Abstract
The researches on MPs in commercial marine fish are very limited although in marine environments microplastic (MPs) pollution is a global problem. In this study, the presence, composition, and characterization of MPs in different tissues (brain, gill, muscle, and gastrointestinal tract) of commercial fish species [red mullet (Mullus barbatus) and pontic shad (Alosa immaculata Bennett 1835)] from the Black Sea were investigated. M. barbatus (demersal) and A. immaculata (pelagic) fish were preferred in the selection of fish species in order to represent demersal and pelagic environments. After dissected the fish, MPs were obtained from the tissues by extraction using the flotation method; then the MPs were counted and categorized according to shape, size, and color. The composition of the MPs was determined via ATR–FTIR spectroscopy. In terms of microplastic abundance in fish tissues, the gastrointestinal tract (40.0%) ranked first in both fish species, while the lowest MPs density was determined in brain tissues (7.0%). After the gastrointestinal tissue, gills were identified as the second tissue with the highest MPs density. Regardless of fish species, MPs characterization was mainly fibrous (51.0%), black colored (49.0%), and 50–200 µm in size (55.0%). Among the nine different polymers determined, polychloroprene (18.8%) and polyamide (15.0%) were found most frequently. This research provides data for tissue-based assessment of MPs in fish. The obtained data showed that MPs (one of the anthropogenic pollutants) are quite high in all tissues regardless of fish species. Moreover, it has emerged that these two fish species are suitable for monitoring microplastics in the study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing use of plastics brings with it the problem of environmental pollution, especially due to single-use and inadequate management of plastic waste. Plastic particles, smaller than 5 mm, are known as microplastics. These are the result of the degradation of plastics in nature or the direct use of materials such as textile fibers.
Microplastics are ubiquitous in nature and are a concern in aquatic environments, as well as for living resources. In the last decade, studies on microplastic-related environmental problems have started to be the focus of attention (Jabeen et al. 2017; Frias et al. 2018; Hanachi et al. 2019; Herrera et al. 2019; Hossain et al. 2019; Amin et al. 2020; Filgueiras et al. 2020). All of the recent studies conducted around the world aimed at determining the microplastic pollution in regional or country-based waters and accumulation in living organisms.
The mixing of microplastics from different sources into water environments poses a threat to aquatic organisms, and possibly humans consuming contaminated fish and seafood (Kor et al. 2020). Potential effects of MPs on aquatic organisms are due to the physical and chemical effects of these ingested plastics (Barboza et al. 2020; Zakeri et al. 2020). Adverse effects of MPs can be caused by: (1) the particles themselves, (2) added materials during the manufacture of plastic products, and (3) pollutants adsorbed to plastic waste in the environment (Zakeri et al. 2020). The literature on MPs toxicity has revealed that these materials can cause physical and chemical toxicity in aquatic organisms, including genotoxicity, oxidative stress, behavioral changes, reproductive impairment, mortality, and a decrease in population growth rate (Hanachi et al. 2019; Barboza et al. 2020; Zakeri et al. 2020). Aquatic organisms can be contaminated with micro- and nano-plastics from water or by feeding contaminated foods or other living organisms (Kolandhasamy et al. 2018; Baalkhuyur et al. 2020; Li et al. 2020).
The presence of microplastics in commercially important fish species poses a potential risk to human health (Hanachi et al. 2019; Zakeri et al. 2020). Currently, more than 660 marine species are known to be affected by plastics (Claessens et al. 2013; Carbery et al. 2018). Usually, in marine biota, ingested MPs are either expelled with feces or they sometimes remain in the gastrointestinal tract, causing damage or a false feeling of fullness in the fish stomach. In some cases, it is divided into smaller sizes and enters the circulatory system through the intestinal wall (Hossain et al. 2019; Zakeri et al. 2020; Wang et al. 2021).
There is increasing evidence that microplastics can be transferred in the food chain. Due to their small size, MPs can be found at different trophic levels such as plankton, bivalves, and fish which are consumed by humans (Lindeque et al. 2020; Sfriso et al. 2020). This situation brings with it increasing concerns about detrimental effects for bioaccumulation from one trophic level to another.
MPs pollution is a global concern emerging in aquatic environments, but the literature is scarce regarding studies on the uptake by commercial marine fish. In the light of this information, the presence and profiles of microplastics in different tissues (brain, gill, gastrointestinal system and muscle) of pontic shad (A. immaculata) and red mullet (M. barbatus) were investigated. In this study, the fish species selection was designed to represent pelagic (pontic shad) and benthic (red mullet) populations and also to include species of economic importance in the Black Sea. This research is the first MPs scanning study in A. immaculata (the first time) and the first for M. barbatus which detects the presence of MPs in brain tissues.
Material and Methods
Fish Sampling Area
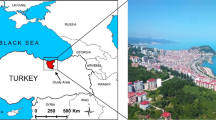
The fisheries activities were carried out with a commercial fishing boat operating along the western coasts of Sinop (İnceburun region) in the Black Sea off the coast of Turkey (Fig. 1). Fish were collected between the months of February and March during the 2019–2020 fishing season. Coordinates of the trawl sampling areas were between 42° 10′ 33'' N–34° 43′ 42'' E and 42° 08′ 48'' N–34° 57′ 36'' E. Water depth of the fishing area varied between 60 and 120 m. Sinop region is an important fishing spot for the Black Sea. Especially bottom trawl fishing is widespread on the western coast of Sinop. Another important feature of the region is that it is located on the migration route of the shoal fish. Shad fish, which is a pelagic species, in winter and spring, can also be captured by bottom trawl, where demersal species are caught. Since both red mullet and shad fish catch were targeted in the study, sampling was done with fishing gear.
A conventional, demersal otter trawl with 800 mesh was used in the study. The codend mesh size was 40 mm diamond mesh. Towing speed ranged between 2.5 and 3 knots, and towing duration was 120 min in all the hauls.
Fish Materials and Lab Works
Fish tissues Red mullet (Mullus barbatus) and pontic shad (Alosa immaculata Bennett 1835) caught by trawling were transported to Atatürk University Fisheries Faculty Laboratories in coolers with ice packs, and then stored in a freezer at − 20° C until analyzed. Fish were thawed and washed with ultrapure distilled water. By determining the morphological characteristics (e.g., total length and weight, visceral weights, etc.) of fish samples, the incidence of microplastics (MPs) was determined on a gravimetric basis for each species (Hossain et al. 2019). In addition to gastrointestinal samples, the brain, gills and muscle tissues were dissected and then the microplastic extraction step applied.
Microplastic Extraction
Microplastic extraction was performed by modifying the methods of Zhang et al. (2020a,b a,b) and Barboza et al. (2020). A total of 82 fishes were chosen from the 2 species (i.e., M. barbatus and A. immaculata) for our research, because these two species are suitable for monitoring the ingestion of marine microplastics in the pelagic zone and the water column (Zhang et al. 2020a). Fish were dissected after thawing at room temperature. The gastrointestinal tract (GT) from the tip of the oesophagus to the vent, dorsal muscle, gills and brain were removed and weighed. Then, the tissues were transferred individually to 500 mL glass bottles and 200 mL of KOH (10%, V/V) was added. GT, muscle and brain samples were incubated at 60 °C for 24 h (Dehaut et al. 2016), and gill samples were incubated at 40 °C for 72 h (Karami et al. 2017) to digest the organic material. The gills were incubated under different conditions of temperature and time interval because the digestion method used for the other tissues was not fully efficient. Density separation was performed to isolate all types of microplastics (Abbasi et al. 2018). As commonly used for fish samples containing a lot of clay, we added 400 mL saturated sodium chloride solution (1.2 g/mL NaCl) after initial filtration of the supernatant, and this was suspended overnight at room temperature (Jabeen et al. 2017). After digestion and suspension, a 0.45 μm membrane filter (Whatman Membrane Filters) was used for filtration. The filters were air-dried at room temperature and placed in glass dishes. Following this procedure, microplastics on the filters were photographed under a Leica S8 APO stereo microscope with an integrated Leica MC170 HD microscope camera, both supplied by Leica Microsystems (40X). Fish tissue samples were prepared in a previously cleaned laboratory with limited access to prevent microplastic contamination. Clean, cotton laboratory coat and nitrile gloves were worn during all laboratory stages. All work surfaces and dissection materials were cleaned with 70% ethanol before use and between individual samples to avoid cross-contamination. The exterior of the fish was washed twice with ultrapure water and once with ethanol to remove all exogenous particles adhering to the fish body surface (Karami et al. 2017). Despite all care, controls were included in the experiment to assess possible contamination from the laboratory atmosphere. These controls were placed in three clean Petri dishes near the work area in all procedures and analyzed as procedural blank controls. Additionally, during digestion procedures, petri dishes containing ultrapure water were analyzed instead of three tissue-free fish samples in parallel with the fish samples (Barboza et al. 2020).
MPs Characterization and Identification
Visual (maximum length, color and shape) evaluation was performed for the items photographed under the stereo microscope. At this stage, each particle was included in the group that was closest in its longest or widest dimension (Frias et al. 2018; Hossain et al. 2019). MP morphotypes obtained in samples from the two fish species were grouped by a software program (ImageJ, https://imagej.nih.gov/ij/) according to color (i.e., blue, black, red/pink, yellow, gray/whitish), shape (i.e., fibers, fragments, pellets) and size (i.e., < 50 μm; 50–200 μm; 200–500 μm; 500–1000 μm; 1000–5000 μm). For the polymer identification of MPs collected from samples of GT, muscle, gill and brain tissues, the attenuated total reflectance (ATR) technique was chosen due to its spectral repeatability and ease of sample preparation. The analysis was performed using an Agilent Cary 630 Fourier Transform Infrared (FTIR) spectrometer operated in ATR mode (Zhang et al. 2020a).
Statistical Analyses
Statistical analyses were performed using the SPSS statistical analysis package (version 20.0), and the statistical significance level was 0.01. For each species, the Student’s t-test was conducted to assess the difference of MPs abundance between M. barbatus and A. immaculata. Microplastic abundance was determined with linear regression analysis. In addition, the correlation analysis was used to test between the abundances of MPs and the fish traits. The relationship between properties such as fish weight-length/intestinal weight and microplastic content was determined with the linear regression analysis. The correlation analysis was conducted to specify the direction and strength of these relationships.
Results
No microplastics were found in any of the blanks of our study. The amounts of MPs of M. barbatus and A. immaculata’s brain, gill, muscle, and gastrointestinal sample contents are given in Table 1. In the evaluation of this table, the total amount of MPs was determined as 168 for M. barbatus and 264 for A. immaculata. Within these total ingredients, the presence of plastic in the tissues of both fish species was determined as the gastrointestinal system, gill, muscle, and brain, respectively, from large to small. Microplastic distribution ratio in M. barbatus tissues was determined as 7.7%, 30.4%, 25%, and 36.9%, while these values were determined as 7.6%, 31.1%, 22.3%, and 39% in A. immaculata, in brain, gill, muscle and gastrointestinal system, respectively (Fig. 2).
A positive and significant (r = 0.66, p < 0.01) relationship was found between MPs and total length (Fig. 3). Another finding was a positive and significant relationship (r = 0.63, p < 0.01) between the total weight and the length. In these analyzes, a high positive correlation (r = 0.99, p < 0.01) was determined between length/weight, and a positive correlation (r = 0.33, p < 0.01) was found between total microplastic content and intestinal weight.
When the colors of MPs were examined, the dominant color was black (28–72%), blue (13–31%), yellow (0–27%), gray/whitish (0–22%), and red/pink was as (0–10%) (Fig. 4).
In the fish tissues, 3 types (fibers, fragments, and pellets) of MPs were identified (Figs. 5, 6, 7, 8). The total number of MPs obtained from fish was 432; 33 of them had been detected in brains, 133 in gills, 101 in muscles, and 165 in gastrointestinal tissues. Of the total microplastics (432), 220 (51%) were evaluated as fiber, 30 (7%) as fragments, and 182 (42%) as pellets (Fig. 9). All types (fiber, fragment, and pellet) were found in all studied tissue samples, and this distribution differed between fish species (Fig. 8). This difference was obtained even in the tissues of the same fish species. As a result, considering the MPs shape distribution among the tissues, the ratio of fiber, fragment, and pellet in M. barbatus brain tissue was 29%, 17%, and 54%, respectively; in gills 24% fiber, 28% pellet; in muscle tissue 65% fiber, 6% fragment and 29% pellets; and finally in gastrointestinal tissues 53% fiber and 47% pellets (Fig. 9).
In A. immaculata, the ratio of fiber, fragment, and pellet in brain tissue is 42%, 10%, and 48%; in gills 41% fiber, 1% fragment, 58% pellet: in muscle tissue 46% fiber, 25% fragment, and 26% pellet, while in gastrointestinal tissues 55% fiber, 5% fragment and 40% pellets values were obtained (Fig. 9).
In dimensional MPs evaluations, the maximum of MPs were measured in the range of 50–200 µm in all fish species and tissues. Gills and gastrointestinal tissues with an incidence of 56% were the most common tissues on tissue-basis (Fig. 10). Again, when tissue evaluations were made between fish species, MPs with a size of 50 µm were not found in the gill and gastrointestinal tissues of M. barbatus. Similarly, very low levels of MPs of 1000–5000 µm were detected in A. immaculata gills and gastrointestinal tissues compared to M. barbatus (Fig. 10).
A total of 87 representative plastic-like particles (20.14%) were selected from the total suspected plastics for analysis by ATR-FTIR. The distribution of analyzed polymers was 10% cellulose, 14% polyester, 15% polyamide, 18% polychloroprene, 13% polyacrylamide, 10% poly lauryl lacta (nylon), 7% polysulfide, 7% ethylene propylene and 6% polytetrafluoroethylene (PTFE), as illustrated in Fig. 11a,b.
Discussion
Recently, attention has been drawn to the problem of microplastics in all aquatic ecosystems and it had been confirmed that this class of pollutants is present everywhere all over the world.
In a previous study, Barboza et al. (2020) concluded that MPs taken up by fish can occur by passive (e.g., gill filtration) or by active (i.e., ingestion by confusion with prey) mechanisms. Microplastic particles, that are too small, can be misidentified by fish or accidentally ingested as prey, and also fish can ingest other contaminated (with these microplastic particles) organisms (Zakeri et al. 2020). Barboza et al. (2020) stated that the MPs can be divided into smaller particles by internalizing microplastics in the fish digestive system. The presence of microplastic in fish may differ according to the nutritional status of the fish (Li et al. 2020). Pontic shad’s food chain contains small fish species (anchovy etc.), and invertebrates (Visnjic-Jeftic et al. 2010), so MPs abundance in A. immaculata can be explained with this species’ lifestyle. The higher level in the fish can be explained with being the main route of MPs uptake, again, its abundance in the gastrointestinal system was due to ingestion, which is effective in MP intake (Miller et al. 2016).
The abundance of MPs in waters allows fish to easily take these particles and store them in their tissues. Recent researches had been showed that most of fish species are susceptible to MPs ingestion. After ingestion by fish, these pollutants can accumulate in the fish gastrointestinal tracts and transport to other fish organs (Wang et al. 2020). The MPs abundance in fish gastrointestinal tract is closely related to the habitat of the fish (Zhang et al. 2020a,b). Until recently, evidence of fish MPs ingestion mostly comes from the gastrointestinal analysis (Wang et al. 2020).
In terms of MPs shapes; the results found in the present study are similar to previous investigations (Amin et al. 2020). In the tissues, the fibers are dominant than other shapes (fragments, pellets), because they are one-dimensional materials and break into smaller pieces easily (Wang et al. 2021).
In addition, it had been reported that human activities play an important role in the microplastic distribution in marine environments (Amin et al. 2020). The high fiber percentage in our study suggests that the high abundance of microplastic here is due to the surrounding wastewater (Li et al. 2020).
Particle size is another common measurement parameter for microplastics (Zhang et al. 2020a). Small-sized MPs were dominant in the present research. The excess of small-sized MPs is a result of the high probability of their unintentional ingestion by fish (Wang et al. 2021). Zitouni et al (2020) reported that MPs smaller than 100 μm were detected in the muscle tissues of a commercial fish species (Serranus scriba). This situation is parallel to our findings.
According to the color analyses results; while blue color was determined as dominant in some studies (Neves et al. 2015; Bessa et al. 2018; Herrera et al. 2019; Barboza et al. 2020), in the current study, black color was found to be dominant, followed by blue color. Bellas et al. (2016) reported that the dominant MPs color in fish species is black. This situation is related to the fish age because young fish prefer the black color more. This color MPs is thought to be due to the fact that more similar to their food (McNeish et al. 2018; Ory et al. 2018; Ferreira et al. 2020). Although phytoplanktons can be transparent, white, or yellow color, most of them are black patches. Plastic products are usually brightly colored but black plastic refracts light and can be used for storage. For this reason, black plastic products are preferred more (Wang et al. 2020).
This observation may be related to the fish age, young fish may prefer black items because this color is similar to their food. The presence of MPs with intense blue color is thought to be due to the fact that there are large amounts of blue microplastics in sea water and these are actively ingested (Barboza et al. 2020). It is known that black, blue and red microplastics are the dominant colors in fish specimens (Lin et al. 2020). Although the color of microplastics is determined by the primary source, transformations by UV radiation, weathering and microbial degradation may change the color (Zhang et al. 2020b).
Our study data showed that polychloroprene was the dominant polymer group in the tissues, followed by polyamide. The intensive use of the polychloroprene polymer for many years (commercially available since 1932) and its resistance to degradation (Johnson, 1976) explains the dominance of this group of MPs. MPs such as polyamide and polyester tend to sink and accumulate in marine sediments. The reason for the high densities of these polymers, especially in the GT tissues, is thought to be because of ingestion of these MPs by the fish as they sink in the water column or after they are deposited in sediments (Wang et al. 2021). Consistent with our research findings, Filgueiras et al. (2020) reported that polyethylene, polyester, polypropylene, polyamide and acrylic are the most common types of polymers found in the marine environment. These polymers have been reported to be abundant in marine areas commonly used for fishing, being deposited in the marine ecosystem by fishing, boating and/or discharges of wastewater (Amin et al. 2020).
Conclusion
There is limited research on the ingestion of MPs particles by Black Sea commercial fish. Again, another important piece of information studied in our research is the presence of MPs in the fish brain. MPs interpretation was also made for the first time in (A. immaculata) in this study. However, our obtained data is an important point to monitor the subsequent changes in the formation of microplastics in the sea. Microplastics were observed in the brain, gill, muscle, and gastrointestinal system contents of two fish species caught from Inceburun region (Black Sea, Sinop, Turkey). Fish length and body condition appear to be relevant variables that explain changes in the number and size of ingested MPs. Histopathological examination of fish tissues contaminated with MPs should be a research priority for future studies.
References
Abbasi S, Soltani N, Keshavarzi B, Moore F, Turner A, Hassanaghaei M (2018) Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere 205:80–87. https://doi.org/10.1016/j.chemosphere.2018.04.076
Amin RM, Sohaimi ES, Anuar ST, Bachok Z (2020) Microplastic ingestion by zooplankton in Terengganu coastal waters, southern South China Sea. Mar Pollut Bull 150:110616. https://doi.org/10.1016/j.marpolbul.2019.110616
Baalkhuyur FM, Qurban MA, Panickan P, Duarte CM (2020) Microplastics in fishes of commercial and ecological importance from the Western Arabian Gulf. Mar Pollut Bull 152:110920. https://doi.org/10.1016/j.marpolbul.2020.110920
Barboza LGA, Lopes C, Oliveira P, Bessa F, Otero V, Henriques B, Guilhermino L (2020) Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci Total Environ 717:134625. https://doi.org/10.1016/j.scitotenv.2019.134625
Bellas J, Martínez-Armental J, Martínez-Cámara A, Besada V, Martínez-Gómez C (2016) Ingestion of microplastics by demersal fish from the Spanish Atlantic and Mediterranean coasts. Mar Pollut Bull 109:55–60. https://doi.org/10.1016/j.marpolbul.2016.06.026
Bessa, F., Barría, P., Neto, J.M., Frias, J. P., Otero, V., Sobral, P., Marques, J.C. (2018). Microplastics in juvenile commercial fish from an estuarine environment. In: Proceedings of the international conference on microplastic pollution in the mediterranean sea, pp 131–135. Springer, Cham
Carbery M, O’Connor W, Palanisami T (2018) Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ Int 115:400–409. https://doi.org/10.1016/j.envint.2018.03.007
Claessens M, Van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of microplastics in sediments and field collected organisms. Marine Pollut Bull 70(1–2):227–233. https://doi.org/10.1016/j.marpolbul.2013.03.009
Dehaut A, Cassone AL, Frère L, Hermabessiere L, Himber C, Rinnert E et al (2016) Microplastics in seafood: benchmark protocol for their extraction and characterization. Environ Pollut 215:223–233. https://doi.org/10.1016/j.envpol.2016.05.018
Ferreira M, Thompson J, Paris A, Rohindra D, Rico C (2020) Presence of microplastics in water, sediments and fish species in an urban coastal environment of Fiji, a Pacific small island developing state. Mar Pollut Bull 153:110991. https://doi.org/10.1016/j.marpolbul.2020.110991
Filgueiras AV, Preciado I, Cartón A, Gago J (2020) Microplastic ingestion by pelagic and benthic fish and diet composition: a case study in the NW Iberian shelf. Mar Pollut Bull 160:111623. https://doi.org/10.1016/j.marpolbul.2020.111623
Frias J, Pagter E, Nash R, O'Connor I, Carretero O, Filgueiras A et al. (2018) Standardised protocol for monitoring microplastics in sediments. Deliverable 4.2. JPI-Oceans BASEMAN Project. https://doi.org/10.25607/OBP-723
Hanachi P, Karbalaei S, Walker TR, Cole M, Hosseini SV (2019) Abundance and properties of microplastics found in commercial fish meal and cultured common carp (Cyprinus carpio). Environ Sci Pollut Res 26(23):23777–23787. https://doi.org/10.1007/s11356-019-05637-6
Herrera A, Ŝtindlová A, Martínez I, Rapp J, Romero-Kutzner V, Samper MD et al (2019) Microplastic ingestion by Atlantic chub mackerel (Scomber colias) in the Canary Islands coast. Mar Pollut Bull 139:127–135. https://doi.org/10.1016/j.marpolbul.2018.12.022
Hossain MS, Sobhan F, Uddin MN, Sharifuzzaman SM, Chowdhury SR, Sarker S, Chowdhury MSN (2019) Microplastics in fishes from the Northern Bay of Bengal. Sci Total Environ 690:821–830. https://doi.org/10.1016/j.scitotenv.2019.07.065
Jabeen K, Su L, Li J, Yang D, Tong C, Mu J, Shi H (2017) Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ Pollut 221:141–149. https://doi.org/10.1016/j.envpol.2016.11.055
Johnson PR (1976) Polychloroprene rubber. Rubber Chem Technol 49(3):650–702. https://doi.org/10.5254/1.3534978
Karami A, Golieskardi A, Ho YB, Larat V, Salamatinia B (2017) Microplastics in eviscerated flesh and excised organs of dried fish. Sci Rep 7(1):1–9. https://doi.org/10.1038/s41598-017-05828-6
Kolandhasamy P, Su L, Li J, Qu X, Jabeen K, Shi H (2018) Adherence of microplastics to soft tissue of mussels: a novel way to uptake microplastics beyond ingestion. Sci Total Environ 610:635–640. https://doi.org/10.1016/j.scitotenv.2017.08.053
Kor K, Ghazilou A, Ershadifar H (2020) Microplastic pollution in the littoral sediments of the northern part of the Oman Sea. Mar Pollut Bull 155:111166. https://doi.org/10.1016/j.marpolbul.2020.111166
Li B, Su L, Zhang H, Deng H, Chen Q, Shi H (2020) Microplastics in fishes and their living environments surrounding a plastic production area. Sci Total Environ 727:138662. https://doi.org/10.1016/j.scitotenv.2020.138662
Lin L, Ma LS, Li HX, Pan YF, Liu S, Zhang L et al (2020) Low level of microplastic contamination in wild fish from an urban estuary. Mar Pollut Bull 160:111650. https://doi.org/10.1016/j.marpolbul.2020.111650
Lindeque PK, Cole M, Coppock RL, Lewis CN, Miller RZ, Watts AJ et al (2020) Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ Pollut 265(A):114721. https://doi.org/10.1016/j.envpol.2020.114721
McNeish RE, Kim LH, Barrett HA, Mason SA, Kelly JJ, Hoellein TJ (2018) Microplastic in riverine fish is connected to species traits. Sci Rep 8(1):1–12. https://doi.org/10.1038/s41598-018-29980-9
Miller K, Santillo D, Johnston P (2016) Plastics in Seafood–full technical review of the occurrence, fate and effects of microplastics in fish and shellfish. Greenpeace research laboratoriess technical report
Neves D, Sobral P, Ferreira JL, Pereira T (2015) Ingestion of microplastics by commercial fish off the Portuguese coast. Mar Pollut Bull 101:119–126. https://doi.org/10.1016/j.marpolbul.2015.11.008
Ory NC, Gallardo C, Lenz M, Thiel M (2018) Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environ Pollut 240:566–573. https://doi.org/10.1016/j.envpol.2018.04.093
Sfriso AA, Tomio Y, Rosso B, Gambaro A, Sfriso A, Corami F et al (2020) Microplastic accumulation in benthic invertebrates in Terra Nova Bay (Ross Sea, Antarctica). Environ Int 137:105587. https://doi.org/10.1016/j.envint.2020.105587
Visnjic-Jeftic Z, Jaric I, Jovanovic L, Skoric S, Smederevac-Lalic M, Nikcevic M, Lenhardt M (2010) Heavy metal and trace element accumulation in muscle, liver and gills of the Pontic shad (Alosa immaculata Bennet 1835) from the Danube River (Serbia). Microchem J 95(2):341–344. https://doi.org/10.1016/j.microc.2010.02.004
Wang S, Zhang C, Pan Z, Sun D, Zhou A, Xie S et al (2020) Microplastics in wild freshwater fish of different feeding habits from Beijiang and Pearl River Delta regions, south China. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.127345
Wang Q, Zhu X, Hou C, Wu Y, Teng J, Zhang C et al (2021) Microplastic uptake in commercial fishes from the Bohai Sea. China Chemosphere 263:127962. https://doi.org/10.1016/j.chemosphere.2020.127962
Zakeri M, Naji A, Akbarzadeh A, Uddin S (2020) Microplastic ingestion in important commercial fish in the southern Caspian Sea. Mar Pollut Bull 160:111598. https://doi.org/10.1016/j.marpolbul.2020.111598
Zhang C, Wang S, Pan Z, Sun D, Xie S, Zhou A et al (2020a) Occurrence and distribution of microplastics in commercial fishes from estuarine areas of Guangdong. South China Chemosphere 260:127656. https://doi.org/10.1016/j.chemosphere.2020.127656
Zhang D, Cui Y, Zhou H, Jin C, Yu X, Xu Y, Li Y, Zhang C (2020b) Microplastic pollution in water, sediment, and fish from artificial reefs around the Ma’an Archipelago, Shengsi. China Sci Total Environ 703:134768. https://doi.org/10.1016/j.scitotenv.2019.134768
Zitouni N, Bousserrhine N, Belbekhouche S, Missawi O, Alphonse V, Boughatass I, Banni M (2020) First report on the presence of small microplastics (≤3 μm) in tissue of the commercial fish Serranus scriba (Linnaeus. 1758) from Tunisian coasts and associated cellular alterations. Environ Pollut 263:114576. https://doi.org/10.1016/j.envpol.2020.114576
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Atamanalp, M., Köktürk, M., Uçar, A. et al. Microplastics in Tissues (Brain, Gill, Muscle and Gastrointestinal) of Mullus barbatus and Alosa immaculata. Arch Environ Contam Toxicol 81, 460–469 (2021). https://doi.org/10.1007/s00244-021-00885-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-021-00885-5