Abstract
Despite a surge in mercury (Hg) pollution from artisanal and small-scale gold mining (ASGM) in Zimbabwe’s drainage basins, little is known about Hg trophodynamics in the country’s major reservoirs. We analyzed fish tissues for total mercury (THg) and stable isotopes of nitrogen and carbon (δ15N and δ13C) to compare patterns of biomagnification between two trophic guilds from a protected reservoir (Chivero) and an ASGM-impacted reservoir (Mazowe) and assessed consequences for human and fish health. Mean dry weight THg concentrations were significantly higher for both piscivorous and herbivorous fishes from Mazowe reservoir compared to fishes from similar feeding guilds in Chivero. Trophic magnification slopes (TMS), inferred from linear regressions between log10[THg] and δ15N, revealed significant Hg biomagnification in Mazowe (TMS = 0.28; p < 0.05) and no evidence for Hg biomagnification in Chivero (TMS = − 0.005; p > 0.05). In Mazowe’s piscivorous fishes, 32% had wet weight THg concentrations that surpassed 0.2 µg/g ww, a threshold for susceptible human populations and biochemical and gene expression alterations in fish. In addition, 17% of Mazowe’s piscivorous fishes surpassed the UNEP THg toxicity threshold for human consumption (0.5 µg/g ww). To reduce exposure to Hg toxicity in humans, the maximum fish consumption for piscivorous species from Mazowe reservoir should not exceed 431 g/week for both adult male and female consumers. Our findings demonstrate the importance of creating freshwater-protected areas to prevent direct Hg contamination of aquatic ecosystems and the need for health agencies to provide fish consumption advisories to vulnerable communities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Globally, pollution of aquatic habitats has been identified as one of the major threats to freshwater biodiversity (Dudgeon et al. 2006; Arthington et al. 2016; Reid et al. 2018; Dudgeon 2019). In recent decades, the impact of metal pollution, particularly mercury (Hg), on aquatic ecosystems has received increased attention (Ikingura and Akagi 1996; Veiga et al. 2006; Driscoll et al. 2013; Zolnikov and Ramirez Ortiz 2018; Niane et al. 2019), largely due to its potential impact on human and ecosystem health. In freshwater ecosystems, Hg bioaccumulates in a range of aquatic organisms including plankton (Watras and Bloom 1992; Rumengan et al. 2003; Stewart et al. 2008), macroinvertebrates (Molina et al. 2010; Razavi et al. 2019), and fish (Razavi et al. 2014; Ouédraogo et al. 2015; Wang and Wang 2019). Biomagnification of Hg is observed from tropical to polar food webs (Lavoie et al. 2013).
Although Hg is often available from natural processes (e.g., volcanic emissions, soil and ocean degassing), anthropogenic activities such as coal combustion (Zhao et al. 2019; Wu et al. 2020), cement production (Won and Lee 2012; Li et al. 2019), and artisanal small-scale gold mining (ASGM; Gbogbo et al. 2017; Mason et al. 2019) have increased its deposition rates in both terrestrial and aquatic ecosystems. ASGM is practiced widely in many African countries where it provides a cheap option for supporting livelihoods in impoverished rural and urban communities. The United Nations Global Mercury Assessment Report (UNEP 2019a) estimated that ASGM contributed 80% of Hg emissions in Sub-Saharan Africa in 2015. ASGM employs rudimentary mining methods such as Hg amalgamation to extract gold from its ore. During the process, an amalgam (a mixture of gold and Hg) is heated at high temperatures to volatilize the Hg leaving purified gold. ASGM activities thus leave behind ecosystems that are highly contaminated by atmospheric Hg deposition (Gerson et al. 2022).
Mercury emissions from gold amalgamation and the disposal of sediment with Hg residues into water bodies often lead to persistent environmental and health impacts on fish assemblages and fish-consuming human communities. Fish from aquatic bodies impacted by ASGM operations have been shown to contain high levels of Hg in both afro-tropical (Hanna et al. 2015; Mason et al. 2019) and neotropical (Montaña et al. 2021; Salazar-Camacho et al. 2021) regions. There is sufficient evidence from both field and laboratory studies to demonstrate the negative effects of high Hg concentrations on fish physiology and behavioral traits (Crump and Trudeau 2009). For example, increased Hg exposure in fish has been linked with disruptions of male and female reproductive functions and a reduction in spawning success (Crump and Trudeau 2009). In addition, studies have shown evidence of reduced predator avoidance and an increase in predator vulnerability in fish exposed to high Hg concentrations under laboratory conditions (Webber and Haines 2003).
Piscivorous fishes, which are often at the apex of most aquatic food webs, have the potential to transfer bioaccumulated Hg to terrestrial food webs including humans and wildlife (Dolbec et al. 2001; Cristol et al. 2008). Mercury intoxication from contaminated sources such as fish is associated with a range of human neurological disorders such as cognitive, vision, somatosensory, and emotional deficits (Santos-Sacramento et al. 2021). In children, both prenatal and postnatal Hg exposure is linked with neurodevelopmental disorders, impaired psychomotor development, low mental indices, and language issues (Grandjean et al. 1999; Harada et al. 2001; Marques et al. 2007; Santos-Lima et al. 2020). Despite the documented neurotoxic effects of Hg on human and animal health (Crump and Trudeau 2009; Budnik and Casteleyn 2019; de Almeida et al. 2019; Santos-Sacramento et al. 2021) and the reported ecological catastrophes from increased Hg contamination in freshwater ecosystems elsewhere (Müller 2018), no studies have been done on Zimbabwe’s waterbodies to determine Hg trophodynamics in aquatic biota and the potential risk of Hg biomagnification on human health.
Protected areas in Zimbabwe cover ca. 50,000 km2, translating to ~ 13% of the country’s total land area (ZPWMA 2017). Most of Zimbabwe’s protected areas were primarily designed for conserving terrestrial biodiversity and are found in regions that receive a mean annual rainfall of < 650 mm (Manatsa et al. 2020). A few exceptions include Kariba, Chivero, Manyame, Kyle, Ngezi, Umfuli, and Sebakwe national and recreational parks that were designed around large reservoirs. However, even for these protected areas with large reservoirs, their design does not provide enough protection for headwater regions, and anthropogenic activities such as ASGM, industrial, and agricultural processes in these unprotected upstream regions often introduce pollutants that are carried to the protected portions of the aquatic systems.
In Zimbabwe, poor regulation of ASGM activities, combined with severe economic hardships have led to an increase in ASGM operations in the country’s major drainage basins (Spiegel 2009), especially those outside the country’s protected area landscape. Maponga and Ngorima (2003) estimated that Zimbabwe has 300,000 miners along 5000 km of its major river basins. Worldwide, ASGM is estimated to release 1,000 tonnes of Hg per year (Spiegel and Veiga 2010), while in Zimbabwe estimates range from 6 tonnes (Maponga and Ngorima 2003) to 21.8 tonnes (Green et al. 2019) per year.
The growing body of knowledge on Hg contamination in Zimbabwe has mainly focused on quantifying Hg in abiotic substrates (Van Straaten 2000; Green et al. 2019; Bose-O’Reilly et al. 2020), examining legislative provisions around ASGM (Maponga and Ngorima 2003; Spiegel 2009), education of local communities on Hg pollution (Metcalf and Veiga 2012), examining factors that drive Hg burden in humans (Mambrey et al. 2020), and violent human conflicts associated with ASGM (Mkodzongi 2020). To understand Hg trophodynamics in Zimbabwe’s aquatic ecosystems, we sampled fishes from a protected and an ASGM-impacted reservoir to: (1) assess Hg exposure between two fish trophic guilds from reservoirs in protected and ASGM-impacted areas; (2) investigate Hg biomagnification using stable isotopes of δ13C and δ15N as indicators of diet and trophic position, respectively; and (3) determine the extent to which current Hg concentrations in economically important fish species pose a health risk to human consumers. Aquatic bodies located inside protected areas receive greater security and surveillance from conservation agencies resulting in reduced direct inputs of Hg from anthropogenic activities such as ASGM. We, therefore, expected to find lower Hg concentrations and biomagnification rates in fishes from the protected reservoir.
Materials and Methods
Study Area
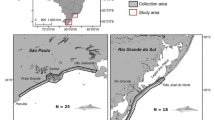
Lake Chivero (17.9033° S, 30.7982° E) and Mazowe Reservoir (17.5217° S, 30.9886° E), hereafter referred to as Chivero and Mazowe, are part of the Manyame and Mazowe catchments, respectively, in north-eastern Zimbabwe (Fig. 1). Chivero is a warm monomictic reservoir that holds up to 250 × 106 m3 of water at full capacity (Magadza 2003) and was constructed across the Manyame River in 1952 to supply potable water to the city of Harare and its surrounding satellite settlements. The reservoir lies at an altitude of 1368 m above sea level and is part of the Chivero Recreational Park, an IUCN category III protected area (ZPWMA 2017). Chivero’s catchment is mainly dominated by urban settlements and heavy industrial sites. Human population increase in Chivero’s catchment has led to significant changes in landcover patterns over the past three decades, particularly an increase in built-up land and a decrease in grassland/shrubland cover (Tendaupenyu et al. 2017).
The burgeoning human population has also put a strain on the existing wastewater treatment infrastructure, resulting in the discharge of partially treated industrial and domestic effluent into the reservoir (Nhapi et al. 2004). Increased nutrient loading from sewage and industrial effluent has led to the hypereutrophic status of Chivero (Mitchell and Marshall 1974; Nhapi et al. 2004) and ecological disasters including massive fish kills due to algal toxins and oxygen depletion from algal die-off (Mhlanga et al. 2006).
Mazowe was constructed across the Mazowe River in 1918 to irrigate citrus plantations and other annual crops in the region (Mitchell and Marshall 1974). The reservoir lies at an altitude of 1,212 m above sea level and holds up to 44.6 × 106 m3 of water at full capacity (Mujere and Mazvimavi 2012). Mazowe is located outside Zimbabwe’s IUCN protected area network and its catchment consists mainly of agricultural land, communal land, large nickel and gold mining operations, and natural vegetation. Part of the drainage also flows through the greenstone belt region that is associated with gold deposits (Masocha et al. 2019) and has attracted many artisanal small-scale gold miners to the region. Mixed agriculture and ASGM are the main sources of livelihood in the catchment (Masocha et al. 2019).
Sample Collection
Field sampling was conducted during the dry season (June and July 2019). A total of 58 fish specimens representing three species and two feeding guilds (piscivorous and herbivorous) were collected (Table S1). Oreochromis niloticus represented the herbivores in both reservoirs while Clarias gariepinus and Micropterus salmoides represented the piscivores. In Chivero, C. gariepinus were the only top predators present. Although C. gariepinus are usually omnivorous, gut analysis studies have shown that in some areas (e.g., Upper Zambezi and the Okavango basins), they tend to be piscivorous and consume a significant amount of fish from pack-hunting strategies (Merron 1993). In addition, other fish Hg toxicology studies in Botswana and Ethiopia have categorized them as ‘piscivorous’ (see Black et al. 2011; Desta et al. 2007). In Mazowe, piscivores were supplemented by M. salmoides because C. gariepinus was not abundant (n = 3), in our samples. Fish were caught from multiple habitats at each locality using electrofishing, rod and reel, and gill netting. Taxonomic identifications were confirmed using diagnostic character descriptions following Skelton (2001) and Marshall (2011).
Standard length (SL, mm) and wet weight (ww, g) measurements were taken on fresh whole fish at each collection site (Table S1). Fillet samples from the axial muscle tissue were dissected using a sterilized dissection board and scalpels. Fillet samples were then packaged in sterile whirl–pak bags, labelled with the species name, collection site, and date. All samples were stored on ice in the field and frozen within 24 h. Samples were then shipped to the United States and stored at − 20℃ prior to THg and stable isotope analyses. The protocol for sample collection (Protocol Number—190,304) was approved by the Institutional Animal Care and Use Committee (IACUC) at SUNY-ESF, while sample collection [(23 (1) (C) (II) 36/2019)] and export (VB/2/13/186) permits were issued by the Zimbabwe Parks and Wildlife Management Authority and the Zimbabwe Veterinary Permits Office, respectively.
THg Analysis and Quality Control
Total mercury (THg) analyses for all fish muscle tissues were done at the State University of New York College of Environmental Science and Forestry (Syracuse, USA). Fish samples were freeze-dried using a PTFE-coated Freezone benchtop freeze dryer (Labconco Corporation, USA). THg was determined as dry weight (dw), by atomic absorption spectrophotometry following guidelines in EPA Method 7473 (USEPA 1998) using a tri-cell DMA-80 automatic mercury analyser (Milestone Inc.). The precision of THg measurements was ascertained by making duplicate measurements on 38% of the fillet samples (n = 22). The average relative percent difference [RPD = (sample 1- sample 2)/average*100%] for duplicate samples was 3.4% (standard deviation, SD = ± 2.6%, range = 0.1–8.8%). Certified Reference Materials (CRM), TORT-3 (Lobster hepatopancreas, National Research Council of Canada), and DORM-4 (Fish protein, National Research Council of Canada) were run after every five samples to check instrument accuracy. Average CRM recovery rates were within the accepted 90–110% range i.e., TORT-3 (average = 102%, SD = ± 4.8%, range = 95–110%, n = 9) and DORM-4 (average = 93%, SD = ± 5.7%, range = 83–103%, n = 16). At the end of each batch run, matrix spikes/matrix spike duplicates were conducted, and the average percentage recovery rate was 94% (SD = ± 12.5%, range = 75–114%, n = 4).
Stable Isotope Analyses and Quality Control
To determine the vertical trophic structure and influence of energy sources on THg accumulation in fish from protected and non-protected reservoirs, carbon and nitrogen stable isotope composition (δ13C and δ15N, respectively) was measured. Samples of ground fish tissue weighing ~ 1 mg were loaded in ultrapure tin capsules and analyzed for stable isotopes using a Thermo Delta V isotope ratio mass spectrometer (IRMS), at Cornell University’s Stable Isotope Laboratory, (Ithaca, New York, USA). Quality control was monitored using four in-house standards that were routinely run to calibrate the instrument. Cayuga Brown Trout (CBT) and a ground corn standard (KCRN) were used for instrument normalization correction. Accuracy and precision were estimated with a deer standard (SD = ± 0.06 ‰ for δ15N and ± 0.03 ‰ for δ13C; n = 17). Duplicate samples (n = 9) representing 16% of the total specimens were run to aid quality control.
Finally, to check instrument linearity and its response to elemental composition measurement, a chemical standard (methionine; SD = ± 0.42 ‰ for δ15N and ± 0.29 ‰ for δ13C) was used. Isotope values were reported in parts per thousand (‰), based on international standards: atmospheric nitrogen (N2) and Vienna Pee Dee Belemnite (V-PDB) for δ15N and δ13C stable isotopes, respectively. Results were expressed in standard delta notation according to the formula in Eq. 1:
where X = 15N or 13C; Rsample = stable isotope ratio (15N/14N, 13C/12C) of the sample; and Rreference = isotope ratio (15N/14N, 13C/12C) of the reference.
Trophic Structure and Hg Trophodynamics
We used stable isotope data to evaluate trophic transfer (trophodynamics) and bioaccumulation of Hg in fish samples from two feeding guilds (herbivores and piscivores) in the two reservoirs. Trophic levels (TrL), of the piscivorous fishes in each reservoir were estimated using δ15N isotopic signatures of O. niloticus as the baseline organism. Oreochromis niloticus is a primary consumer that was present in both reservoirs. Previous research (Cabana and Rasmussen 1996) demonstrated that fractionation rates of stable isotopic signatures (δ15N and δ13C) in freshwater aquatic producers such as phytoplankton are influenced by spatial and temporal variability in plankton biomass and growth rate. As a result, the use of primary consumer δ15N isotopic signatures as baseline indicators (instead of primary producers) is accepted in aquatic trophic structuring studies (Vander Zanden and Rasmussen 1999; Ouédraogo et al. 2015; Razavi et al. 2014), as their large body size and greater longevity reduces the influence of external variables on stable isotope fractionation rates. Trophic levels of piscivorous fishes were estimated following the formula in Eq. 2:
where δ15Nsample and δ15Nbaseline represent mean δ15N for the sample and the baseline organisms respectively, 3.4 ‰ represents the δ15N isotopic enrichment per trophic level (Post 2002), and λ = 2, i.e., the trophic position of the baseline organism. Although there is evidence that δ15N fractionation rates vary based on diet and the primary form of nitrogen excretion (Vander Zanden and Rasmussen 2001; Vanderklift and Ponsard 2003), no specific nitrogen fractionation estimates have been developed for afrotropical freshwater fishes. Studies on biomagnification patterns in afrotropical freshwater fishes (Hanna et al. 2015; Ouédraogo et al. 2015) have also used the 3.4‰ fractionation rate.
Vertical trophic structure of fish samples from the two reservoirs was determined by constructing δ13C versus δ15N biplots. To account for ontogenic dietary shifts in fish samples that had wide specimen size ranges (O. niloticus and C. gariepinus), we first plotted [THg] ~ SL for each species to check for positive relationships between the two variables i.e., whether [THg] increased with fish length. We then plotted SL ~ δ15N to check for trophic positioning and ontogenic diet shifts between juvenile and adult fish for each species. It must be noted that, although we did the same analysis for M. salmoides, specimens for the latter species were generally large adults (SL range = 177 to 384 mm), Table S1.
Biomagnification of THg in both reservoirs was quantified by calculating the trophic magnification slope (TMS, Lavoie et al. 2013), a linear regression between log10[THg] ~ δ15N, (with log10[THg] as the dependent variable and δ15N as the predictor) for the two feeding guilds in each reservoir. The TMS estimates the average increase in THg concentration per unit δ15N. A TMS > 0 indicates significant biomagnification of THg across trophic levels, with higher TMS values representing greater THg biomagnification (Lavoie et al. 2013; Li et al. 2021). Linear regressions to obtain TMS values followed the formula in Eq. 3:
where THg represents the total mercury concentration, b represents the slope of the equation or the trophic magnification slope (TMS), and a is the intercept. In addition, trophic magnification factors (TMF), which represent the average factor change in THg concentration between two trophic levels (Ouédraogo et al. 2015), were calculated as the antilogarithm of b as shown in Eq. 4:
where b is the slope of the TMS regression (Eq. 2) and 3.4‰ is the trophic discrimination factor for δ15N (Post 2002).
Hg Exposure Risk to Fish and Human Health
To assess the risk of Hg exposure to human health, fish tissue dry weight concentrations were converted to wet weight concentrations using the formula in Eq. 5:
where THg(ww) and THg(dw) are Hg concentrations in wet and dry tissues, respectively, and percent sample moisture is the difference between sample wet weight and sample dry weight expressed as a percentage. As the use of theoretical conversion factors underestimates chemical contamination in fish muscle tissue (Cresson et al. 2017), we made individual calculations of the percentage moisture content considering the intra- and interspecific variation between sample wet and dry mass ratios. The average percentage moisture content for the three species was as follows: C. gariepinus – 77.1%, M. salmoides – 77.9%, and O. niloticus – 72.1%.
The United Nations Environment Program (UNEP), in partnership with the International Labour Organisation (ILO) and the World Health Organization (WHO), has determined an Hg consumption threshold value of (0.5 µg/g ww), above which Hg exposure can have harmful effects on human health (UNEP 2002; 1990). A separate THg threshold (0.2 µg/g ww) has been established for susceptible populations i.e., those that may experience severe adverse effects at comparable or lower exposure levels than the general population (WHO 2008). To assess the risk of Hg exposure to human health, we compared THg (ww) values for the two feeding guilds (piscivores and herbivores) and reservoirs (Chivero and Mazowe) to the two THg threshold values i.e., (0.2 µg/g ww and 0.5 µg/g ww).
In addition, we made estimates of the maximum amount of fish meat that can be consumed per week without adverse effects on human health based on the provisional tolerable weekly intake (PTWI). PTWI is the amount per unit body weight of a harmful substance that can be ingested over a lifetime without negative health effects (JEFCA 2007). For Hg, the PTWI has been set at 1.6 µg/kg of body weight to protect vulnerable populations from neurotoxic effects (JEFCA 2007). Mean THg (ww) concentrations and the mean weights of different fish species were used to estimate the maximum amount of fish meat in grams, that can be safely consumed on a weekly basis for both adult men and women. The maximum amount of fish meat (MAF, WHO 2008) was estimated using the formula in Eq. 6:
where BW = average body weight (65 kg and 70 kg for adult women and men respectively, Ferreira da Silva and de Oliveira Lima 2020), I = tolerable weekly intake of fish (µg/kg body weight), and C = metal concentration in fish (µg/g). Maximum amount of fish meat, MAF (g) for each fish species, and gender was converted to mean number of fish that could be safely consumed per week by dividing the MAF (g) with the mean weight (g) of each fish species in each reservoir.
To determine the exposure risk of observed THg concentrations on fish health, THg concentrations for individual fish in the two feeding guilds were compared to two reference values: 0.2 µg/g ww, which represents the upper Hg toxicity threshold for alteration of biochemical and gene expression processes, and 0.3 µg/g ww, which represents the upper Hg toxicity threshold for behavioral, reproductive, and histological changes in fish (Lepak et al. 2016).
Statistical Analyses
We explored the data for normality and homogeneity of variance using the Shapiro–Wilk and Levene’s tests, respectively. To account for size differences and their effect on Hg concentrations in conspecific species from the two reservoirs, we compared mean standard lengths of C. gariepinus and O. niloticus specimens using t-tests. On average, fishes from Mazowe were significantly larger (Welch’s t-test: p < 0.05) than those from Chivero for both C. gariepinus and O. niloticus (Figs. S1a and S2a). We standardized THg concentrations by regressing [THg] and standard length (SL). THg ~ SL was only significant for O. niloticus from Chivero, therefore, data were combined for the two reservoirs to create regression equations for size adjusting (Figs. S1b and S2b). Mean differences in [THg adj] (size adjusted for C. gariepinus and O. niloticus) were then compared across the two trophic guilds (herbivorous and piscivorous) and reservoirs (Chivero and Mazowe) using Welch’s two sample t-tests for unequal variances. We used a t-test to confirm that C. gariepinus and M. salmoides belonged to the same trophic guild (Fig. S3), and then combined unadjusted M. salmoides with adjusted C. gariepinus data to quantify [THg] in the piscivorous guild for Mazowe. A significance level of (α = 0.05) was used for all statistical tests. All calculations and graph illustrations were implemented in R statistical software, version 4.03 (R Core Team 2020) using the package ggplot2 (Wickham 2016), and OriginPro (version 2021b, Northampton, MA, USA).
Results
Fish THg Concentrations
Mean dry weight THg(adj) concentration in Mazowe ranged from 0.12 µg/g ± 0.05 SD in herbivorous species to 1.27 µg/g ± 0.94 SD in piscivorous species. In Chivero, mean dry weight THg(adj) concentration ranged from 0.09 µg/g ± 0.01 SD in herbivorous species to 0.15 µg/g ± 0.09 SD in piscivorous species (Table 1). Mean dry weight THg (adj) concentrations were significantly higher (Welch’s t-test: p < 0.05), in herbivorous species from Mazowe compared to those from Chivero (Fig. 2a). A similar pattern was evident in piscivorous species, with mean dry weight THg (adj) concentrations significantly higher (Welch t-test: p < 0.05) in fishes from Mazowe compared to those from Chivero (Fig. 2b). Summary statistics for THg (adj) concentrations and standard length for the two feeding guilds (piscivores and herbivores) and reservoirs (Mazowe and Chivero) are presented in Table 1.
Differences in mean dry weight adjusted total mercury concentrations (THg µg/g dw) in fishes from the two feeding guilds a herbivores and b piscivores from the two reservoirs. Black diamonds indicate the mean [THg] dw concentration and error bars represent 95% confidence interval for the mean. Different letters on boxplots indicate significant differences
Trophic Structure and Hg Trophodynamics
Biplots of δ13C versus δ15N for both reservoirs showed the range of carbon sources and trophic positions for fish species in the two trophic guilds. Piscivorous species had the highest δ15N isotopic ratios in both Mazowe (M. salmoides, max δ15N = 14.59 ‰) and Chivero (C. gariepinus, max δ15N = 20.08 ‰; Fig. 3). The common herbivorous species in both reservoirs (O. niloticus) had consistently low δ15N ratios in Chivero (δ15N range = 14.3–16.87 ‰) and Mazowe (δ15N range = 9.16–10.90 ‰) and occupied a lower trophic position in both reservoirs (Fig. 3). Although piscivorous species recorded the highest max δ15N ‰ in Chivero, their mean isotope ratios for both nitrogen (15.17 ‰ ± 4.44 SD) and carbon (− 19.56 ‰ ± 2.16 SD) were lower than those of herbivorous species for both isotopes, i.e., δ15N (15.74 ‰ ± 0.83 SD) and δ13C (− 17.78 ‰ ± 1.11 SD; Fig. 3).
Biplots δ13C versus δ15N in two trophic guilds from the two reservoirs. The ratio of δ15N indicates trophic position while δ13C indicates dietary carbon source. Unconsolidated trophic positions for all specimens in each trophic guild are shown in grey symbols for both piscivores and herbivores, while mean trophic positions (± SD for δ13C and δ15N) of the two trophic guilds are represented by XY error plots and black symbols
In both reservoirs, fish were supported by a variety of carbon sources as evidenced by the δ13C values that ranged from − 24.7 to − 16.2 ‰ in Chivero and − 28.4 to − 21.1‰ in Mazowe. Calculated trophic levels (TrL) revealed that the mean trophic level for piscivorous fishes in Mazowe (2.85) was significantly higher (Welch’s t-test, p < 0.05) than the mean trophic level (1.83) for piscivorous fishes in Chivero (Table 2). In Mazowe, the TMS slope from log10[THg] ~ δ15N regression (0.28) was greater than zero (Fig. S4a), confirming the occurrence of significant (p < 0.05) THg biomagnification. In Chivero, there was no evidence of THg biomagnification (TMS = − 0.0049; p < 0.05; Fig. S4b). In addition, the rate of change in THg concentration between trophic levels (i.e., TMF) was higher in Mazowe (8.95) compared to Chivero (1.08; Table 2), indicating that THg biomagnifies by a factor of ~ 9 between the two trophic levels in Mazowe and a factor of ~ 1 in Chivero. Plots between SL ~ δ15N did not reveal evidence of ontogenic diet shifts for piscivorous species in the two reservoirs, C. gariepinus (Fig. S5a) and M. salmoides (Fig. S5b). Although O. niloticus specimens from Chivero were significantly smaller in size than those from Mazowe (Fig. S2a), they had higher δ15N ratios compared to larger specimens from Mazowe (Fig. S5c).
Hg Exposure Risk to Fish and Human Health
Two piscivorous samples, i.e., M. salmoides, (0.73 µg/g ww) and C. gariepinus (0.58 µg/g ww), accounting for 9.1% of all fish samples from the Mazowe reservoir, had wet weight THg concentrations that exceeded the recommended UNEP threshold for human consumption (0.5 µg/g ww, Fig. 4). In addition, seven samples of Mazowe’s piscivorous fishes, accounting for 32% of all fishes from that reservoir, exceeded the WHO threshold concentration for susceptible populations (0.2 µg/g ww, Fig. 4), and the Hg toxicity threshold beyond which biochemical and gene expression are altered in fish (0.2 µg/g ww, Fig. 4). Three piscivorous samples from Mazowe were above the Hg toxicity limit for behavioral, reproductive, and histological alterations in fish (0.3 µg/g ww, Fig. 4).
Total wet weight mercury concentration [THg (µg/g) ww] in fish muscle tissue and the corresponding δ15N values for the two feeding guilds (piscivores and herbivores), compared to Hg threshold reference values (TRV) for human  and fish
and fish  health. TRV-1 = WHO Hg threshold in fish for susceptible human populations and, the threshold for biochemical and gene expression in fish; TRV-2 = threshold for behavioral, reproductive, and histological changes in fish; TRV-3 = UNEP threshold for Hg in fish destined for human consumption. Fish Hg threshold reference values follow Lepak et al. (2016)
health. TRV-1 = WHO Hg threshold in fish for susceptible human populations and, the threshold for biochemical and gene expression in fish; TRV-2 = threshold for behavioral, reproductive, and histological changes in fish; TRV-3 = UNEP threshold for Hg in fish destined for human consumption. Fish Hg threshold reference values follow Lepak et al. (2016)
In addition, a quantitative estimation of safe fish consumption rates that minimize risk to human health revealed that piscivorous fishes from Mazowe should be consumed in low quantities per week (Table 3). For both adult men and women, the weekly intake of C. gariepinus and M. salmoides from Mazowe should not exceed 330 g and 431 g, respectively. MAF estimates for the herbivorous species, O. niloticus, which was common in both reservoirs, were generally larger, i.e., 3,467 g and 3,733 g per week of fish meat from the two reservoirs for both adult men and women (Table 3).
Discussion
Fish THg Concentrations
Our study provides the first attempt to analyze Hg trophodynamics in Zimbabwe’s aquatic ecosystems and offers a quantitative examination of how anthropogenic activities, such as ASGM, influence THg concentration and biomagnification patterns in freshwater fishes. Studies on non-mined and relatively undisturbed afrotropical aquatic systems including wetlands (Black et al. 2011), natural lakes (Campbell et al. 2003, 2004; Machiwa 2004; Desta et al. 2007; Poste et al. 2012), and reservoirs (Agorku et al. 2009) have consistently reported low THg concentrations in afrotropical freshwater fishes. In contrast, there is evidence of high THg concentrations in fishes from ASGM impacted freshwater systems in both afrotropical (Taylor et al. 2005; Hanna et al. 2015; Mason et al. 2019) and neotropical (Ferreira da Silva and de Oliveira Lima 2020; Montaña et al. 2021) regions. Our findings corroborate these earlier studies by providing more evidence that, on average, fishes from ASGM impacted sites have higher THg concentrations, especially for species feeding at higher trophic levels. For example, the mean THg (ww) for C. gariepinus from four ASGM impacted ponds in the Rwamagasa artisanal gold mining area in Tanzania ranged from 0.28 µg/g to 1.67 µg/g (Taylor et al. 2005; Hanna et al. 2015), while the mean THg (ww) for O. niloticus in ASGM impacted regions of Cote d'Ivoire was 0.025 µg/g (Mason et al. 2019).
THg concentrations in both piscivorous and herbivorous fishes from Chivero were lower than expected. Although the reservoir is located inside a protected area, non-point pollution from upstream anthropogenic activities has led to nutrient loading (Mitchell and Marshall 1974; Magadza 2003) and the hypereutrophic status of the lake (Nhapi et al. 2004). There is evidence that high primary productivity in hypereutrophic systems can lower Hg bioaccumulation rates as the rapid growth rate of phytoplankton lowers Hg uptake, resulting in bloom dilution of Hg (Ouédraogo and Amyot 2013; Poste et al. 2015; Razavi et al. 2015). Bloom dilution lowers cellular Hg concentrations in producer communities leading to low Hg levels in consumer organisms such as fish. In addition, growth dilution could also be occurring at higher trophic levels in Chivero, i.e., in fish communities that make up the reservoir’s primary and secondary consumers. Fish growth rates in productive tropical lakes have been demonstrated to be consistently high, resulting in reduced Hg concentrations in fishes at higher trophic levels (Razavi et al. 2014; Poste et al. 2015). Thus, in addition to absence of direct Hg contamination from ASGM operations, phytoplankton bloom dilution and fish growth dilution in Chivero’s producer and consumer communities could be partly responsible for the observed low THg patterns in the reservoir’s fishes.
Recent studies on metal pollution in Chivero have reported heavy to extreme sediment contamination by heavy metals including Fe, Cd, Zn, Cr, Ni, and Cu (Utete et al. 2018). Although we did not find literature on sediment Hg contamination for Chivero, it is possible that the reservoir’s stratification regime might temporarily prevent Hg from contaminated sediments in the hypolimnion to be mixed with epilimnetic water where most fish are found. The influence of such a temporal disconnect between stratified layers on fish Hg contamination has been demonstrated in other eutrophic reservoirs (Liu et al. 2011). Chivero is a warm monomictic lake that is stratified throughout much of the summer season due to density differences between cold hypolimnetic and warm epilimnetic water. Our sampling was conducted in summer when the reservoir was most likely stratified. Future studies should therefore investigate the temporal/seasonal variations in fish THg concentrations to account for the variation introduced by limnological mixing cycles, as previous work has shown that fish THg concentrations show seasonal variability (Bastos et al. 2007; Kassegne et al. 2018). For example, in hypereutrophic Chivero, algal die-off has been reported to create hypoxia and fish kills (Mhlanga et al. 2006). During stratification, the decomposition of sinking organic matter from the epilimnion can further reduce hypolimnetic oxygen concentrations. The resulting hypoxic conditions favor increased methylation of Hg to MeHg (Matthews et al. 2013). This pool of hypolimnetic MeHg is then distributed into the entire water column during lake turnover, exposing the lake’s ichthyofauna to high concentrations of MeHg (Matthews et al. 2013).
Trophic Structure and Hg Trophodynamics
Both herbivorous and piscivorous fish from Chivero recorded higher δ13C and δ15N ratios compared to fish from similar feeding guilds in Mazowe (Fig. 3). In freshwater systems, carbon isotopic ratios have been routinely used to differentiate between pelagic and benthic carbon sources. Organisms that are dependent on benthic carbon sources typically have a greater enrichment of δ13C compared to those reliant on pelagic sources (Hecky and Hesslein 1995). It is possible that O.niloticus (the primary consumer) is feeding on different carbon substrates in the two lakes. In Chivero, the significantly smaller size of O. niloticus (Fig S2a), might imply a preference for detritus and benthic invertebrates, whereas the significantly larger specimens from Mazowe might be using pelagic phytoplankton as their primary food source. The high δ15N observed in fish from Chivero might be a direct influence of anthropogenic nitrogen inputs into the reservoir. Chivero is a hypereutrophic lake that has received a huge amount of nutrient pollution over the years (Magadza 2003, Nhapi 2004). At the time of sampling, we observed partially treated sewage flowing into the reservoir from one of its main tributaries, the Marimba River (Figs. S6a and b).
TMS analysis showed that there was significant Hg biomagnification in Mazowe and no evidence of the same in Chivero (Figs. S4a and b), and the same pattern was observed for TMF, which was larger by a factor of ~ 9 in Mazowe compared to Chivero (TMF = ~ 1; Table 2). Given the role played by ASGM activities through directly releasing Hg in the Mazowe catchment, the relatively high Hg biomagnification rates were expected. Recent studies of Hg biomagnification on aquatic ecosystems in other ASGM impacted sites in the neotropics (Azevedo-Silva et al. 2016, TMS range = 0.19–0.21; Montaña et al. 2021; TMS = 0.22), have produced similar Hg biomagnification patterns to those reported here.
The absence of Hg biomagnification in Chivero was unexpected. Previous studies on similar tropical hypereutrophic lakes in East Africa have reported low, but nonetheless positive TMS values (i.e., 0.08, 0.09 and 0.08 for lakes George, Mburo and Saka, respectively; Poste et al. 2015). Biodilution processes, i.e., bloom and growth dilution (see the first section of the Discussion), could be responsible for the absence of biomagnification in Chivero. In addition, trophic level analysis for fish species in both reservoirs revealed that both the mean and median trophic level for piscivorous fish in Chivero was significantly lower than that of Mazowe (Table 2). Previous work has shown that apex predatory fish in tropical systems with longer food chains accumulate more toxicants than those found in systems with shorter food chains (Razavi et al. 2014; Ouédraogo et al. 2015). Thus, the lower trophic level position of piscivorous fish in Chivero might be indicative of a shorter food chain, and the latter is associated with low Hg biomagnification.
Variation of dietary preferences between piscivores from the two reservoirs could also be responsible for the different biomagnification patterns. Mazowe’s piscivores were dominated by M. salmoides, an introduced centrachid with a diet mainly comprised of nekton, especially fish (Cochran and Adelman 1982). In Chivero, piscivorous fish were exclusively comprised of C. gariepinus, a native clariid with a diet mainly composed of fish, detritus, and insects (Winemiller and Kelso-Winemiller 1996). Evidence of a broad dietary range for C. gariepinus in Chivero was supported by the range of δ13C ratios in sampled fish (Fig. 3). In addition, differences in δ15N ratios between Chivero’s C. gariepinus samples exceeded 2.8‰ (Fig. 3), a threshold beyond which an omnivorous diet is considered dominant (Jepsen and Winemiller 2002; Razavi et al. 2014). Overall, absence of ASGM activities, the omnivorous feeding pattern of Chivero’s piscivores, the shorter food chain, and Hg biodilution processes occurring in the reservoir are all likely to be the major factors contributing to the recorded absence of Hg biomagnification.
The negative relationship between SL ~ δ15N for O. niloticus specimens from the two reservoirs (Fig. S5c), could be indicative of ontogenic dietary shifts. O. niloticus specimens from Chivero were significantly smaller than those from Mazowe (Fig. S2a). Previous studies (Poste et al. 2012), have confirmed ontogenic dietary shifts in this species, with juvenile fish having a diet that includes primary consumers such as benthic invertebrates while larger-adult fish tend to have an exclusively herbivorous diet comprising of phytoplankton and detritus.
Hg Exposure Risk to Fish and Human Health
Although our study only quantified THg concentrations in fish muscle tissue, previous work has shown that more than 95% of Hg in fish muscle tissues is in the form of MeHg (Bloom 1992). Fish muscle THg concentration can therefore be used as a proxy to assess the risk of human exposure to MeHg. Our results indicate that piscivorous fishes from the ASGM-impacted reservoir (Mazowe) had wet weight THg concentrations exceeding both the WHO threshold for susceptible human populations (0.2 µg/g ww) and the threshold for biochemical and gene expression alteration in fish (0.2 µg/g ww; Fig. 4). Susceptible populations are those that may experience severe adverse effects at comparable or lower exposure levels than the general population (WHO 2008). These include pregnant women and the developing fetus, infants, and individuals with pre-existing ailments of the liver, kidneys, and lungs. Our results indicate that consumption of piscivorous fishes from ASGM impacted sites should be avoided by individuals from susceptible groups to reduce Hg toxicity risk (Fig. 4). In the protected reservoir (Chivero), THg concentrations for both piscivorous and herbivorous fish species were below all examined thresholds used in this study. Based on these results, we provide the first fish consumption data to inform advisories for communities dependent on fish meat from the two reservoirs, which mainly include residents from Mazowe District and the Harare Metropolitan Province. MAF results for Mazowe fishes revealed that low consumption of piscivorous fishes is recommended for both adult men and women to avoid potential health risks (Table 3). Fish consumption has many health benefits and is a major way in which low-income communities in both rural and urban areas acquire essential nutrients such as protein, macro, and micronutrients (Béné 2006; Mcclanahan et al. 2015). Given the critical contribution of fish to human health, to reduce human exposure to Hg, we recommend consumption of species at lower trophic levels, and for local environmental management agencies to support efforts by the Minamata Convention on Mercury (UNEP 2019b) to reduce Hg use in ASGM.
In addition, our results reveal how anthropogenic activities such as ASGM have increased the threat to freshwater biodiversity conservation through the introduction of toxicants such as Hg in aquatic systems. Although the protected area status of Chivero might be partly responsible for the observed low THg concentrations and absence of Hg biomagnification, the role played by the trophic status of the reservoir through bloom and growth dilution cannot be overlooked. It remains unclear whether we would have obtained similar results for Chivero’s fishes if the trophic status of the reservoir was in the meso-oligotrophic range. In both reservoirs, upstream anthropogenic activities seem to have a major influence on Hg trophodynamics. Freshwater ecosystems make up a small component of the country’s protected area network, often being partially included in terrestrial protected areas. The few protected areas that include large reservoirs, such as Chivero, have been poorly designed, with narrow boundaries around the reservoir (see Fig. 1) and no consideration of upstream processes that may introduce pollutants into the protected portion. Nutrient pollution, for example, can alter the trophic state and the resulting length of the food chain, which are both important determinants of Hg bioaccumulation in freshwater biodiversity. The creation of specially designed freshwater protected areas that extend into headwater areas, thereby considering the connected nature of fluvial systems, should be part of a suite of preventative measures to address the Hg contamination problem.
Conclusions
Economic hardships and unpredictable climate patterns in Zimbabwe have acted as catalysts for the expansion of ASGM operations in the country as people seek alternative livelihood options. The use of Hg in ASGM operations in Zimbabwe has led to contamination of aquatic ecosystems and the scale of the problem remains largely unquantified. In this study, we use fish from two trophic guilds in each reservoir (protected and ASGM-impacted), to provide the first quantitative evidence for patterns of THg concentration and biomagnification in fishes from Zimbabwe’s aquatic ecosystems. Reservoirs outside Zimbabwe’s protected area network are directly impacted by Hg pollution from ASGM operations and piscivorous fish from such water bodies should be consumed with caution, as they increase the risk of exposure to Hg toxicity in humans.
Although we did not find significant THg concentrations and evidence of THg biomagnification in fishes from the protected reservoir (Chivero), pollution levels (e.g., other toxicants and nutrients) from upstream activities in the Chivero catchment remain very high. In addition, there is a need to explore Hg contamination in upstream portions of the catchment as well as in-lake mitigation processes such as biodilution and limnological mixing cycles, and other broader factors such as seasonal variation on fish THg concentration. Future studies should aim to include specimens that are more representative of the aquatic foodwebs in the reservoirs to get a clearer picture of Hg trophic transfer patterns.
Our findings demonstrate the need for the country’s biodiversity conservation institutions to reconsider approaches to protect freshwater biodiversity, as aquatic ecosystems located outside protected area boundaries are disproportionately impacted by direct anthropogenic activities such as ASGM. In particular, the creation of specially designed freshwater protected areas extending to headwaters would ensure reasonable protection of aquatic biodiversity and habitats in the country. Furthermore, we provide baseline evidence for the potential threat of Hg contamination in fish species of commercial importance and call upon public health agencies to work on providing detailed fish consumption advisories to the general fish-consuming public.
References
Agorku E, Voegborlo R, Adimado A (2009) Total mercury levels in nine species of freshwater fish from two hydroelectric reservoirs and a crater lake in Ghana. Environ Monit Assess 153:383–389. https://doi.org/10.1007/s10661-008-0365-8
Arthington AH, Dulvy NK, Gladstone W, Winfield IJ (2016) Fish conservation in freshwater and marine realms: status, threats, and management. Aquat Conserv: Mar Freshw Ecosyst 26:838–857. https://doi.org/10.1002/aqc.2712
Azevedo-Silva C, Almeida R, Carvalho DP, Ometto JPHB, de Camargo PB, Dorneles PR, Azeredo A, Bastos WR, Malm O, Torres JPM (2016) Mercury biomagnification and the trophic structure of the ichthyofauna from a remote lake in the Brazilian Amazon. Environ Res 151:286–296. https://doi.org/10.1016/j.envres.2016.07.035
Bastos W, De Almeida R, Dórea JG, Barbosa AC (2007) Annual flooding and fish-mercury bioaccumulation in the environmentally impacted Rio Madeira (Amazon). Ecotoxicology 16:341–346. https://doi.org/10.1007/s10646-007-0138-0
Béné C (2006) Small-scale fisheries: assessing their contribution to rural livelihoods in developing countries. FAO Fisheries Circular No. 1008. FAO, Rome
Black FJ, Bokhutlo T, Somoxa A, Maethamako M, Modisaemang O, Kemosedile T, Cobb-Adams C, Mosepele K, Chimbari M (2011) The tropical African mercury anomaly: lower than expected mercury concentrations in fish and human hair. Sci Total Environ 409:1967–1975. https://doi.org/10.1016/j.scitotenv.2010.11.027
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49(5):1010–1017. https://doi.org/10.1139/f92-113
Bose-O’Reilly S, Lettmeier B, Shoko D, Roider G, Drasch G, Siebert U (2020) Infants and mothers’ levels of mercury in breast milk, urine and hair, data from an artisanal and small-scale gold mining area in Kadoma. Zimbabwe. Environ. Res 184:109266. https://doi.org/10.1016/j.envres.2020.109266
Budnik LT, Casteleyn L (2019) Mercury pollution in modern times and its socio-medical consequences. Sci Total Environ 654:720–734. https://doi.org/10.1016/j.scitotenv.2018.10.408
Cabana G, Rasmussen J (1996) Comparison of aquatic food chains using nitrogen isotopes. PNAS USA 93(20):10844–10847. https://doi.org/10.1073/pnas.93.20.10844
Campbell LM, Osano O, Hecky RE, Dixon DG (2003) Mercury in fish from three rift valley lakes (Turkana, Naivasha, and Baringo), Kenya. East Africa Environ Pollut 125:281–286. https://doi.org/10.1016/S0269-7491(03)00053-8
Campbell LM, Balirwa JS, Dixon DG, Hecky RE (2004) Biomagnification of mercury in fish from Thruston Bay, Napoleon Gulf, Lake Victoria (East Africa). Afr J Aquat Sci 29(1):91–96. https://doi.org/10.2989/16085910409503796
Cochran P, Adelman I (1982) Seasonal aspects of daily ration and diet of largemouth bass, Micropterus salmoides, with an evaluation of gastric evacuation rates. Environ Biol Fishes 7:265–275. https://doi.org/10.1007/BF00002501
Cresson P, Travers-Trolet M, Rouquette M, Timmerman CA, Giraldo C, Lefebvre S, Ernande B (2017) Underestimation of chemical contamination in marine fish muscle tissue can be reduced by considering variable wet:dry weight ratios. Mar Pollut Bull 123:279–285. https://doi.org/10.1016/j.marpolbul.2017.08.046
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, White AE (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335. https://doi.org/10.1126/science.1154082
Crump K, Trudeau V (2009) Mercury-induced reproductive impairment in fish. Environ Toxicol Chem 28:895–907. https://doi.org/10.1897/08-151.1
de Almeida RP, Ferrari R, dos Santos L, Conte Junior C (2019) Mercury in aquatic fauna contamination: a systematic review on its dynamics and potential health risks. J Environ Sci 84:205–218. https://doi.org/10.1016/j.jes.2019.02.018
Desta Z, Borgstrøm R, Rosseland BO, Dadebo E (2007) Lower than expected mercury concentration in piscivorous African sharptooth catfish. Sci Total Environ 376:134–142. https://doi.org/10.1016/j.scitotenv.2007.01.091
Dolbec J, Mergler D, Larribe F, Roulet M, Lebel J, Lucotte M (2001) Sequential analysis of hair mercury levels in relation to fish diet of an Amazonian population, Brazil. Sci Total Environ 271:87–97. https://doi.org/10.1016/S0048-9697(00)00835-4
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983. https://doi.org/10.1021/es305071v
Dudgeon D (2019) Multiple threats imperil freshwater biodiversity in the Anthropocene. Curr Biol 29:960–967. https://doi.org/10.1016/j.cub.2019.08.002
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc 81:163–182. https://doi.org/10.1017/S1464793105006950
Ferreira da Silva S, de Oliveira Lima M (2020) Mercury in fish marketed in the Amazon Triple Frontier and health risk assessment. Chemosphere 248:125989. https://doi.org/10.1016/j.chemosphere.2020.125989
Gbogbo F, Otoo SD, Huago RQ, Asomaning O (2017) High levels of mercury in wetland resources from three river basins in Ghana: a concern for public health. Environ Sci Pollut Res 24:5619–5627. https://doi.org/10.1007/s11356-016-8309-2
Gerson JR, Szponar N, Zambrano AA, Bergquist B, Broadbent E, Driscoll CT, Erkenswick G, Evers DC, Fernandez LE, Hsu-Kim H, Inga G, Lansdale KN, Marchese MJ, Martinez A, Moore C, Pan WK, Purizaca RP, Sánchez V, Silman M, Ury EA, Vega C, Watsa M, Bernhardt ES (2022) Amazon forests capture high levels of atmospheric mercury pollution from artisanal gold mining. Nat Commun 13(559):1–10. https://doi.org/10.1038/s41467-022-27997-3
Grandjean P, White RF, Nielsen A, Cleary D, De Oliveira Santos EC (1999) Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ Health Perspect 107:587–591. https://doi.org/10.1289/ehp.99107587
Green CS, Lewis PJ, Wozniak JR, Drevnick PE, Thies ML (2019) A comparison of factors affecting the small-scale distribution of mercury from artisanal small-scale gold mining in a Zimbabwean stream system. Sci Total Environ 647:400–410. https://doi.org/10.1016/j.scitotenv.2018.07.418
Hanna DEL, Solomon CT, Poste AE, Buck DG, Chapman LJ (2015) A review of mercury concentrations in freshwater fishes of Africa: patterns and predictors. Environ Toxicol Chem 34:215–223. https://doi.org/10.1002/etc.2818
Harada M, Nakanishi J, Yasoda E, Pinheiro MN, Oikawa T, De Assis GG, Da Silva CB, Kizaki T, Ohno H (2001) Mercury pollution in the Tapajos River basin, Amazon: mercury level of head hair and health effects. Environ Int 27:285–290. https://doi.org/10.1016/S0160-4120(01)00059-9
Hecky R, Hesslein R (1995) Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. J N Am Benthol Soc 14(4):631–653
Ikingura JR, Akagi H (1996) Monitoring of fish and human exposure to mercury due to gold mining in the Lake Victoria goldfields. Tanzania Sci Total Environ 191:59–68. https://doi.org/10.1016/0048-9697(96)05178-9
JEFCA, (2007) Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), World Health Organisation. Available at: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=3083. Accessed: 11 October 2021
Jepsen D, Winemiller K (2002) Structure of tropical river food webs revealed by stable isotope ratios. Oikos 96: 46–55. http://www.jstor.org/stable/3547276
Kassegne AB, Okonkwo JO, Esho TB, Mekonnen KN, Malehase T, Asfaw SL (2018) Distribution of total mercury in surface sediments and African catfish (Clarias gariepinus) from Akaki River catchment and Aba Samuel Reservoir, downstream to the mega-city Addis Ababa. Ethiopia Emerg Contam 4:32–39. https://doi.org/10.1016/j.emcon.2018.10.003
Lavoie RA, Jardine TD, Chumchal MM, Kidd KA, Campbell LM (2013) Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ Sci Technol 47:13385–13394. https://doi.org/10.1021/es403103t
Lepak J, Hooten MB, Eagles-Smith CA, Tate MT, Lutz MA, Ackerman JT, Willacker JJJr, Jackson AK, Evers DC, Wiener JG, (2016) Assessing potential health risks to fish and humans using mercury. Sci Total Environ 571:342–354. https://doi.org/10.1016/j.scitotenv.2016.03.031
Li X, Li Z, Wu T, Chen J, Fu C, Zhang L, Feng X, Fu X, Tang L, Wang Z, Wang Z (2019) Atmospheric mercury emissions from two pre-calciner cement plants in Southwest China. Atmos Environ 199:177–188. https://doi.org/10.1016/j.atmosenv.2018.11.011
Li C, Xu Z, Luo K, Chen Z, Xu X, Xu C, Qiu G (2021) Biomagnification and trophic transfer of total mercury and methylmercury in a sub-tropical montane forest food web, southwest China. Chemosphere 277:130371. https://doi.org/10.1016/j.chemosphere.2021.130371
Liu B, Yan H, Wang C, Li Q, Guédron S, Spangenberg JE, Feng X, Dominik J (2011) Insights into low fish mercury bioaccumulation in a mercury-contaminated reservoir, Guizhou China. Environ Pollut 160:109–117. https://doi.org/10.1016/j.envpol.2011.09.023
Machiwa J (2004) Total mercury concentration in common fish species of Lake Victoria. Tanzania Tanzan J Sci 30:11–20. https://doi.org/10.4314/tjs.v30i2.18357
Magadza CHD (2003) Lake Chivero: a management case study. Lakes Reserv: Res Manag 8:69–81. https://doi.org/10.1046/j.1320-5331.2003.00214.x
Mambrey V, Rakete S, Tobollik M, Shoko D, Moyo D, Schutzmeier P, Steckling-Muschack N, Muteti-Fana S, Bose-O’Reilly S (2020) Artisanal and small-scale gold mining: a cross-sectional assessment of occupational mercury exposure and exposure risk factors in Kadoma and Shurugwi, Zimbabwe. Environ Res 184:109379. https://doi.org/10.1016/j.envres.2020.109379
Manatsa D, Mushore TD, Gwitira I, Wuta M, Chemura A, Shekede MD, Mugandani R, Sakala LC, Ali LH, Mupuro J (2020) Revision of Zimbabwe’s agro-ecological zones. Produced by the Government of Zimbabwe under Zimbabwe National Geospatial and Space Agency (ZINGSA) for the Ministry of Higher and Tertiary Education, Innovation Science and Technology Development. Zimbabwe Government Printers, Harare
Maponga O, Ngorima CF (2003) Overcoming environmental problems in the gold panning sector through legislation and education: The Zimbabwean experience. J Clean Prod 11:147–157. https://doi.org/10.1016/S0959-6526(02)00034-3
Marques RC, Garrofe Dórea J, Rodrigues Bastos W, de Freitas RM, de Freitas FM, Malm O (2007) Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon). Brazil Int J Hyg Environ Health 210:51–60. https://doi.org/10.1016/j.ijheh.2006.08.001
Marshall B (2011) The fishes of Zimbabwe and their biology. South African Institute for Aquatic Biodiversity, Grahamstown
Masocha M, Dube T, Mambwe M, Mushore TD (2019) Predicting pollutant concentrations in rivers exposed to alluvial gold mining in Mazowe Catchment, Zimbabwe. Phys Chem Earth, Parts a/b/c 112:210–215. https://doi.org/10.1016/j.pce.2018.12.007
Mason RP, Baumann Z, Hansen G, Yao KM, Coulibaly M, Coulibaly S (2019) An assessment of the impact of artisanal and commercial gold mining on mercury and methylmercury levels in the environment and fish in Cote d’Ivoire. Sci Total Environ 665:1158–1167. https://doi.org/10.1016/j.scitotenv.2019.01.393
Matthews DA, Babcock DB, Nolan JG, Prestigiacomo AR, Effler SW, Driscoll CT, Todorova SG, Kuhr KM (2013) Whole-lake nitrate addition for control of methylmercury in mercury-contaminated Onondaga Lake. NY Environ Res 125:52–60. https://doi.org/10.1016/j.envres.2013.03.011
Mcclanahan T, Allison EH, Cinner JE (2015) Managing fisheries for human and food security. Fish Fish 16:78–103. https://doi.org/10.1111/faf.12045
Merron G (1993) Pack-hunting in two species of catfish, Clarias gariepinus and C. ngamensis, in the Okavango Delta. Botswana J Fish Biol 43:575–584. https://doi.org/10.1111/j.1095-8649.1993.tb00440.x
Metcalf SM, Veiga MM (2012) Using street theatre to increase awareness of and reduce mercury pollution in the artisanal gold mining sector: a case from Zimbabwe. J Clean Prod 37:179–184. https://doi.org/10.1016/j.jclepro.2012.07.004
Mhlanga L, Day J, Chimbari M, Siziba N, Cronberg G (2006) Observations on limnological conditions associated with a fish kill of Oreochromis niloticus in Lake Chivero following collapse of an algal bloom. Afr J Ecol 44:199–208. https://doi.org/10.1111/j.1365-2028.2006.00625.x
Mitchell DS, Marshall BE (1974) Hydrobiological observations on three Rhodesian reservoirs. Freshw Biol 4:61–72. https://doi.org/10.1111/j.1365-2427.1974.tb00939.x
Mkodzongi G (2020) The rise of “Mashurugwi” machete gangs and violent conflicts in Zimbabwe’s artisanal and small-scale gold mining sector. Extr Ind Soc 7:1480–1489. https://doi.org/10.1016/j.exis.2020.10.001
Molina CI, Gibon FM, Duprey JL, Dominguez E, Guimarães JRD, Roulet M (2010) Transfer of mercury and methylmercury along macroinvertebrate food chains in a floodplain lake of the Beni River, Bolivian Amazonia. Sci Total Environ 408:3382–3391. https://doi.org/10.1016/j.scitotenv.2010.04.019
Montaña CG, Liverpool E, Taphorn DC, Schalk CM (2021) The cost of gold: Mercury contamination of fishes in a Neotropical river food web. Neotrop Ichthyol 19(3):e200155. https://doi.org/10.1590/1982-0224-2020-0155
Mujere N, Mazvimavi D (2012) Impact of climate change on reservoir reliability. Afr Crop Sci J 20:545–551
Müller S (2018) Corporate behaviour and ecological disaster: Dow chemical and the Great Lakes mercury crisis, 1970–1972. Bus Hist 60:399–422. https://doi.org/10.1080/00076791.2017.1346611
Nhapi I, Siebel MA, Gijzen HJ (2004) The impact of urbanisation on the water quality of Lake Chivero, Zimbabwe. Water Environ J 18:44–49. https://doi.org/10.1111/j.1747-6593.2004.tb00492.x
Niane B, Guédron S, Feder F, Legros S, Ngom PM, Moritz R (2019) Impact of recent artisanal small-scale gold mining in Senegal: Mercury and methylmercury contamination of terrestrial and aquatic ecosystems. Sci Total Environ 669:185–193. https://doi.org/10.1016/j.scitotenv.2019.03.108
Ouédraogo O, Amyot M (2013) Mercury, arsenic and selenium concentrations in water and fish from sub-Saharan semi-arid freshwater reservoirs (Burkina Faso). Sci Total Environ 444:243–254. https://doi.org/10.1016/j.scitotenv.2012.11.095
Ouédraogo O, Chételat J, Amyot M (2015) Bioaccumulation and trophic transfer of mercury and selenium in African sub-tropical fluvial reservoirs food webs (Burkina Faso). PLoS ONE 10:e0123048. https://doi.org/10.1371/journal.pone.0123048
Post DM (2002) Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Poste A, Muir DCG, Mbabazi D, Hecky RE (2012) Food web structure and mercury trophodynamics in two contrasting embayments in northern Lake Victoria. J Great Lakes Res 38:699–707. https://doi.org/10.1016/j.jglr.2012.09.001
Poste AE, Muir DCG, Guildford SJ, Hecky RE (2015) Bioaccumulation and biomagnification of mercury in African lakes: The importance of trophic status. Sci Total Environ 506–507:126–136. https://doi.org/10.1016/j.scitotenv.2014.10.094
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Razavi NR, Qu M, Jin B, Ren W, Wang Y, Campbell LM (2014) Mercury biomagnification in subtropical reservoir fishes of eastern China. Ecotoxicology 23:133–146. https://doi.org/10.1007/s10646-013-1158-6
Razavi NR, Qu M, Chen D, Ren W, Zhong Y, Wang Y, Campbell LM (2015) Effect of eutrophication on mercury (Hg) dynamics in subtropical reservoirs from a high Hg deposition ecoregion. Limnol Oceanogr 60:386–401. https://doi.org/10.1002/lno.10036
Razavi NR, Cushman SF, Halfman JD, Massey T, Beutner R, Cleckner LB (2019) Mercury bioaccumulation in stream food webs of the Finger Lakes in central New York State, USA. Ecotoxicol Environ Saf 172:265–272. https://doi.org/10.1016/j.ecoenv.2019.01.060
Reid AJ, Carlson AK, Creed IF, Eliason EJ, Gell PA, Johnson PTJ, Kidd KA, MacCormack TJ, Olden JD, Ormerod SJ, Smol JP, Taylor WW, Tockner K, Vermaire JC, Dudgeon D, Cooke SJ (2018) Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol Rev 94:849–873. https://doi.org/10.1111/brv.12480
Rumengan IFM, Rumampuk D, Limbong D, Arai T, Miyazaki N (2003) THg contents in plankton collected from Talawaan Watershed, north Sulawesi, Indonesia. Otsuchi Marine Science 28: 80–83. https://doi.org/10.15083/00038785
Salazar-Camacho C, Salas-Moreno M, Paternina-Uribe R, Marrugo-Negrete J, Díez S (2021) Mercury species in fish from a tropical river highly impacted by gold mining at the Colombian Pacific region. Chemosphere 264:128478. https://doi.org/10.1016/j.chemosphere.2020.128478
Santos-Lima C, Mourão D, Carvalho CF, Souza-Marque B, Vega CM, Gonçalves RA, Argollo N, Menezes-Filho JA, Abreu N, Hacon S (2020) Neuropsychological effects of mercury exposure in children and adolescents of the Amazon Region, Brazil. Neurotoxicology 79:48–57. https://doi.org/10.1016/j.neuro.2020.04.004
Santos-Sacramento L, Arrifano GP, Lopes-Araújo A, Augusto-Oliveira M, Albuquerque-Santos R, Takeda PY, Souza-Monteiro JR, Macchi BM, doNascimento JLM, Lima RR, Crespo-Lopez ME, (2021) Human neurotoxicity of mercury in the Amazon: a scoping review with insights and critical considerations. Ecotoxicol Environ Saf 208:111686. https://doi.org/10.1016/j.ecoenv.2020.111686
Skelton PH (2001) A complete guide to the freshwater fishes of Southern Africa, 2nd edn. Struik Publishers, Cape Town
Spiegel SJ (2009) Resource policies and small-scale gold mining in Zimbabwe. Resour Policy 34:39–44. https://doi.org/10.1016/j.resourpol.2008.05.004
Spiegel SJ, Veiga MM (2010) International guidelines on mercury management in small-scale gold mining. J Clean Prod 18:375–385. https://doi.org/10.1016/j.jclepro.2009.10.020
Stewart AR, Saiki MK, Kuwabara JS, Alpers CN, Marvin-DiPasquale M, Krabbenhoft DP (2008) Influence of plankton mercury dynamics and trophic pathways on mercury concentrations of top predator fish of a mining-impacted reservoir. Can J Fish Aquat Sci 65:2351–2366. https://doi.org/10.1139/F08-140
Taylor H, Appleton JD, Lister R, Smith B, Chitamweba D, Mkumbo O, Machiwa JF, Tesha AL, Beinhoff C (2005) Environmental assessment of mercury contamination from the Rwamagasa artisanal gold mining centre, Geita District, Tanzania. Sci Total Environ 343:111–133. https://doi.org/10.1016/j.scitotenv.2004.09.042
Tendaupenyu P, Magadza CHD, Murwira A (2017) Changes in landuse/landcover patterns and human population growth in the Lake Chivero catchment, Zimbabwe. Geocarto Int 32:797–811. https://doi.org/10.1080/10106049.2016.1178815
United Nations Environment Programme (UNEP) (2002) Global mercury assessment. UNEP Chemicals, Geneva, p 270
United Nations Environment Programme (UNEP) (1990) Methylmercury - Environmental Health Criteria 101. https://wedocs.unep.org/20.500.11822/29413
United Nations Environment Programme (UNEP) (2019a) Global Mercury Assessment 2018. UN Environment Programme, Chemicals and Health Branch, Geneva, Switzerland
United Nations Environment Programme (UNEP) (2019b) Minamata Convention on Mercury: Text and Annexes, Nairobi, Kenya
United States Environmental Protection Agency (USEPA) (1998) Method 7473 (SW-846): Mercury in solids and solutions by thermal decomposition, amalgamation, and atomic absorption spectrophotometry, Revision 0. Washington, DC
Utete B, Phiri C, Mlambo SS, Maringapasi N, Muboko N, Fregene TB, Kavhu B (2018) Metal accumulation in two contiguous eutrophic peri-urban lakes, Chivero and Manyame. Zimbabwe Afr J Aquat Sci 43:1–15. https://doi.org/10.2989/16085914.2018.1429249
Van Straaten P (2000) Mercury contamination associated with small-scale gold mining in Tanzania and Zimbabwe. Sci Total Environ 259:105–113. https://doi.org/10.1016/S0048-9697(00)00553-2
Vander Zanden, J, Rasmussen J (1999) Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80: 1395–1404. https://www.jstor.org/stable/177083
Vander Zanden J, Rasmussen J (2001) Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066. https://doi.org/10.4319/lo.2001.46.8.2061
Vanderklift M, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182. https://doi.org/10.1007/s00442-003-1270-z
Veiga MM, Maxson PA, Hylander LD (2006) Origin and consumption of mercury in small-scale gold mining. J Clean Prod 14:436–447. https://doi.org/10.1016/j.jclepro.2004.08.010
Wang X, Wang WX (2019) The three “B” of fish mercury in China: bioaccumulation, biodynamics and biotransformation. Environ Pollut 250:216–232. https://doi.org/10.1016/j.envpol.2019.04.034
Watras CJ, Bloom NS (1992) Mercury and methylmercury, in individual zooplankton: implications for bioaccumulation. Limnol Oceanogr 37:1313–1318. https://doi.org/10.4319/lo.1992.37.6.1313
Webber H, Haines T (2003) Mercury effects on predator avoidance behavior of a forage fish, golden shiner (Notemigonus crysoleucas). Environ Toxicol Chem 22:1556–1561. https://doi.org/10.1002/etc.5620220718
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York
Winemiller K, Kelso-Winemiller L (1996) Comparative ecology of catfishes of the Upper Zambezi River floodplain. J Fish Biol 49:1043–1061. https://doi.org/10.1111/j.1095-8649.1996.tb01777.x
Won JH, Lee TG (2012) Estimation of total annual mercury emissions from cement manufacturing facilities in Korea. Atmos Environ 62:265–271. https://doi.org/10.1016/j.atmosenv.2012.08.035
World Health Organization (WHO) (2008) Guidance for identifying populations at risk from mercury exposure. Issued by: UNEP DTIE Chemicals Branch and WHO Department of Food Safety, Zoonoses and Foodborne Diseases, Geneva
Wu Z, Ye H, Shan Y, Chen B, Li J (2020) A city-level inventory for atmospheric mercury emissions from coal combustion in China. Atmos Environ 223:117245. https://doi.org/10.1016/j.atmosenv.2019.117245
Zhao S, Pudasainee D, Duan Y, Gupta R, Liu M, Lu J (2019) A review on mercury in coal combustion process: content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog Energy Combust Sci 73:26–64. https://doi.org/10.1016/j.pecs.2019.02.001
Zolnikov TR, Ramirez Ortiz D (2018) A systematic review on the management and treatment of mercury in artisanal gold mining. Sci Total Environ 633:816–824. https://doi.org/10.1016/j.scitotenv.2018.03.241
ZPWMA (2017) Zimbabwe Parks and Wildlife Management Authority, Investment Prospectus, Harare
Acknowledgements
We thank Rebecca Rundell (SUNY ESF), Melanie Stiassny (AMNH), and Jeremy Wright (New York State Museum) for their reviews and comments during the development of the manuscript. Abby Webster (SUNY ESF) is gratefully acknowledged for technical assistance with THg analyses. We also thank Patrick Mutizamhepo (UZ), for field technical support and the University of Zimbabwe, Biological Sciences Department, for providing sampling equipment used during fieldwork.
Funding
Fieldwork was funded by the Rufford Small Grant for Nature Conservation (Offer number—27654-1) awarded to JM. Start-up funds to NR. Razavi from SUNY-ESF funded mercury and stable isotope analyses. JM is grateful to the US Institute of International Education for the Fulbright Foreign Student Ph.D. fellowship. Fulbright provided no input into the study design, interpretation of results, or the writing of this article. The following grants and fellowships were also awarded to JM from the SUNY College of Environmental Science and Forestry: The W.A Dence Fellowship Award, the Moore Family Fund for Wildlife Conservation, and Graduate Assistantships in the Department of Environmental Biology.
Author information
Authors and Affiliations
Contributions
JM Project conceptualization, Funding acquisition for fieldwork, Investigation, Data curation, Formal analyses, Project administration, Writing—original draft, Writing—review and editing. TD Investigation, Writing—review and editing. DJS Resources, Writing—review and editing, Supervision. NRR Conceptualization, Funding acquisition for all laboratory work, Resources, Supervision, Field and lab methodology, Writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no relevant financial or non-financial interests to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Makaure, J., Dube, T., Stewart, D. et al. Mercury Exposure in Two Fish Trophic Guilds from Protected and ASGM-Impacted Reservoirs in Zimbabwe and Possible Risks to Human Health. Arch Environ Contam Toxicol 84, 199–213 (2023). https://doi.org/10.1007/s00244-023-00977-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-023-00977-4