cut-plane selection. Am Heart J 135:995–1003
Kasprzak JD, Salustri A, Roelandt JR, Ten Gate FJ (1998) Three-dimensional echocardiography of the aortic valve: feasibility, clinical potential, and limitations. Echocardiography 15:127–138
King DH, Smith EO, Huhta JC, Gutgesell HP (1985) Mitral and tricuspid valve anular diameter in normal children determined by two-dimensional echocardiography. Am J Cardiol 55:787–789
Macnab A, Jenkins NP, Bridgewater BJ, et al (2004) Three-dimensional echocardiography is superior to multiplane transoesophageal echo in the assessment of regurgitant mitral valve morphology. Eur J Echocardiogr 5:212–222
Menzel T, Mohr-Kahaly S, Kolsch B, et al (1997) Quantitative assessment of aortic stenosis by three-dimensional echocardiography. J Am Soc Echocardiogr 10:215–223
Poutanen T, Ikonen A, Vainio P, Jokinen E, Tikanoja T (2000) Left atrial volume assessed by transthoracic three dimensional echocardiography and magnetic resonance imaging: dynamic changes during the heart cycle in children. Heart 83:537–542
Poutanen T, Ikonen A, Vainio P, Jokinen E, Tikanoja T (2001) Transthoracic three-dimensional echocardiography is as good as magnetic resonance imaging in measuring dynamic changes in left ventricular volume during the heart cycle in children. Eur J Echocardiogr 2:31–39
Riggs TW, Lapin GD, Paul MH, Muster AJ, Berry TE (1983) Measurement of mitral valve orifice area in infants and children by two-dimensional echocardiography. J Am Coll Cardiol 1:873–878
Roelandt JRTC, Yao J, Kasprzak JD (1998) Three-dimensional echocardiography. Curr Opin Cardiol 13:383–396
Roman MJ, Devereux RB, Kramer-Fox R, O’Loughlin J (1989) Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 64:507–512
Sairanen H, Louhimo I (1992) Dimensions of the heart and great vessels in normal children. A postmortem study of cardiac ventricles, valves and great vessels, Scand J Thorac Cardiovasc Surg 26:83–92
Sheil ML, Jenkins O, Sholler GF (1995) Echocardiographic assessment of aortic root dimensions in normal children based on measurement of a new ratio of aortic size independent of growth. Am J Cardiol 75:711–715
Skjaerpe T, Hegrenaes L, Hatle L (1985) Noninvasive estimation of valve area in patients with aortic stenosis by Doppler ultrasound and two-dimensional echocardiography. Circulation 72:810–818
Snider AR, Serwer GA, Ritter SB (1997) Echocardiography in Pediatric Heart Disease, 2nd edn. Mosby, St. Louis
Sutaria N, Northridge D, Masani N, Pandian N (2000) Three dimensional echocardiography for the assessment of mitral valve disease. Heart 84:II7–II10
Tei C, Pilgrim JP, Shah PM, Ormiston JA, Wong M (1982) The tricuspid valve annulus: study of size and motion in normal subjects and in patients with tricuspid regurgitation. Circulation 66:665–671
Acknowledgment
This study was supported by the Finnish Foundation for Cardiovascular Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poutanen, T., Tikanoja, T., Sairanen, H. et al. Normal Mitral and Aortic Valve Areas Assessed by Three- and Two-Dimensional Echocardiography in 168 Children and Young Adults. Pediatr Cardiol 27, 217–225 (2006). https://doi.org/10.1007/s00246-005-1056-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-005-1056-7