Abstract
This study examined the distribution and toxic potential of conventional and emerging contaminants in composite sediment samples from 15 stormwater ponds in the Minneapolis–St. Paul, MN metropolitan area. Previously, coal tar-based sealants were shown to be a major source of polycyclic aromatic hydrocarbons to these ponds, and concentrations of carcinogenic benzo[a]pyrene (B[a]P) equivalents were influencing management options about pond maintenance. For the second component of this study, a complex mixture of 13 metal(loid)s, 4-nonylphenols, 8 brominated diphenyl ethers (BDEs), and total polybrominated diphenyl ethers (PBDEs) were detected in all surficial samples. Contaminants with detection frequencies ≥ 20% included: silver (46.7%), beryllium (20.0%), chloride (60.0%), bis(2-ethylhexyl)phthalate (60.0%), 10 per- and polyfluoroalkyl substances (PFASs; 26.7–80.0%), 4-nonylphenol monoethoxylate (66.7%), 4-nonylphenol diethoxylate (40.0%), bifenthrin (20.0%), total permethrins (33.3%), and 24 other BDE congener groups (20.0–93.3%). Five stormwater ponds had contaminants exceeding benchmarks likely to be associated with harmful effects to benthic organisms. Ponds with watersheds dominated by either commercial and/or industrial land uses had significantly higher (p < 0.05) concentrations of zinc, 4-nonylphenol, six BDEs (28 + 33, 47, 99, 100, 154, and 209), and total PBDEs than those dominated by residential land uses. Multivariate statistical analyses verified that updated B[a]P equivalents were an effective chemical proxy for making management decisions about excavated pond sediment. Jurisdictions that do not test their stormwater pond sediments prior to maintenance dredging should consider the environmental ramifications of applying this potentially contaminated material to land.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The application and use of stormwater ponds was pioneered in Minnesota during the 1980s when untreated stormwater runoff was one of the leading sources of nonpoint source pollution to Minnesota’s waterways (Crane et al. 2010). Since that time, stormwater ponds have become a popular best management practice (BMP) for the treatment of urban stormwater runoff in developed countries (Weiss et al. 2008). The ponds act to slow the flow of water to allow suspended sediment and particle-associated contaminants to settle out. In addition, these ponds are used for stormwater detention and flood control (Minnesota Stormwater Manual contributors 2017). Although new stormwater ponds are constructed, natural ponds and/or wetlands have historically been used for the same purposes when stormwater was diverted to them. New stormwater diversions to natural waterways are no longer allowed in Minnesota, but some historical diversions are still in place. Constructed ponds usually have a design life of approximately 20–30 years, and large-scale sediment removal is suggested every 8–12 years (Crane et al. 2010). In the United States, publicly owned stormwater ponds are considered part of the Municipal Separate Storm Sewer System (MS4) when located within a MS4 boundary. MS4s are regulated through National Pollutant Discharge Elimination System permits administered by state agencies through the federal Clean Water Act (USEPA 2018). For example, the Minnesota Pollution Control Agency (MPCA) regulates the stormwater discharges of 252 municipalities, universities, and other entities. A single municipality may have oversight of hundreds of stormwater ponds through their MS4 permit, for which the MPCA requires pond sediments to be analyzed for a suite of polycyclic aromatic hydrocarbons (PAHs), arsenic, and copper prior to sediment removal actions (Berger et al. 2017). However, other jurisdictions in the United States and Canada vary widely in how they manage stormwater pond sediments (Polta 2004), including some that have no requirements for chemical testing of pond sediments prior to excavation and land application (e.g., South Carolina; Weinstein et al. 2010).
Municipalities in Minnesota have been stymied by the discovery of high concentrations of carcinogenic PAHs in many stormwater ponds, which would require expensive disposal of the excavated sediment in specially lined landfills (Crane 2014; Crane et al. 2010). This finding has resulted in delayed maintenance of stormwater ponds that are filling up with sediment and losing water storage capacity. Although PAHs are commonly found in urban sediments due to their hydrophobic properties and ubiquitous sources (Burgess et al. 2003), the reasons for the high PAH concentrations in Minnesota’s stormwater ponds were unknown. In the first component of this study, a contaminant mass balance receptor model was used to determine that coal tar-based sealant (CT-sealant) particulate washoff and dust sources were the most important sources of PAHs (67.1%) to 15 stormwater pond sediments in the Minneapolis–St. Paul, MN (Twin Cities) metropolitan area, followed by vehicle-related sources (29.5%) and pine wood combustion particles (3.4%; Crane 2014). After 29 municipalities in Minnesota passed local bans on the use of CT-sealants, the State of Minnesota became the second state in the country on January 1, 2014 to prohibit the statewide sale and use of CT-sealants (Crane 2014). Nationwide, sediment PAH concentrations are expected to decrease over several decades after local and/or statewide bans on CT-sealants have been enacted (Mahler et al. 2012; Pavlowsky 2012; Van Metre and Mahler 2014).
Contaminants of emerging concern (CECs) could pose additional challenges for the management of stormwater pond sediments. CECs have varying definitions (Diamond et al. 2011; Sauvé and Desrosiers 2014) but will be generally defined here as “synthetic or naturally occurring chemicals that are not regulated or commonly monitored in the environment but have the potential to enter the environment and cause adverse ecological or human health impacts” (Sutton et al. 2017). In the Twin Cities metropolitan area, 123 CECs were recently detected in stormwater samples, including commercial-consumer compounds, veterinary and human pharmaceuticals, lifestyle and personal care compounds, pesticides (e.g., acetochlor and atrazine), and other compounds (Fairbairn et al. 2018). Stormwater runoff elsewhere also has been found to contain other CECs, such as alkylphenols (APs, Zgheib et al. 2011; Sébastian et al. 2015), brominated diphenyl ethers (BDEs, Sébastian et al. 2015), nonylphenol ethoxylates (NPEs, Sébastian et al. 2015), and pyrethroids (Weston et al. 2009). Markiewicz et al. (2017) reviewed the sources of organic pollutants (including several CECs) in road and traffic environments, and they prioritized a list of significant sources of organic pollutants to Swedish road runoff as: PAHs > alkanes C20–C40 > APs > phthalates > aldehydes > phenolic antioxidants > bisphenol A > oxygenated-PAHs > naphtha C5–C12 > amides > amines. Also in Sweden, several phthalates, 4-nonylphenol (4-NP), and NPEs have been detected in three stormwater sedimentation chambers from urban areas (Björklund et al. 2009). While information is emerging on a wide variety of chemical substances transported in urban runoff, little data exist regarding the detection and distribution of CECs in stormwater pond sediments.
A short list of CECs that would be of potential concern in Minnesota’s stormwater pond sediments due to their persistence, toxicity to aquatic life, and use in a range of consumer products and/or commercial/industrial applications include per- and polyfluoroalkyl substances (PFASs), APs and NPEs, pyrethroids, and flame retardant compounds, such as polybrominated diphenyl ethers (PBDEs) and specific BDE congeners. All of these CECs are classified as endocrine-disrupting compounds (EDCs; Rudel et al. 2003; Brander et al. 2016; Braun 2017).
PFASs are a class of manufactured, fluorinated, organic compounds with novel physical and chemical properties that make them both lipid- and water-repellent and extremely persistent in the environment (Lindstrom et al. 2011; OECD 2013). PFASs have been detected in the environment, wildlife, and humans (Buck et al. 2011). The two PFAS compounds that are most important in terms of past production are perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). The 3M Company produced these compounds at their Cottage Grove facility in the southeast portion of the Twin Cities until 2002 (Paul et al. 2009). By 2015, eight companies, including 3M, eliminated PFOA and long-chain PFAs from their emissions and products (https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program, accessed 14 September 2018).
Nonylphenols (NPs) are one group of APs that are used mainly to produce NPEs, both of which are high production chemicals. NPEs are nonionic surfactants, which have a hydrophilic head that attracts water and a hydrophobic tail that attracts poorly soluble substances like oils and greases (USEPA 2010). Their use in consumer detergents has largely been eliminated, although NPEs are used in other industrial applications and consumer products that lead to releases to the aquatic environment (USEPA 2010). The environmental fate of these compounds has been reviewed by Ying et al. (2002). Chokwe et al. (2017) provided a review of the distribution, exposure pathways, sources, and toxicity of NPs and NPEs in the environment. In 2014, the U.S. Environmental Protection Agency (EPA) proposed a Significant New Use Rule, under the Toxic Substances Control Act, to require manufacturers to provide 90-day notice before commencing or resuming any significant new use of 15 NP/NPEs that are no longer used in commerce, including 4-NP and 4-NP diethoxylate (4-NP2EO; EPA 2014). Canada and the U.S. proposed NPs and NPEs as chemicals of mutual concern in the Great Lakes basin in 2014, although insufficient information was available to apply the Binational Considerations for further action (Identification Task Team 2015).
Pyrethroids are the synthetic version of pyrethrins, which are derived from a species of chrysanthemum (Palmquist et al. 2012). Both groups of compounds are broad-spectrum insecticides used worldwide to control a variety of agricultural and urban pests (Li et al. 2017), but pyrethroids are much more photostable and persistent in the environment (ATSDR 2003). Both types of compounds are neurotoxins that work by binding to sodium channels in the target organisms, resulting in hyperexcitation of the nerve cell that eventually shuts down insects’ nervous systems (Davies et al. 2007). Piperonyl butoxide often is added as a synergist to block the insects’ detoxification enzymes and subsequently increase the efficacy of these compounds (ATSDR 2003). First generation pyrethroids (e.g., allethrin) are mainly used for indoor applications, whereas photostable second generation pyrethroids (e.g., bifenthrin, permethrin) are registered for use in agricultural and nonagricultural applications outdoors (Li et al. 2017). The use, environmental fate, and ecotoxicology of pyrethroid insecticides has been described by Palmquist et al. (2012), whereas Li et al. (2017) have provided an overview of the global occurrence of pyrethroids in sediment along with their high toxic impacts on benthic invertebrates.
PBDEs are organobromine compounds that are similar to PCBs in their chemical structure, environmental persistence, and bioaccumulation propensity (Hooper and McDonald 2000). Commercial mixtures of PBDEs (i.e., pentaBDE, octaBDE, and decaBDE) have been used as flame retardants to slow the burning of plastics, foam, fabrics, electronics, and other products (USEPA 2009). The use of certain PBDE homologs has been restricted in Minnesota and nationwide (Cordner et al. 2013). The sole U.S. manufacturer of pentaBDE and octaBDE voluntarily ceased production in 2004 (USEPA 2009). Beginning in 2008, the State of Minnesota prohibited the manufacturing, processing, or distribution of products which contained more than 0.1% of pentaBDE or octaBDE (2007 Minn. Stat. 325E.386; https://www.revisor.mn.gov/statutes/cite/325E.386; accessed 18 September 2018). The U.S. EPA negotiated with the two U.S. producers of decaBDE, as well as the largest U.S. importer, to phase out this product by the end of 2013 (USEPA 2014). In 2015, Minnesota restricted the future use of four flame retardants, including: decaBDE, tris(1,3-dichloro-2-propyl)phosphate (TDCPP), tris(2-chloroethyl)phosphate (TCEP), and hexabromocyclododecane (HBCDD). Beginning on July 1, 2018, this law banned the manufacture or importation of children’s products and residential upholstered furniture containing these chemicals greater than 1000 parts per million, and a ban on the sale of such products in Minnesota will take effect on July 1, 2019 (2015 Minn. Stat. 325F.071; https://www.revisor.mn.gov/statutes/cite/325F.071; accessed 18 September 2018). The replacement flame retardants for commercial mixtures of pentaBDE, octaBDE, and decaBDE are of emerging concern. For example, hexabromobenzene (HBB), pentabromoethylbenzene (PBEB), and other emerging halogenated and nonhalogenated flame retardants have been detected in mostly low levels in the livers of top predator glaucous gulls in the Eastern Canadian Arctic (Verreault et al. 2018). These results indicate these compounds are bioaccumulating in biota.
This paper provides another component of the study reported by Crane (2014), which featured the source apportionment and distribution of PAHs in 15 stormwater ponds from the Twin Cities, MN metropolitan area. Ancillary data for total organic carbon (TOC), black carbon, and particle size classes also were provided in Crane (2014). In this paper, additional analytical data obtained from split, composite sediment samples will be described. The objectives of this part of the study were to: (1) determine the distribution of a suite of conventional contaminants and CECs in the pond sediments; (2) evaluate whether ecological and human health benchmarks for sediment were exceeded, including an update of the benzo[a]pyrene (B[a]P) equivalent calculations provided in Crane (2014); (3) determine the influence of major urban watershed land uses (i.e., residential, commercial, and industrial) on contaminant concentrations; (4) utilize multivariate statistical techniques to determine the subset of contaminants that accounted for most of the variance in the data set and to identify similar groups of ponds; and (5) evaluate implications for stormwater management, including dredge disposal options and applications to other jurisdictions. The conventional contaminants included 19 metal(loid)s, chloride, and semivolatile organic compounds (SVOCs), such as phthalates. The CECs included 13 PFASs, 2 APs, 2 NPEs, 14 pyrethroids, 6 pyrethrins, piperonyl butoxide, 40 BDE groups, and 4 non-BDE brominated flame retardants (BFRs). This study appears to be the most comprehensive one to date regarding CECs in urban stormwater pond sediments.
Materials and Methods
Sample Collection
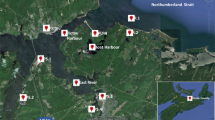
The sampling design and collection of individual and composite sediment samples from 15 stormwater ponds in the Twin Cities, MN metropolitan area were described in Crane (2014). In brief, five ponds each from primarily residential, commercial, and industrial land use areas were sampled with a modified drop corer during October 2009 (Fig. 1; Table S1). Composite samples were comprised of approximately equal portions of three discrete samples of the upper 15 cm of sediment collected from an equidistant transect across each pond. Each composite sample was mixed in a 1.9-L precleaned Pyrex mixing cup. Field replicates were collected from two ponds.
Location of composite stormwater pond sites (SWP09-) in the Minneapolis–St. Paul, MN metropolitan area (Crane 2014)
The composite (“C”) samples were distributed to four different sample jars at each pond. The jars included a 250-mL Nalgene jar [metal(loid)s, mercury, and chloride], a 125-mL, precleaned amber wide-mouth glass jar (SVOCs), a 125-mL HDPE jar (PFASs), and a 250-mL amber glass jar with a Teflon-lined lid (other CECs). The jars were stored on ice in coolers while in the field.
The samples were transported to the MPCA each day where they were stored until ready for shipment to three analytical laboratories. The sediment samples were either refrigerated (3 °C) or frozen (− 18 °C) in the case of the 250-mL CEC jars (to preserve the samples for pyrethroids). All of the samples, and their respective sample tracking forms, were transported in chilled coolers to two local laboratories and via overnight courier to AXYS Analytical Services Ltd. in Sidney, BC, Canada for the PFASs and other CECs.
Analytical Extraction and Instrumental Analysis
Metal(loid)s and Chloride
The Minnesota Department of Health’s Environmental Laboratory in St. Paul, MN was used to analyze sediment samples for a suite of 18 metal(loid)s, mercury, and chloride. Metal(loid)s were analyzed by inductively coupled plasma—mass spectrometry using reference method SW6020. Mercury was analyzed by atomic absorption cold vapor spectrometry using reference method SW7471. Chloride was analyzed using ultraviolet or visible molecular absorption spectrometry by EPA Method 325.2. Percent moisture was determined by gravimetry on each sample using reference method ASTM D2216.
SVOCs
The analysis of SVOCs, including phthalate ester plasticizers, was conducted by Pace Analytical Services, Inc. in Minneapolis, MN. The samples (30 g) were extracted using EPA Method 3550. Six surrogate standards were added to the sample extracts, which were analyzed by EPA Method 8270C using an Hewlett Packard (HP) 5973 gas chromatography/mass spectrometry (GC/MS) system. Moisture was determined using ASTM D2216.
CECs
The preparation and analysis of all CEC samples followed standard operating procedures developed by AXYS Analytical Services, Ltd. Once AXYS received the samples, a turn-around-time of 45 days was expected for PFASs and 8 weeks for the other parameters. The pyrethroid samples were stored at − 20 °C before sample preparation and analysis.
Sediment samples were analyzed for 13 PFASs by AXYS Method MLA-041. In this method, the environmentally relevant conjugate base anions were analyzed. An accurately weighed sample (up to 5 g dry wt.) was spiked with isotopically labeled quantification standards and extracted in acetic acid and basic methanol. The extract was cleaned up using ultra-pure carbon powder followed by solid phase extraction cartridges. After spiking with labeled internal standards, the extract was analyzed using high performance liquid chromatography coupled to a triple quadrupole MS (LC–MS/MS). Analyte concentrations were determined by isotope dilution/internal standard method, comparing the area of the quantification ion to that of the 13C-labelled standard and correcting for response factors. Linear quantification equations with 1/X2 weighting fit were determined from a multipoint calibration series prepared alongside the samples.
Alkylphenols and NPEs were determined by AXYS Method MLA-004 for 4-NP, 4-n-octylphenol, 4-nonylphenol monoethoxylate (4-NP1EO), and 4-NP2EO. A surrogate standard (13C-p-NP) was added to an approximately 5-g sediment sample, digested in methanolic potassium hydroxide, extracted with hexane at pH 6, and acetylated to convert the analytes to their acetate derivatives. The extract was then cleaned up on a silica column, reduced in volume, and spiked with d10-pyrene recovery standard before analysis. The extracts were analyzed by high-resolution GC/low-resolution MS (HRGC/LRMS) using a Restek RtX-5 capillary column (30 m, 0.25-mm i.d. × 0.25-µm film thickness) or equivalent for chromatographic separation. The MS was operated at a unit mass resolution in the electron ionization mode using multiple ion detection acquiring at least two characteristic ions for each target analyte and surrogate standard. Target analyte concentrations were determined by isotope dilution (internal standard) quantification procedures. The totals of 4-NPs, 4-NP1EOs, and 4-NP2EOs were each quantified as the sum of several individual marker peaks in the HRGC/LRMS chromatogram.
Sediment samples were analyzed for 14 pyrethroids, 6 pyrethrins, and the synergist piperonyl butoxide by AXYS Method MLA-046. Approximately 2 g (wet weight) of the samples were spiked with 13C-labeled cypermethrin surrogate and Soxhlet extracted with dichloromethane. Each extract was split into unequal portions: 1/10th for analysis and 9/10th for backup. The portion for analysis was cleaned up using chromatography columns. The resulting extracts were reduced in volume and spiked with recovery (internal) standard before instrumental analysis. The quantification procedures accounted for the splitting of the extract, and the final results are in terms of the original sample size extracted. The samples were analyzed on a HRGC equipped with a DB-5 capillary column and coupled to a high-resolution MS (HRMS). The HRMS was operated in a static (≥ 8000) mass resolution in the electron ionization mode using voltage selected ion detection. The initial calibration was performed using a five-point calibration series of solutions that encompassed the working concentration range. The mid-level calibration solution was analyzed as a bracketing calibration, run before and after the batch of samples. Due to matrix interferences from hydrocarbons, the pyrethroid samples required repeat analysis from a smaller sample size that had additional surrogate quantification solution added. The data were not blank corrected. Because isotope dilution/internal standard quantification procedures were used, the sample data were recovery corrected for possible losses during extraction and clean-up.
All of the pyrethroid standards were obtained as a technical mixture of the stereoisomers, except for bifenthrin and fenpropathrin. The concentrations of the individual isomers in the technical mixture were assigned by assuming the same response factors. Pyrethrins were obtained as a technical mixture of the six naturally occurring components, where the concentrations of the individual components were estimated to be equal. The concentrations of pyrethroids were reported as the sum of the concentrations of the observed isomers. Since the first eluting isomer of flucythrinate (flucythrinate A) coelutes with the last eluting isomer of cypermethrin (cypermethrin D), an additional ion for flucythrinate was monitored in the channel for cypermethrin. Due to conversion of tralomethrin to deltamethrin in the GC injector, these two analytes were detected and reported as a sum. The pyrethrins were reported as the sum of cinerin I, cinerin II, jasmolin I, jasmolin II, pyrethrin I, and pyrethrin II. These compounds degrade rapidly when exposed to light, heat, or microbial action (ATSDR 2003). Consequently, the accuracy of quantification is unknown for these pyrethrins.
Forty BDE groups and four non-BDE BFRs were analyzed by AXYS Method MLA-033, which is based on EPA Method 1614. The semiquantitative analysis of the four non-BDE compounds included: 1,2-bis(2,4,6-tribromophenoxy)ethane (BTBPE), decabromodiphenylethane (DBDPE), HBB, and PBEB. Approximately 10 g (dry weight) of the sediment samples were spiked with 13C-labeled BDE surrogates and Soxhlet extracted with dichloromethane. The extract was cleaned up on a series of chromatographic columns, which could include layered acid/base silica, Florisil, and alumina. During extract cleanup, samples 03-C, 05-C, and 12-C-R were inadvertently lost. These samples were repeated from fresh aliquots of sediment, and the data were reported separately in two batches. Most of the samples required additional cleanup and a dilution due to the presence of interferences. The final extract was spiked with isotopically labeled recovery (internal) standards before instrumental analysis. The extracts were analyzed by HRGC/HRMS.
Quality Assurance/Quality Control
Quality control (QC) samples were included in each batch of field samples to assess the precision and accuracy of the results. These samples varied by laboratory and could include analytical duplicates, laboratory blanks, surrogate standards, laboratory control samples, and matrix spike/matrix spike duplicates. The QC sample results for each group of analytes were acceptable, with the exceptions noted in the electronic supplementary material (ESM; Tables S2–S16).
Calculated Benchmarks
Ecological and human health-based benchmarks were calculated on groups of similar classes of chemicals, as well as on a mixture of contaminants for benthic invertebrates. Mean probable effect concentration quotients (PEC-Qs) were calculated on a dry weight basis to distill data from a mixture of metal(loid)s (i.e., arsenic, cadmium, chromium, copper, lead, nickel, and zinc) and ∑PAH12 into one unitless index (Crane and Hennes 2007). The 12 parent PAHs included in ∑PAH12 were identified in Crane (2014) and were not normalized to TOC (Crane and Hennes 2007). The calculation of mean PEC-Qs involved three steps for chemicals with reliable probable effect concentrations (PECs, MacDonald et al. 2000). These PECs were adopted by the MPCA as Level II sediment quality targets (SQTs) for benthic invertebrates (Crane et al. 2000, 2002; Crane and Hennes 2007). First, individual PEC-Qs were calculated by dividing each chemical or chemical group concentration by the corresponding PEC value. Second, the individual PEC-Q values for metal(loid)s were summed and divided by seven to yield the mean PEC-Qmetal(loid)s. Third, the mean PEC-Qmetal(loid)s and PEC-Q∑PAH12 values were summed and divided by two to yield the mean PEC-Q value for each composite sample.
Toxic units (TUs) were calculated for detected concentrations of bifenthrin and permethrin, which were summed to provide TUs for total pyrethroids. The bifenthrin and total permethrin data were first normalized to organic carbon (OC) using the mean TOC data from Crane (2014) for split sediment samples. Next, the 10-d whole sediment median lethal concentration values (LC50’s) for the amphipod Hyalella azteca, that were determined from spiked sediment toxicity tests for both pyrethroids, were obtained from the peer reviewed literature for two different test temperature ranges (i.e., 23 °C and 15/18 °C; Tables S17 and S18). Mean LC50 values were then calculated (Tables S17 and S18). The TUs for each pyrethroid were calculated as:
Nonylphenol toxic equivalents (TEQs) were calculated using Canadian methods for the protection of freshwater aquatic life (CCME 2002). Concentration values of APs and NPEs less than the associated reporting limits were substituted as zero. The total NP TEQs were calculated by summing the products of the toxic equivalency factors (CCME 2002) by the concentrations of the respective APs and NPEs.
The human health-based B[a]P equivalents provided in Crane (2014) were recalculated based on updated procedures (Berger et al. 2017) and a combination of overlapping carcinogenic PAH (cPAH) data (Crane 2014). The number of cPAHs included in the calculation has been reduced from 24 cPAHs to 17 cPAHs (Berger et al. 2017). Because benzo[b]fluoranthene and benzo[j]fluoranthene often coelute as benzo[b&j]fluoranthene, the number of cPAHs was reduced to 16 for the recalculated values. In addition, the Kaplan–Meier method (Helsel 2010) is now being used to calculate B[a]P equivalents on data sets with ≥ 20% detects, instead of using substitution methods to sum the B[a]P equivalents for each sample (Berger et al. 2017). The same potency equivalency factors (Table S19; Crane 2014; Berger et al. 2017) were used to multiply by the individual cPAH concentrations to determine B[a]P equivalent values. For each composite sediment sample, concentration data for seven cPAHs from the ∑PAH34 data set (Crane 2014; Table S19) were combined with the data for nine other cPAHs from the original cPAH data set (Crane 2014; Table S19) in order to calculate revised B[a]P equivalents. This step was performed, because the laboratory that performed the ∑PAH34 analyses had a more rigorous clean-up step that resulted in lower reporting limits than the laboratory that performed the original cPAH analyses (Crane 2014). Thus, some cPAHs that were reported as nondetects in the original cPAH data set were detected in the ∑PAH34 data set.
Statistical Analyses
The data were compiled in an internal Microsoft Access 2010 sediment quality database, and either the queried or original data files were used for subsequent statistical analyses. Data that were either J-flagged from the analytical laboratory, or were determined to be estimated based on a QA/QC review of the data, were treated as detected values in the statistical analyses. Summary statistics were run in either Microsoft Excel® 2010 or 2016 (Microsoft Corporation, Redmond, WA), SigmaPlot 13.0 (Systat Software, Inc., San Jose, CA), or the U.S. EPA’s ProUCL 5.1 software. The Kaplan–Meier method (Helsel 2012) was used in ProUCL 5.1 to calculate total values of PFAS and PBDE compounds containing ≥ 20% detects (i.e., the Kaplan–Meier mean was multiplied by the number of compounds to determine total values). The Kaplan–Meier method (Helsel 2012) also was used in ProUCL 5.1 to calculate summary statistics for censored data sets with ≥ 20% detects. Box plots, quantile–quantile plots, and Dixon’s outlier tests at the 5% significance level were determined using ProUCL 5.1 for both 100% detected parameters and those with ≥ 20% detects. Box plots, bar charts, and linear regression graphs were assembled for selected parameters in SigmaPlot 13.0. Shapiro–Wilk normality tests and Pearson product moment correlations (including the use of transformed variables to meet normality requirements) were run in SigmaPlot 13.0.
The data were compared to corresponding ambient sediment quality data collected from 54 mostly randomly selected lakes located across Minnesota (Crane 2017). In particular, the 95% upper tolerance limits with 95% coverage (UTL95-95) values for a select group of contaminants (Crane 2017) were used. Percentage exceedances of the ambient UTL95-95 values would most likely be due to natural geologic and/or anthropogenic influences on the surrounding watershed (Crane 2017).
Hypothesis testing was used to compare selected data sets by major watershed land uses. The null hypothesis was identified as no significant (p > 0.05) differences in either contaminant concentrations or the detection frequency of 10 PFAS compounds. These scenarios encompassed stormwater ponds located in primarily residential (n = 5), commercial (n = 5), and industrial (n = 5) watersheds. This approach utilized the Shapiro–Wilk normality test and the Brown-Forsythe equal variance test in SigmaPlot 13.0 to determine if the raw or transformed data for each parameter passed (p > 0.05). If so, a one-way Analysis of Variance (ANOVA) was run. Statistically significant results (p < 0.05) triggered pairwise multiple comparisons run by the Holm-Sidak method. A Kruskal–Wallis one-way ANOVA on Ranks was run on data that did not pass the normality and/or equal variance tests. If the result was statistically significant (p < 0.05), the Tukey test was used to conduct pairwise multiple comparisons.
Multivariate statistical methods were used for two main purposes. First, to determine if B[a]P equivalents were one of the most important factors accounting for variance in the data set when several detected parameters were considered. Second, to determine if ponds within the same land use category had similar chemical profiles. Principal components analysis (PCA) was run in SigmaPlot 13.0 to reduce the complexity of numerous parameters down to its most important components. Although PCA is a large sample statistical technique that is best used on a minimum of 50 samples (Osborne and Costello 2004), it was used on the composite sediment samples from the 15 stormwater ponds for the focused purposes described here. In PCA, a large number of correlated variables are transformed to a new set of uncorrelated reference variables (i.e., principal components). The first principal component accounts for the largest amount of variability in the data, and each succeeding component accounts for as much of the remaining variability as possible. All of the detected and calculated data selected for PCA analyses underwent a range transformation (also known as the maximum-minimum transformation; Johnson et al. 2004) to reduce variance in the data. This step resulted in unitless data so that a covariance matrix could be run for each PCA scenario. The significance level for hypothesis testing was 0.05. The number of components was varied, and the Henze–Zinker normality test was used with a p value to reject of 0.05. Scree plots, component loading plots, and component score plots were produced for each PCA scenario. The principal component scores were used to prepare three-dimensional plots in SigmaPlot 13.0. To designate discrete groups on the plots, the principal component scores were run through hierarchical cluster analysis (HCA) using PAleontological STatistics (PAST) version 3.20 software. The best HCA option for identifying discrete groups was the Paired Group (UPGMA) algorithm with the Gower similarity index (as also used in Crane 2017).
Minnesota Pesticide Sales Data for Pyrethroids
Annual sales data of pyrethroids in Minnesota were obtained from the Minnesota Department of Agriculture’s pesticide sales database (http://www.mda.state.mn.us/chemicals/pesticides/useandsales.aspx; MDA 2013). These data are based on pesticide registrant reporting requirements. The data were converted to kg active ingredient, as well as the percentage of pyrethroids in different sales categories. An important caveat to these data is that not all of the pesticides sold in Minnesota may be used in the same year they are sold or in some cases may never be used in Minnesota (MDA 2013). Although sales data specific to the Twin Cities metropolitan area were not available, these data can be used to provide general indications of the sale of pyrethroids for agricultural and nonagricultural (including urban) purposes.
Pyrethroids Used to Control Mosquitoes in the Twin Cities
The Metropolitan Mosquito Control District (MMCD) utilized permethrin and resmethrin as part of their mosquito control strategy (MMCD 2010) during the time of sample collection. The amount of active ingredient that was applied on a yearly basis was obtained from the MMCD, and the locations of targeted areas during the 2009 application season were compared to the study pond areas for potential overlap.
Results and Discussion
Distribution of Contaminants
A mixture of conventional contaminants and CECs were detected in stormwater pond sediments from the Twin Cities metropolitan area. Summary statistics for contaminants with ≥ 20% detections are provided in Table 1, while those with more censored data are provided in Table S20.
Metal(loid)s
Thirteen metal(loid)s were detected in all 15 stormwater ponds (Table 1). Of this group, copper and zinc exceeded their corresponding ambient UTL95-95 values in 60% of ponds, followed by cadmium and nickel in 40% of ponds (Table 1). Although some metals (e.g., copper, nickel) are naturally elevated in portions of northeast Minnesota (Crane 2017), this is not the case in the Twin Cities area where urban sources of metal(loid)s prevail. In particular, vehicles are an important source of heavy metals to urban road dust and stormwater runoff (Hwang et al. 2016). Nickel is released in large amounts during the combustion of oil (Pacyna and Pacyna 2001), and it is a common contaminant released from coal combustion and automotive parts (Landers et al. 2008). The exceedances of copper, zinc, and cadmium were consistent with the composition of urban runoff in Maryland where Davis et al. (2001) determined building siding was an important source for all three metals (as well as lead). In addition, copper from brake pads, zinc from tire wear, and atmospheric deposition of cadmium and copper (as well as lead) also were important sources of metals to urban stormwater in Maryland (Davis et al. 2001). Cadmium, zinc, and nickel also are used in brake plating and could contribute stormwater-relevant concentrations (McKenzie et al. 2009). In 2015, a Memorandum of Understanding to reduce the use of copper and other materials in motor vehicle brake pads was signed by the U.S. EPA, states, and the automotive industry after California and Washington passed requirements to reduce the use of copper in brake pads (https://www.epa.gov/npdes/copper-free-brake-initiative, accessed 10 May 2018). This copper-free brake initiative will reduce copper in brake pads to < 5% by weight in 2021 and 0.5% by 2025. This voluntary initiative will also reduce mercury, lead, cadmium, asbestiform fibers, and chromium-six salts in brake pads. Thus, this action will reduce future runoff loads of these metals to stormwater ponds across the United States.
The concentrations of the other metal(loid)s included in this study were similarly compared to corresponding UTL95-95 values, and potential sources of more prevalent metals were determined. Smaller exceedances of the UTL95-95 values were observed for aluminum (13.3%), lead (13.3%), chromium (6.7%), and cobalt (6.7%; Table 1). Historical trends of lead in traffic area runoff have shown a sharp decrease in recent decades due to the phase-out of leaded gasoline (Huber et al. 2016), which is reflected in historical sediment cores collected from urban lakes across the U.S. (Mahler et al. 2006). However, there continue to be other vehicle-related and building sources of lead in the environment as previously discussed. Pond samples 02-C, 06-C, and 12-C had statistical outliers for cobalt, arsenic, and chromium, respectively (Table 2). Concentrations of arsenic, barium, manganese, mercury, and vanadium were all less than the corresponding UTL95-95 values, indicating these values were within 95% of the population values expected to fall with 95% confidence for a set of Minnesota lake sediments (Crane 2017). The majority of silver and beryllium analyses were not detected in the pond sediments (Table 1), and the high exceedances of the UTL95-95 values for these metals were due to reporting limits that exceeded the corresponding UTL95-95 values. Molybdenum was only detected in two ponds, and there were no detections of antimony, selenium, and thallium (Table S20). The metal(loid)s were an important component of the mean PEC-Q values, which exceeded the corresponding UTL95-95 value in 60% of the ponds (Table 1). In addition, the mean PEC-Q value of 3.0 in pond 05-C was a statistical outlier (Table 2).
The particle size distribution and organic carbon results from split sediment samples (Crane 2014) were compared with the chemical parameters detected in all of the composite sediment samples. Of immediate interest are the correlations with the metal(loid)s. Natural log (ln) transformations were used, where needed, so that all of the parameters passed the Shapiro–Wilk normality test (Table S21). The ln median size of particles had the greatest number (n = 8) of significant (p < 0.05) Pearson correlation coefficients with metal(loid)s (Fig. 2; Table S22). These correlations were negative, as were the significant (p < 0.05) Pearson correlation coefficients between % sand and five metal(loids). The other correlations for this group of parameters were positive. TOC had the least number (n = 1, mercury) of significant (p < 0.05) Pearson correlation coefficients (Fig. 2; Table S22). Aluminum, cobalt, and nickel all had the highest number (n = 5) of significant (p < 0.05) Pearson correlation coefficients, while arsenic and lead were not correlated to any particle size and organic carbon parameters (Fig. 2; Table S22).
Detailed size fractions of the particle size data (Crane 2014) also were compared with the metal(loid)s and the other chemical parameters detected in all of the composite sediment samples. The results of the Shapiro–Wilk normality tests (using transformed parameters, where needed) and Pearson product moment correlations are provided in Tables S23 and S24, respectively. Of note is that chromium, which did not have significant (p > 0.05) Pearson correlation coefficients to the general particle size fractions, had a significant (p < 0.05) Pearson correlation coefficient to % medium silt (5.0–20 µm; Table S24). For ln aluminum, cobalt, and nickel, all of these metals had significant (p < 0.05) Pearson correlation coefficients to % medium clay (0.08–0.2 µm), % coarse clay (0.2–2 µm), % fine silt (2.0–5.0 µm), and % medium silt (Table S24). Vanadium also had significant (p < 0.05) correlations to % fine silt and % medium silt (Table S24). In addition, barium had a significant (p < 0.05) correlation to % medium silt (Table S24). For the detailed particle size classes, % medium silt had the greatest number (n = 6) of significant correlations with metals (Table S24). Other significant (p < 0.05) correlations are noted in Table S24 for transformed values of % fine sand (100–250 µm) and % medium sand (250–500 µm). There were no significant (p > 0.05) correlations between the detailed particle size fractions and ln arsenic, ln cadmium, copper, lead, manganese, mercury, or zinc (Table S24).
Several significant correlations were observed between detected metals (Fig. 2; Table S22). Highly significant (p < 0.001) Pearson correlation coefficients were observed for nine pairs of metals, including cobalt and nickel (r = 0.927), ln aluminum and vanadium (r = 0.907), ln cadmium and chromium (r = 0.870), ln aluminum and barium (r = 0.844), ln cadmium with nickel (r = 0.837) and copper (r = 0.810), barium and vanadium (r = 0.797), chromium and nickel (r = 0.776), and ln cadmium and mercury (r = 0.765; Fig. 2; Table S22). Similar strong correlations were observed in a statewide survey of ambient sediment quality in Minnesota lakes for cobalt and nickel (Spearman rho = 0.887), as well as between aluminum and vanadium (r = 0.886; Crane and Hennes 2016). Cobalt compounds naturally occur in copper and nickel minerals (Shedd 2017), which accounts for their strong correlations. In addition, cobalt, nickel, and iron all have similar chemical and physical properties (Zhou and Guo 2015). Chromium, cobalt, and nickel had the largest number of significant (p < 0.05) correlations with eight metals each (Table S22). Lead, ln arsenic, manganese, and zinc were not significantly (p > 0.05) correlated to any other metal(loid)s (Fig. 2; Table S22).
Total PAH data and Equilibrium Partitioning Sediment Benchmark (ESB) Toxic Unit values from Crane (2014), as well as the other sediment benchmarks presented in this paper, were compared to the metal(loid)s and the other detected chemical parameters. The results of the Shapiro–Wilk normality tests (using transformed parameters, where needed) and Pearson product moment correlations are provided in Tables S25 and S26, respectively. Both ln ∑PAH12 and ln ∑PAH34 were significantly (p < 0.05) correlated to copper and zinc (Table S26), which indicated co-occurrence of these common urban stormwater contaminants. The ln of ESB Toxic Units was also significantly (p < 0.05) correlated to zinc (Table S26). Subsequent sections will describe significant correlations between certain metal(loid)s with 4-NP, BDE congeners, total PBDEs, and other sediment benchmarks.
Chloride
Chloride is not often analyzed in sediments due to its aqueous solubility. However, it was detected in 60% of pond sediments with concentrations ranging from 190 to 1100 mg/kg dry wt. (Table 1; Fig. S1). The highest chloride concentration was found at site 04-C (Fig. S1), which receives runoff from the parking lots for several retailers, commercial buildings, and a fast food restaurant in Lakeville, MN. Elevated chloride levels have also been found in surficial wetland sediments from the Twin Cities metropolitan area, in Alexandria, MN, and in agricultural areas of Minnesota (Crane and Hennes 2016). Deicing salt was the most likely source of chloride to these Twin Cities wetlands (Crane and Hennes 2016), as well as to the study ponds. Chloride is of concern, because it can make surface water and groundwater more saline (Snodgrass et al. 2017), resulting in adverse ecological effects (Bartlett et al. 2012; Van Meter and Swan 2014) and drinking water impacts (MPCA 2016a). It also can cause density stratification in small lakes (MPCA 2016a). Brand et al. (2010) observed decreased survival of gray treefrog embyos (Hyla versicolor), a common species to Minnesota, after elevated amounts of chloride leached from stormwater pond sediments into the overlying water of treatment microcosms. Although the Twin Cities metropolitan area has a chloride management plan to reduce the usage of salt (MPCA 2016a), it does not currently consider chloride concentrations in sediment. Lerman and Weiler (1970) showed that the amount of chloride accumulated in the upper 30 cm of Lake Ontario sediments was on the order of 1–2% of the amount of chloride in lake water. High salt concentrations were found in sediment porewater from an urban stormwater pond located in Ontario, Canada, which increased the concentrations of inorganic ligands (chlorides) capable of complexing with cadmium (Mayer et al. 2008). More work is needed to assess fluxes of chloride to bulk sediment and pore water, particularly in waters exceeding aquatic life criteria for chloride.
SVOCs (Phthalates)
Phthalates were included in this study as an indicator of plastic pollution. Bis(2-ethylhexyl)phthalate (DEHP) was the only phthalate detected in nine of the ponds (i.e., 60%; Table 1; Fig. S2). Concentrations of DEHP in the stormwater pond sediments were one to two orders of magnitude higher than downstream of the Twin Cities in Sturgeon Lake (Lee et al. 2005), a backwater lake to Navigation Pool 3 of the Mississippi River. DEHP is a common pollutant in urban stormwater (Björklund et al. 2009; Zgheib et al. 2012). This high production chemical is used to increase the flexibility or durability of polyvinyl chloride, plastics, paints, cosmetics, wood varnish, and medical supplies (MDH 2011). DEHP also is detected frequently in recycled plastics (Pivnenko et al. 2016). Because DEHP is not incorporated into the plastic matrix, it slowly leaches into the environment (Carlisle et al. 2009). Nonpoint sources of DEHP contribute to its ubiquitous distribution in the environment (Wang et al. 2014). DEHP is of concern, because it is classified as a Group B2 probable human carcinogen (USEPA 1988), and it is an EDC (Rudel et al. 2003; Sutton et al. 2017). The U.S. EPA’s Toxic Release Inventory was assessed for point source release data of DEHP in Minnesota (https://iaspub.epa.gov/triexplorer/tri_release.facility, accessed 14 May 2018). During 1998 to 2005, yearly releases of DEHP from one to three businesses in the Twin Cities metropolitan area were reported, with most of it deposited in landfills. During 2006 to 2015, there were no releases of DEHP. In 2016, the 3M Cottage Grove plant in Cottage Grove, MN (i.e., southeast metro) released 20 kg of DEHP in surface water discharges. The minimum and maximum reporting limits of five other phthalates and other SVOCs analyzed in the stormwater pond sediments are provided in Table S20. The reporting limits of the SVOCs tended to be high due to the laboratory practice of diluting sample extracts to remove interferences.
PFASs
The PFASs displayed a range of detection frequencies (Table 1). PFOS was the most frequently detected compound (80%), and it also had the highest mean concentration of 0.84 ng/g dry wt. (Table 1). The highest PFOS concentration of 2.25 ng/g dry wt. was observed in pond 07-C (Fig. 3), which receives drainage from two strip malls, a fast food restaurant, other restaurants, a large retail store, road and parking lot runoff, and a residential area. Nine other PFASs were detected in four to ten ponds (Table 1; Figs. S3–S5), and the Kaplan–Meier mean concentration of total PFASs was estimated as 3.0 ng/g dry wt. (Table 1). These results mostly supported Zhao et al.’s (2012) findings in fractionated sediments that the concentrations of perfluorosulfonates were higher than perfluorocarboxylates (PFCAs) with the same chain length. This was especially the case for PFOS and perfluorooctane sulfonamide (PFOSA), in addition to perfluorohexane sulfonate (PFHxS) and perfluorohexanoate (PFHxA; Tables 1 and S20). The chain length also can impact the type of partitioning in sediments. Longer-chain PFASs are more likely to bind to particles through hydrophobic interactions, while the sorption of lower-chain PFASs may be driven by electrostatic interactions (Zhao et al. 2012). Although no PFASs were detected in all of the ponds, a Pearson product moment correlation was run between detected samples of PFOS, perfluorododecanoate (PFDoA), and ln PFOSA with black carbon, ln mean TOC, and detailed/general particle size fractions. The only significant (p < 0.05) correlation was between PFDoA and ln mean TOC (r = 0.858).
Three short-chain PFASs (C4–C7) were not detected in any of the pond sediments (Table S20), including perfluorobutane sulfonate (PFBS), perfluoroheptanoate (PFHpA), and PFHxA. In particular, the short-chain PFASs containing a carboxylic acid group (e.g., PFHpA and PFHxA) are relatively hydrophilic compared to long-chain PFCAs (Yeung et al. 2013). For example, PFHpA was detected in approximately 45% of stormwater runoff samples collected in the Minneapolis–St. Paul, MN area after six storm events (Xiao et al. 2012a). Ahrens et al. (2009) observed that short-chain PFCAs (C ≤ 7) preferentially diffused into pore water, whereas long-chain PFCAs (C ≥ 11) were found only in sediment cores collected from Tokyo Bay, Japan. PFHxA was frequently detected in surficial sediments in Lake Huron (Codling et al. 2018a), whereas perfluorobutanoic acid (PFBA) and PFHxA dominated surficial sediments in Lakes Erie and Ontario (Codling et al. 2018b; Table S27). Codling et al. (2018b) attributed this dominance to interstitial water included in the sediment analyses. However, bulk sediment analyses provide the concentrations of contaminants associated with the solid phase. Porewater measurements would be needed to verify elevated levels of PFBA and PFHxA in Codling et al.’s (2018b) study. PFBA and perfluoropentanoate (PFPeA; another short-chain PFCA) were detected in 27% of the stormwater ponds sampled in this study (Table 1; Fig. S5).
The high detection frequency of PFOS, and frequent detection of other short and long-chain PFASs, was consistent with local production sources and historical/current uses in a variety of consumer goods and commercial products. PFOS was used to make Scotchgard™ (a fabric and carpet protector; Richardson 2007), to treat fast-food packaging (Schaider et al. 2017), and it also was used in old-generation, aqueous, film-forming foams for firefighting until PFOS was phased out in 2002 (Rotander et al. 2015; Hu et al. 2016). PFOA was produced to make the fluoropolymers used in non-stick cookware (Teflon®) and other consumer products (Oliaei et al. 2006). Local production of eight-carbon PFOS- and PFOA-related PFASs occurred at the 3M Chemolite plant in the southeast metro (Cottage Grove, MN). The 3M Chemolite Plant, now known as the 3M Cottage Grove plant, produced these compounds between 1950 and 2002, and their wastewater was discharged into Pool 2 of the Mississippi River (Oliaei et al. 2013). After 2002, 3M switched to producing four-carbon perfluorinated compounds based on PFBS (OECD 2002). This plant also manufactured PFBA until 1998 for an industrial customer to make photographic film (http://multimedia.3m.com/mws/media/437903O/frequently-asked-questions-about-pfba.pdf, accessed 8 May 2018). PFBA is also a breakdown product of other PFASs used in stain-resistant fabrics, paper food packaging, and carpets (http://www.health.state.mn.us/divs/eh/risk/guidance/gw/pfbainfo.pdf, accessed 8 May 2018). PFAS-related contamination from 3M was extensive in the east metro region. Historically, 3M disposed of PFAS-contaminated wastes and sludges at two dumps in the east metro (i.e., Oakdale and Woodbury, MN), at the 3M Chemolite plant, and at the Washington County Landfill, MN (Oliaei et al. 2013). In 2004, PFASs were found in some drinking water supplies in the east metro, for which PFBA was the most mobile compound detected in both groundwater and drinking water samples (Oliaei et al. 2013). 3M’s wastewater treatment plant was the primary source of PFASs to the Mississippi River (Xiao et al. 2012a), and 3M also disposed of wastewater treatment plant sludges at the Pine Bend Landfill across the river from the plant (Oliaei et al. 2013). Fish consumption advisories for six Twin Cities area lakes and part of the Mississippi River were updated in May 2018 for PFOS contamination (http://www.health.state.mn.us/news/pressrel/2018/fish050318.html, accessed 24 May 2018).
This study marks the first investigation of PFASs in stormwater pond sediments in the Twin Cities area. The concentrations of PFOS in this study (i.e., < 0.33 to 2.25 ng/g dry wt.) were comparable to surficial sediment (0–5 cm) concentrations in the Mississippi River upstream of the 3M Cottage Grove Plant (as far upstream as Minneapolis, MN) and some areas further downstream from the plant (Monson 2013). Immediately downstream from the plant outfall, PFOS concentrations ranged from 6.4 to 80.2 ng/g dry wt. in the upper 5 cm of sediment (Monson 2013). Twelve other PFASs were detected in the most contaminated samples downstream of 3M, including the only detections of PFBS, PFHxA, and PFHpA (Monson 2013). These three compounds were not detected in the stormwater pond sediments (Table S20). A previous study of the top 10 cm of sediment in the vicinity of the 3M plant showed a PFOS concentration of 1.6 ng/g dry wt. just upstream of the plant (similar to this study), 99 ng/g dry wt. immediately downstream of 3M’s outfall, and decreasing concentrations at three sites downstream (Oliaei et al. 2006). Additional PFAS comparisons between this study and three Great Lakes surficial sediment studies (Codling et al. 2014, 2018a, b) are provided in the ESM (p. S2) and Table S27.
APs and NPEs
The distribution of APs and NPEs in the stormwater pond sediments was dominated by 4-NP (Table 1). It was detected in all of the ponds within a wide range of concentrations (32.2–85,000 ng/g dry wt.; Fig. S6). The highest concentration (statistical outlier; Table 2) was observed in pond 12-C. The other 4-NP concentrations ranged from 32.2 to 6300 ng/g dry wt., which was similar to the range of 4-NPs detected in surficial sediments above and below wastewater treatment plants (WWTPs) in Minnesota (Ferrey 2011). The Kaplan–Meier means for 4-NP1EO and 4-NP2EO were over an order of magnitude lower than the mean value of 7611 ng/g dry wt. for 4-NP (Table 1). Octylphenol was not detected in any of the pond sediments (Table S20), which was consistent with Ferrey’s (2011) findings. The prevalence of 4-NP is due to the degradation of high production NPEs in the environment (Chokwe et al. 2017 and references therein). Surfactant usage patterns are predominately NPEs (80–85%) compared with octylphenol ethoxylates (USEPA 2010). These patterns, in addition to the higher hydrophobicity of NPs, can also explain the absence of octylphenol in these samples. The relevant distributions of APs and NPEs in sewage sludge, stormwater, and sediments from other studies are further described in the ESM (pp. S2-S3).
Potential correlations between 4-NP and other parameters detected in all of the composite sediment samples were assessed using transformed data, where necessary. Ln 4-NP was not significantly (p > 0.05) correlated to black carbon, ln mean TOC, summary particle size parameters (Fig. 2; Table S22), or detailed particle size classes (Table S24). AP compounds and 4-NP also were not correlated with TOC in Swedish stormwater samples (Björklund et al. 2009; Kalmykova et al. 2013). Ln 4-NP was significantly (p < 0.05) correlated to ln cadmium (r = 0.584), and it was highly significantly (p < 0.001) correlated to zinc (r = 0.788; Fig. 2; Table S22). Because the 4-NP value for pond 12-C was a large statistical outlier, it was removed and the Pearson product moment correlation was re-run. Doing so did not alter the results, except that the Pearson correlation coefficient with ln cadmium was no longer significant (p > 0.05; r = 0.417). Ln 4-NP (all data) also was significantly (p < 0.05) correlated to both ln ∑PAH12 (r = 0.723) and ln ESB Toxic Units (r = 0.726), as well as being highly significantly (p < 0.001) correlated to ln ∑PAH34 (r = 0.76; Table S26). It is not known if these significant Pearson correlations were associated with releases from the same types of sources. Kalmykova et al. (2013) indicated sources of alkylphenol ethoxylates (APEs) in stormwater could be due to concrete and plastic materials, lubricants, tire rubber, and car care products. Because zinc and PAHs are used in the production of automobile tires (Depaolini et al. 2017), which abrade onto roads and are transported in stormwater runoff, this could be a possible reason for the correlation with 4-NP if it was a degradation product of APEs used in tires. Proprietary surfactants also are used in the production of some CT-sealants, but the identity of these surfactants and whether they include APEs is not public information. Other significant correlations with 4-NP will be described in the PBDE section.
Pyrethroids and Pyrethrins
The detection of pyrethroids was limited to bifenthrin and total permethrins (Table 1) in a subset of commercial and industrial stormwater ponds (Table 3; Fig. S7). The mean concentration of bifenthrin (8.4 ng/g dry wt.) was slightly higher than that of total permethrins (5.8 ng/g dry wt.; Table 1), although the detected sample sizes were low (3 and 5 samples, respectively). Pyrethrins were not detected in any of the samples (Table S20). Pyrethrins degrade rapidly in sunlight and are not persistent in the environment (ATSDR 2003).
The highest bifenthrin (32.7 ng/g dry wt.) and total permethrin (41.9 ng/g dry wt.) concentrations were observed in sample 05-C from Hampshire Pond, which is located in an industrial area of Bloomington, MN (Table 3). The city recycled de-icing sands and sealcoat aggregates from city streets at an outdoor upland site by this pond from about 1992 to 2010. Pre-wash water was pumped from Hampshire Pond to the screening equipment, and the post-wash water ran off into a two-cell sediment basin system that was mostly full by 2009. The overflow water from the sediment basin would have flowed into Hampshire Pond. This site is currently used to compost organic material from mostly fall street sweeping operations. Bifenthrin and total permethrins that were transported in stormwater runoff or aerial drift from lawns or buildings to city streets would be likely scenarios by which these pyrethroids could associate with street materials that were then washed and recycled by Hampshire Pond. In comparison, bifenthrin and cypermethrin were measured in street sweepings from San Diego, CA by different types of sweepers and concentrations ranged from 94.51 ± 33.66 ng/g dry wt. for bifenthrin and 21.64 ± 5.88 ng/g dry wt. for cypermethrin (Weston Solutions, Inc. 2010). Thus, the collection and recycling of products from streets is a useful way to reduce pyrethroid transport to surface waters. However, it would be prudent to analyze pyrethroids in compost generated from organic material in fall street sweeping operations to assess potential contamination of this material.
The analysis of sediment samples for pyrethroid compounds was complicated by interferences from hydrocarbon compounds (e.g., PAHs). These interferences resulted in higher reporting limits. For bifenthrin, the reporting limits were 7 to 18 times higher than the laboratories typical detection limit of 0.6 ng/g dry wt. For total permethrins, the reporting limits were 5 to 16 times higher than the typical detection limit of 0.2 ng/g dry wt. Matrix interferences and higher pyrethroid reporting limits also occurred in a sediment study of stormwater-impacted urban streams in the Seattle, WA metropolitan area (Anderson 2011). Thus, stormwater-impacted sediments can present additional analytical challenges for effective clean-up of the sediment matrix. This can be a particular problem in urban areas impacted by CT-sealants, which were a major source of PAHs to the study ponds (Crane 2014).
Pyrethroids strongly sorb to sediment particles due to their low water solubility and high octanol–water partition coefficients (log Kow; Li et al. 2017). Although there were only five detected total permethrin concentrations in this study, these data were found to be have significant (p < 0.05) Pearson product moment correlations to black carbon (r = 0.951), mean TOC (r = 0.935), % silt (r = 0.930), % sand (r = − 0.921), % medium silt (r = 0.917), and % fine clay (r = 0.915). A larger sample size of detected concentrations would be advantageous for assessing correlations over a broader range of TOC values, which were limited to 2.25–8.54%.
Bifenthrin and total permethrins comprised the majority (88.4%) of all pyrethroid and pyrethrin compounds sold in Minnesota during 2009 when the sediment samples were collected (Table S28). Most of the bifenthrin sales during this time were for crop chemicals (95.3%), followed by other nonagricultural categories (4.7%), such as home, turf and ornamental, and garden and lawn applications (Table S28). In contrast, most of the permethrin sales were for formulating products (73.6%) and other nonagricultural categories (15.4%), including animal care products (e.g., flea and tick control products), structural applications, and products for the garden and lawn, home, and turf and ornamental plants (Table S28). Although sales data do not provide an indication of the amount of products used in Minnesota (MDA 2013), the dominance of permethrin and bifenthrin in the nonagricultural sales data (Fig. S8) are consistent with these compounds being detected in the study ponds. In addition, none of the study ponds were included in areas where pyrethroids were applied to control mosquitoes in 2009.
Although pyrethroids have been studied in the surficial sediments of several states and countries [most notably California (Amweg et al. 2006; Li et al. 2017)], data for Minnesota waterways are limited. Bifenthrin was detected in the surficial sediments of five of nine stream sites in Minnesota, with the highest concentrations (2.5 and 4.1 ng/g dry wt.) in urban streams (Hladik and Kuivila 2012; Rogers et al. 2016). These concentrations are an order of magnitude lower than observed in this study. Resmethrin was also detected (3.8 ng/g dry wt.) in an agricultural stream in southern Minnesota (Hladik and Kuivila 2012), whereas it was not detected in the pond sediments. Bifenthrin has been detected more often than permethrin in urban streams from multiple states (Anderson 2011; Hladik and Kuivila 2012), as well as in mostly urban storm drains in Illinois (Ding et al. 2010). In contrast, total permethrins were detected more often in the stormwater ponds (33%) than bifenthrin (20%), although this finding is based on a smaller sample size (n = 15) and deeper sediment core depth (i.e., 0–15 cm) than used in these other studies. Both bifenthrin and permethrin exhibit moderate to long persistence in sediments under aerobic or anaerobic conditions, with greater degradation of bifenthrin under anaerobic conditions (Gan et al. 2005). Such anaerobic conditions would be typical for stormwater pond sediments.
PBDEs
Several BDE compounds were frequently detected in the sediment samples. Eight congener groups were detected in all of the samples, including BDEs-17 + 25, 28 + 33, 47, 49, 99, 100, 154, and 209 (Table 1). Total PBDEs were calculated by the Kaplan–Meier method for all sites (Table 1). For pond 08-C, the concentrations of BDE-209 and Total PBDEs (due to BDE-209) were statistical outliers (Table 2; Fig. S9). This pond receives drainage from a large shopping mall and other commercial and residential buildings. Summary statistics for 24 other BDE congeners with ≥ 20% detects are provided in Table 1. The list of eight BDE congeners with < 20% detects is provided in Table S20. BDEs-47, 99, and 209 were the dominant congeners, comprising mean (SD) percentages of total PBDEs of 5.2 (2.4), 5.2 (3.2), and 70.3 (7.9)%, respectively. These congeners also dominated the distribution of BDEs in sediments from tributaries flowing into Lake Ontario (Kolic et al. 2004), as well as in sediments from Lake Superior (Song et al. 2004), Lakes Ontario and Erie (Song et al. 2005a), and Lakes Michigan and Huron (Song et al. 2005b). BDEs-47 and 99 were the two main congeners in the pentaBDE commercial mixture, before it was voluntarily withdrawn from the U.S. market in 2004 (USEPA 2009). BDE-209 was the major component of the decaBDE commercial mixture, which was withdrawn from the U.S. market at the end of 2013 (USEPA 2012). The debromination of decaBDE appears to occur very slowly in natural sediments of boreal lakes (Orihel et al. 2016), and slow rates of debromination may occur in these stormwater pond sediments, too. In comparison to a statewide survey of ambient sediment quality in Minnesota (Crane 2017), mean concentrations of BDEs-47, 99, 206, 209, and total PBDEs in the stormwater pond sediments were 1.8, 1.8, 1.1, 3.3, and 2.6 times greater, respectively, than observed statewide. This same subgroup of compounds exceeded the UTL95-95 values (Crane 2017) in 6.7 (for BDE-206) to 40.0% (for BDE-209) of pond samples due to urban sources of BDEs (Table 1). Some of the commonly detected BDE congeners in the stormwater pond sediments (i.e., BDEs-47, 49, 99, and 100) also corresponded to the most prevalent congeners in fish tissue in Minnesota, particularly in predator fish (Crane 2017). Thus, the distribution of BDE congeners is widespread throughout Minnesota (Crane 2017), with higher concentrations observed in urban stormwater pond sediments.
For the non-BDE BFRs included in this study, estimated summary statistics are provided in Table 1. The highest estimated mean concentration was noted for HBB (50.2 µg/kg dry wt.; Table 1). PBEB had the lowest estimated mean concentration at 3.7 pg/g dry wt. (Table 1). Site 05-C had the highest estimated HBB and PBEB concentrations of 387,000 and 15.1 pg/g dry wt., respectively. At the time of sampling, this industrial pond was located by an upland site used to store and process seasonal quantities of deicing sands (during spring), chip seal rock from residential streets (mostly summer), and street sweepings (during fall) from the City of Bloomington, MN (Crane 2014). As previously discussed in the pyrethroid section, there was the possibility of unintentional releases of overflow water from the aforementioned activities into the pond. Comparisons between the four non-BDE BFRs included in this study and other sediment studies are provided in the ESM (p. S3).
The subset of eight detected BDE congener groups and Total PBDEs were assessed for correlations with other parameters detected in all of the samples. None of these compounds were significantly correlated (p > 0.05) to ln mean TOC or general and specific particle size fractions (Fig. 2; Tables S22 and S24). Natural log transformations of BDE-17 + 25 and BDE-49 were the only BDE congeners with significant (p < 0.05) Pearson correlation coefficients to black carbon (Fig. 2; Table S22). Khairy and Lohmann (2017) determined that black carbon is the dominant factor in the solid-water partitioning of BDE congeners in the Passaic River, NJ. Ln BDE-17 + 25 and ln BDE-49 also were significantly (p < 0.05) correlated to ln cadmium and copper in the stormwater pond sediments (Fig. 2; Table S22). Either significant (p < 0.05) or highly significant (p < 0.001) Pearson correlation coefficients were observed for natural log transformations of all eight BDE congener groups and total PBDEs with zinc, ln 4-NP, ln ∑PAH12, ln ∑PAH34, ln ESB Toxic Units, as well as between these BDE congener groups and Total PBDEs (Fig. 2; Tables S22 and S26). Only ln BDE-209 was not significantly (p > 0.05) correlated to ln BDE-28 + 33 (Table S22). Similarly, Moon et al. (2007) observed that BDE-209 did not correlate to BDE-28 in marine sediments from industrialized bays of Korea, probably due to different sources and environmental behavior. Long-chain APs are used in the production of flame retardants (http://www.greenspec.co.uk/building-design/alkylphenols-environment-human-health/, accessed 17 August 2018), which could contribute to the significant correlations between these parameters. In particular, highly significant (p < 0.001) Pearson correlation coefficients ranged from 0.809 to 0.901 for ln BDE-154 and lower congeners compared to ln 4-NP (Table S22). Zinc had the highest Pearson correlation coefficients for ln BDE-28 + 33 (r = 0.77) and ln BDE-47 (r = 0.771; Table S22). Metallic compounds, such as zinc or antimony oxides, are often added to enhance the efficiency of brominated flame retardants through the formation of metal oxohalides on the surface of the material (Lassen et al. 2014). The use of zinc oxides in this case could contribute to the positive correlations observed in Fig. 2 and Table S22 for ln BDE compounds and ln total PBDEs. The highly significant (p < 0.001) correlations between ln ∑PAH12 and ln ∑PAH34 with seven of the BDE congener groups (excluding ln BDE-209) were unexpected, but both groups of compounds are ubiquitous in the environment and have vehicle-related sources (Li et al. 2003; Boonyatumanond et al. 2007; Lagalante et al. 2009; Harrad and Mohamed 2011). These correlations were particularly strong for ln BDE-28 + 33 when compared to ln ∑PAH12 (r = 0.897) and ln ∑PAH34 (r = 0.904; Table S26).
Comparisons to Sediment Benchmarks
The sediment chemistry data of eight metal(loid)s, ∑PAH12 (Crane 2014), and mean PEC-Qs were compared to ranges of Level I and Level II SQT values (Crane and Hennes 2007; Crane et al. 2000, 2002). The Level I SQTs are intended to identify contaminant concentrations below which harmful effects on benthic invertebrates are unlikely to be observed, whereas the Level II SQTs are intended to identify contaminant concentrations above which harmful effects on benthic invertebrates are likely to be observed (Crane and Hennes 2007; Crane et al. 2000, 2002). Sites were ranked from low to high mean PEC-Qs in a heat map comparison to SQT values (Fig. 4). Three stormwater ponds (i.e., 05-C, 09-C, and 12-C) exceeded the Level II SQTs for mean PEC-Qs (0.6) and ∑PAH12 (23.0 mg/kg dry wt.; Figure 4), implying harmful effects on benthic organisms were likely in these sediment samples. ∑PAH12 was the main contributor of the elevated mean PEC-Q values for these samples. The other mean PEC-Q values were between the Level I and Level II SQT ranges (i.e., > 0.1 to ≤ 0.6; Fig. 4). These sediments had moderate sediment quality and could exhibit a higher incidence of sediment toxicity than sediment below the Level I SQT value. Of the metal(loid)s, cadmium had the greatest number of exceedances (14) of the corresponding Level I SQT value of 0.99 mg/kg dry wt (Fig. 4). Mercury and arsenic were of least concern in the stormwater ponds, with nearly all values below the corresponding Level I SQT values (Fig. 4). The rest of the metals had a mix of samples below and above the corresponding Level I SQT values (Fig. 4).
Sediment quality guidelines and benchmarks from other jurisdictions were used to provide general comparisons to chemicals lacking SQT values. In comparison to the New York State Department of Environmental Conservation freshwater sediment guidance values for DEHP (NYSDEC 2014), the stormwater pond DEHP values were much less than their Class A value of < 360 mg/kg dry wt. when adjusted for the site-specific TOC values of this study. Class A sediments are considered to be of low risk to aquatic life in New York (NYSDEC 2014) and are comparable to the Level I SQTs in narrative intent (Crane et al. 2000, 2002; Crane and Hennes 2007). TOC-normalized bifenthrin and permethrin concentrations were compared with integrated sediment benchmarks based on H. azteca and Chironomus spiked-sediment bioassay data (Nowell et al. 2016). All three detected, TOC-normalized bifenthrin samples (i.e., 05-C, 09-C, and 12-C), as well as the permethrin sample for 05-C, exceeded the corresponding threshold effect benchmarks (Table 3). However, these concentrations were less than the likely effect benchmarks (Table 3). These samples corresponded to the likelihood of intermediate toxicity to benthic invertebrates from pyrethroids. This finding is further supported by the results of mesocosm experiments that showed TOC-normalized bifenthrin concentrations lower than this study reduced larval macroinvertebrate abundance, richness, and biomass (Rogers et al. 2016).
The TU approach also was used to assess potential toxicity of H. azteca due to pyrethroids. Bifenthrin was the main contributor to pyrethroid TUs (Table 3). A similar finding has been observed in other urban studies in the United States (Amweg et al. 2006; Holmes et al. 2008; Hintzen et al. 2009; Ding et al. 2010; Kuivila et al. 2012; Nowell et al. 2013). None of the pyrethroid TUs exceeded 1.0 when based on mean LC50’s for two overlying water temperature ranges (i.e., 23 °C and 15/18 °C; Table 3). Pyrethroids are more toxic to H. azteca at colder temperatures (Holmes et al. 2008; Weston and Lydy 2010), as reflected in the higher TUs at 15/18 °C (Table 3). When the upper standard deviations of the LC50 values were used in the calculations, the TU of 05-C was 1.1 at 23 °C and rounded up to 1.0 at 15/18 °C (Table 3). Under this scenario, the pyrethroid TUs also approached 1.0 at 23 °C for 09-C and 12-C (Table 3). Amweg et al. (2006) observed that sediment samples from urban creeks in California were nontoxic at pyrethroid TUs < 1, whereas those with TUs > 2 were consistently toxic. Weston et al. (2013) has since shown that some populations of nontarget H. azteca in California have developed genetic resistance to pyrethroids and a different mode of toxicity (i.e., oxidative stress at high concentrations). In their study of three laboratory cultures and seven wild populations of H. azteca, there was more than 550-fold variation in sensitivity to widely used pyrethroid insecticides (Weston et al. 2013). Their study has implications for ensuring consistent populations of H. azteca, which is a complex of several species, are used by various toxicity laboratories for generating data for regulatory agencies. In regards to this study, all three of the stormwater pond samples with the highest TUs (i.e., 05-C, 09-C, and 12-C) had mean PEC-Qs and ∑PAH12 concentrations greater than the corresponding Level II SQT values. Thus, PAHs would be the most likely chemical group to contribute to benthic invertebrate toxicity in these samples. Because PAH concentrations will likely decrease over time due to Minnesota’s ban on CT-sealants, bifenthrin should be assessed to determine if concentrations are increasing to levels associated with sediment toxicity.
Total NP TEQs were compared with Canadian interim sediment quality guidelines (ISQGs; CCME 2002), which were adjusted to site-specific mean TOC values. Three samples exceeded the site-specific ISQG values for ponds 10-C, 12-C, and 13-C by 1.1, 13.0, and 1.8 times, respectively (Table 4). For these stormwater ponds, concentrations of NP and its ethoxylates were likely to cause adverse effects to benthic organisms, especially in the pond with the highest exceedance (i.e., 12-C). However, elevated concentrations of ∑PAH12 in these three ponds also could contribute to sediment toxicity, especially in pond 12-C where the Level II SQT for ∑PAH12 was exceeded (Fig. 4).
The update of the B[a]P equivalent calculations resulted in somewhat higher concentrations compared with those used in Crane (2014; Table 5). The percentage of detects was increased enough to allow B[a]P equivalents to be calculated for four samples that were previously listed as nondetects in Crane (2014; i.e., 02-C, 04-C, 06-C, and 14-C). The ln B[a]P equivalent values were either significantly (p < 0.05) or highly significantly (p < 0.001) correlated to zinc, ln 4-NP, seven of the BDEs, and ln total PBDEs (Table S26). The highest Pearson correlation coefficient (r = 0.931) was observed with ln BDE-28 + 33 (Table S26; Fig. S10).
The updated B[a]P equivalents were compared to the MPCA’s Soil Reference Values (SRVs), which are soil contaminant concentrations above which an unacceptable risk to human health is predicted under two different exposure scenarios for residential and industrial land uses (MPCA 2016b, c). Management decisions about upland disposal options for stormwater pond sediment are based on these comparisons. Ten ponds exceeded the Residential SRV value of 2 mg/kg compared to nine ponds in Crane (2014; Table 5). The same nine ponds exceeded the Industrial SRV of 3 mg/kg in both Crane (2014) and the updated calculations (Table 5). Three ponds (i.e., 05-C, 09-C, and 12-C) had much higher exceedances of the Industrial SRV value than the other six ponds (Table 5), and these ponds also exceeded the Level II SQT values for both ∑PAH12 and mean PEC-Qs (Fig. 4). Dredged sediment from ponds exceeding the Industrial SRV is treated as hazardous waste, and disposal would be restricted to lined landfills or other contained systems (Stollenwerk et al. 2014). Sample 08-C was the only pond with B[a]P equivalents between the Residential and Industrial SRV values (Table 5); dredged sediment from this pond could be used on properties with an industrial use category (Stollenwerk et al. 2014). Five ponds (i.e., 02-C, 04-C, 06-C, 14-C, and 15-C) had low concentrations of B[a]P equivalents below the Residential SRV value (Table 5), and excavated sediment from these ponds could be used as unregulated fill with no special management (Berger et al. 2017). However, pond 06-C exceeded the Residential SRV value of 9 mg/kg for arsenic, but it was below the Industrial SRV value of 20 mg/kg. Thus, sediment from this pond would have more restricted disposal options. None of the ponds exceeded the Residential SRV value for copper of 100 mg/kg. The MPCA recently updated the B[a]P equivalent SRV values with new toxicity data from the Minnesota Department of Health, although these draft values have not been finalized, yet. Once finalized, the number of ponds exceeding the Industrial SRV value will likely be reduced. Until that time, the original SRV values will remain in use.
Major Land Use Comparisons
The impact of watershed land uses on the distribution of contaminants detected in all 15 stormwater ponds was assessed. Nine of the 25 chemical parameters listed in Table 6 had significant (p < 0.05) one-way ANOVA results, indicating the differences in mean values among major land use categories (i.e., residential, commercial, and industrial) were greater than would be expected by chance. The nine significant parameters included zinc, 4-NP, six BDEs (i.e., 28 + 33, 47, 99, 100, 154, and 209), and total PBDEs (Table 6). All of these parameters had either significant (p < 0.05) or highly significant (p < 0.001) Pearson correlation coefficients with each other (Table S22). Multiple pairwise comparisons showed significant (p < 0.05) differences in the mean values of all nine parameters for ponds dominated by industrial land uses versus residential land uses (Table S29). Similar results were observed for ponds dominated by commercial land uses versus residential land uses, except for zinc (which was not significant; Table S29). For the significant pairwise comparisons, mean concentrations were always higher in the industrial and commercial pond groups, respectively, compared to those in the residential ponds (Fig. 5). Zinc also was higher in stormwater pond sediments from other commercial (Liebens 2001; Crawford et al. 2010) and industrial (Egemose et al. 2015; Sharley et al. 2017) areas, as well as being positively correlated with the extent of development (Blaszczak et al. 2018), compared with residential or less developed areas.
Although DEHP and PFASs were not detected in all of the stormwater pond sediments, their detection frequency varied by land use category. DEHP was detected more often in the commercial (80%) and industrial (60%) ponds, at higher concentrations, compared with the residential ponds (40%). However, this difference was not significant (p > 0.05) based on a Kruskal–Wallis one-way ANOVA on ranks. A higher percentage of 10 PFASs were detected in commercial (66%) and industrial (52%) pond sediments than residential (30%) ponds (Table S30), for which the difference was significant (p < 0.05) between commercial and residential ponds. PFOS, perfluorononanoate (PFNA), and PFDoA were detected in all five commercial ponds (Table S30). Watershed land uses also influenced the distribution of six PFASs in stormwater runoff from the Twin Cities metropolitan area (Xiao et al. 2012b). While the distribution of PFASs in residential stormwater runoff was similar to that in rainfall, nonatmospheric sources contributed PFASs in stormwater runoff from commercial and industrial areas, particularly for PFOS and PFOA (Xiao et al. 2012b).
Multivariate Statistical Analyses
PCA analysis of the composite sediment samples was done to reduce a subset of contaminants detected in all of the samples to the ones that accounted for most of the variance in the data set. PAH metrics from Crane (2014) were considered, as well, including ∑PAH12 and PAH ESB Toxic Units. A total of 26 PCA scenarios were run using different suites of chemicals and number of principal components. The options that passed the normality test, included three principal components, utilized a representative set of detected parameters, and explained a higher proportion of variance were considered further. The best PCA option included arsenic, cadmium, chromium, copper, lead, mercury, nickel, zinc, 4-NP, BDE-49, BDE-209, B[a]P equivalents, ∑PAH12, and ESB Toxic Units (Fig. 6). The scree plot, component loading plots, and component score plots from the PCA analysis are provided in Figs. S11 and S12. Three principal components (PCs) accounted for 75.8% of the variance in the data (Fig. 6). The Eigenvectors of the covariance matrix in PC1 were highest for BDE-49, B[a]P equivalents, and ESB Toxic Units. The Eigenvectors in PC2 were highest for mercury, cadmium, and nickel, whereas lead contributed the highest value to PC3. BDE-209 was reduced from the first three PCs. The unexplained variance ranged from 5.2% for cadmium to 88.0% for BDE-209. Arsenic (61.0%) and 4-NP (46.9%) had the next highest percentages of unexplained variance. No outliers (p > 0.05) were noted from the PCA component scores.
The most similar ponds were not always linked by watershed land use categories (Figs. 6 and S13). The two most contaminated ponds, 05-C and 09-C, were grouped together in the HCA analysis and encompassed industrial and commercial land uses, respectively. These two ponds had the highest mean PEC-Qs and ∑PAH12 concentrations (Fig. 4), the highest B[a]P equivalents (Table 5), the highest and third highest ESB Toxic Units (Crane 2014), respectively, and elevated concentrations of cadmium, copper, lead, nickel, and zinc (Fig. 4). While pond 05-C (Hampshire Pond) appeared to be impacted more by runoff from street sweeping piles and sorting operations, pond 09-C (Helen St. Pond) received drainage from primarily commercial and some residential areas in North St. Paul, MN (Crane 2014). Pond 12-C (2nd St. Pond) was another highly contaminated pond, which was unique from the other ponds (Fig. 6). It was located in a former wood treating Superfund site in New Brighton, MN that had been remediated (Crane 2014). Besides elevated PAH-related parameters and metals, pond 12-C also had the highest 4-NP and chromium concentrations of this study (Table 2). Three other ponds were grouped singly, including the industrial ponds 13-C and 15-C, as well as the residential pond 02-C (Fig. 6). The rest of the ponds were grouped into two clusters spanning more than one watershed land use category (Fig. 6).
Implications for Stormwater Management
The multivariate statistical analyses confirmed that making management decisions primarily based on B[a]P equivalents was appropriate for the upland disposal of dredged sediment from this group of stormwater ponds. In the PCA analysis, the Eigenvector for B[a]P equivalents was important for the first principal component and 91.4% of the variance for B[a]P equivalents was explained by using three principal components. The broad mixture of conventional contaminants and CECs (e.g., PFASs, pyrethroids, 4-NP, BDEs) in the pond sediments with the highest B[a]P equivalents (i.e., 05-C, 09-C, and 12-C) would be sequestered with the restricted landfill options for these ponds. The other ponds with exceedances of Residential and/or Industrial SRV values for B[a]P equivalents and arsenic would also garner restricted sediment disposal, which would reduce releases of CECs to the environment. This restriction is especially important for pond 08-C, which had the highest BDE-209 and Total PBDE concentrations in this study (Table 2).
Restrictions and phase-outs of certain chemicals, as well as product bans, should result in future reductions of PAHs, BDEs, copper, and long-chain PFASs in stormwater pond sediments in Minnesota. Van Metre and Mahler (2014) determined that the 2006 ban on CT-sealants in Austin, TX resulted in reductions of ∑PAH16 by approximately 71% and 35% from 2001 to 2014 in two of three sediment cores collected from the principal lake receiving Austin’s urban runoff. Potential reductions of B[a]P equivalents in either lake or stormwater pond sediments in the Twin Cities area after the 2014 CT-sealant ban have not been assessed yet, because it may be too early to conduct such a study. Particles will continue to abrade from old CT-sealed driveways and parking lots, with subsequent transport by stormwater runoff, until replacement sealants are applied that are much lower in PAHs. Reductions of some BDE congeners have been found in the environment as a result of product phase-outs and bans. In San Francisco Bay, CA, concentrations of BDE-47 in sediment have dropped by over one-third from 2002 to 2012, whereas BDE-209 has not shown any temporal trend yet (Sutton et al. 2015). Lower brominated BDEs have been reduced by 46–74% in fish fillets collected from the Great Lakes between 2006/2007 and 2012 (Gandhi et al. 2017). In response to the national copper-free brake initiative described earlier, copper loads in urban runoff in California are expected to decrease 46 to 57% by 2024 (California Stormwater Quality Association 2016). Similarly, less copper from vehicle sources can be expected to be emitted into stormwater runoff in Minnesota over time, resulting in reduced loadings to stormwater pond sediments. Between 2009 and 2013, substantial reductions in PFOS concentrations have been observed in pelagic fish fillets from Pool 2 of the Upper Mississippi River after the voluntary phase-out of PFOS in 2002 (Newsted et al. 2017). Because sediments may accumulate in stormwater ponds for over a decade before removal actions, some ponds will continue to contain a legacy of contaminants before implementing pond maintenance.
As PAH concentrations, and subsequently B[a]P equivalents, likely decrease in Minnesota stormwater pond sediments over time, municipalities may be able to consider less restrictive disposal options for pond sediment. Changes in disposal options also could occur as the result of updates to the MPCA’s SRV values for B[a]P equivalents, particularly if the Industrial SRV value becomes less restrictive in the future. Municipalities will still face challenges with where to place the volume of excavated sediment from ponds with unrestricted disposal. In addition, other emerging and unmeasured contaminants that would have previously been sequestered in a landfill may then be released to the environment in future scenarios where the excavated sediment is classified as unregulated fill. The MPCA does not have human health-based SRV values for several of the CECs included in this study, except for a limited number of PFASs. The Residential SRV values for PFBA, PFOA, and PFOS are all much greater than the concentrations observed in this study, implying unrestricted disposal of dredged material based on just those contaminants. However, these SRV values will likely be modified in the future as new toxicity data becomes available. Once there is more toxicity data available for 4-NP and 4-NP1EO, deriving SRVs would provide a way to evaluate the potential impacts to people from exposure to these CECs in sediment. The MPCA does not usually consider ecological risks in upland disposal of stormwater pond sediment.
Current evaluations of stormwater pond sediments for arsenic, copper, and a suite of 17 carcinogenic and 10 noncarcinogenic PAHs are sufficient for Minnesota municipalities and other MS4 permittees to analyze in their stormwater pond sediments before excavation and disposal. MPCA guidance recommends additional parameters be included in areas with site-specific contaminant sources (Berger et al. 2017). Although pyrethroids are increasing in usage in Minnesota, there are analytical challenges with measuring these compounds in the complex organic matrices of stormwater pond sediments. A future follow-up study may be prudent to determine concentrations of CECs and chloride in stormwater pond sediments from a larger number of ponds. In particular, APs, NPEs, pyrethroids, PBDE replacement flame retardants, short-chain PFASs, bisphenol A, and chloride could be included, along with arsenic, copper, and the suite of required PAHs. In addition, microplastics have not been studied in stormwater ponds to determine prevalence and potential for deposition to sediments. Microplastics in freshwater (Driedger et al. 2015; McCormick et al. 2014, 2016; Baldwin et al. 2016) and soil (de Souza Machado et al. 2018; Hurley et al. 2018) are an emerging problem that can affect the transport and fate of hydrophobic organic contaminants and metals (Alimi et al. 2018; Lee et al. 2019). Because there are inconsistent regulations (or lack thereof) across the United States and Canada for the disposal of stormwater pond sediments (Polta 2004), this study has demonstrated there could be releases of a complex mixture of contaminants to the environment if the sediment is just land-applied without analytical testing.
Other stormwater management techniques, in tandem with the use of stormwater ponds or separately, may provide lower cost options for removing more contaminants from stormwater. Adsorption filtration is being studied for the removal of dissolved, colloidal, and particulate pollutants from stormwater. Björklund and Li (2018) determined in laboratory experiments that pine bark and chitosan-covered bark quickly removed > 80% of organic compounds frequently detected in stormwater, including PAHs, APs, and phthalates. The field applicability of this technique needs to be tested. Iron-enhanced sand filters have been shown to significantly remove 14 of the 48 most detected CECs in stormwater samples from the Twin Cities area (Fairbairn et al. 2018). In Europe, infiltration basins and constructed wetlands were ranked as the best BMPs for endocrine-disrupting chemicals and other priority pollutants in stormwater (Scholes et al. 2008). Other stormwater BMPs, such as rain gardens and tree trenches, can reduce contaminant inflows to stormwater ponds and allow microbial degradation of some organic compounds to occur (e.g., lower-ring PAHs: LeFevre et al. 2012).
Conclusions
The stormwater pond sediments from this study included a complex mixture of contaminants, which spanned low to high levels of concern for aquatic biological effects and upland disposal options based on human health risks. Previously published work from this study showed that CT-sealants were a major source of PAHs to the study pond sediments, and concentrations of B[a]P equivalents were influencing management options about how excavated sediment from these ponds could be disposed. In this component of the study, a broad mixture of conventional contaminants and CECs were detected in some or all of the surficial pond sediments collected in 2009 from the Minneapolis–St. Paul, MN metropolitan area. Given the historic production of PFOS, PFOA, and other long-chain PFASs by 3M Corporation in the southeastern Twin Cities area, and use of these compounds in consumer products, it was not surprising to find detections of these compounds in the 15 stormwater ponds. Commercial ponds had significantly more (p < 0.05) detections of a group of 10 PFCAs than those with residential watershed land uses. The detection of bifenthrin and permethrin in certain ponds with commercial and/or industrial land uses provided the first data of pyrethroids in Minnesota stormwater pond sediments, although the analysis of pyrethroids was stymied by matrix interferences in the sediment samples. It may be more productive to analyze for pyrethroids in depositional areas of stream or lake sediments to target sediments with less hydrocarbon interferences, or employ other analytical procedures to reduce the impact of interferences. Watershed land uses were a factor in the distribution of zinc, 4-NP, six BDE compounds, and total PBDEs, with the lowest concentrations in residential ponds. This group of compounds also displayed significant (p < 0.05) Pearson product moment correlations amongst most of these variables, along with total PAH groups and B[a]P equivalents. Any follow-up studies should examine whether these relationships still hold after more time has elapsed from phase-outs and bans of commercial BDE mixtures, as well as the statewide ban on CT-sealants. The updated B[a]P equivalents were an effective chemical proxy for use in making management decisions about disposal options for stormwater pond sediments in this study. The nine ponds with the highest B[a]P equivalents that would prompt restricted disposal of excavated sediments also would encapsulate sediments with elevated metals, DEHP, chloride, and CECs, such as PFASs, PBDEs, bifenthrin, permethrin, 4-NP, and NPEs. If PAHs decrease in the future in response to Minnesota’s ban on CT-sealants, then additional consideration is needed regarding managing CECs in stormwater pond sediments. Other jurisdictions can use these results for considering how they manage excavated sediment from their stormwater ponds, too.
Abbreviations
- ANOVA:
-
Analysis of variance
- APs:
-
Alkylphenols
- APEs:
-
Alkylphenol ethoxylates
- ASTM:
-
American Society for Testing and Materials
- B[a]P:
-
Benzo[a]pyrene
- BDE:
-
Brominated diphenyl ether
- BFRs:
-
Brominated flame retardants
- BMP:
-
Best management practice
- BTBPE:
-
1,2-Bis(2,4,6-tribromophenoxy)ethane
- CA:
-
California
- CECs:
-
Contaminants of emerging concern
- cPAHs:
-
Carcinogenic polycyclic aromatic hydrocarbons
- CT-sealant:
-
Coal tar-based sealant
- DBDPE:
-
Decabromodiphenylethane
- DEHP:
-
Bis(2-ethylhexyl)phthalate
- EDCs:
-
Endocrine-disrupting compounds
- EPA:
-
Environmental Protection Agency
- ESB:
-
Equilibrium Partitioning Sediment Benchmark
- ESM:
-
Electronic supplementary material
- GC:
-
Gas chromatography
- HBB:
-
Hexabromobenzene
- HBCDD:
-
Hexabromocyclododecane
- HCA:
-
Hierarchical cluster analysis
- HRGC/LRMS:
-
High resolution gas chromatography/low-resolution mass spectrometry
- HRMS:
-
High resolution mass spectrometer
- ISQGs:
-
Interim sediment quality guidelines
- K ow :
-
Octanol-water partition coefficient
- LC:
-
Liquid chromatography
- LC50 :
-
Median lethal concentration
- LEB:
-
Likely effect benchmark
- ln:
-
Natural log
- MMCD:
-
Metropolitan Mosquito Control District
- MN:
-
Minnesota
- MPCA:
-
Minnesota Pollution Control Agency
- MS:
-
Mass spectrometry or spectrometer
- MS4:
-
Municipal Separate Storm Sewer System
- NPs:
-
Nonylphenols
- 4-NP:
-
4-Nonylphenol
- NPEs:
-
Nonylphenol ethoxylates
- 4-NP1EO:
-
4-Nonylphenol monoethoxylate
- 4-NP2EO:
-
4-Nonylphenol diethoxylate
- OC:
-
Organic carbon
- OH:
-
Ohio
- PAEs:
-
Phthalate esters
- PAH:
-
Polycyclic aromatic hydrocarbon
- PBDE:
-
Polybrominated diphenyl ether
- PBEB:
-
Pentabromoethylbenzene
- PC:
-
Principal component
- PCA:
-
Principal components analysis
- PCBs:
-
Polychlorinated biphenyls
- PEC:
-
Probable effect concentration
- PEC-Qs:
-
Probable effect concentration quotients
- PFASs:
-
Per- and polyfluoroalkyl substances
- PFBA:
-
Perfluorobutanoate
- PFBS:
-
Perfluorobutane sulfonate
- PFCAs:
-
Perfluorocarboxylates
- PFDA:
-
Perfluorodecanoate
- PFDoA:
-
Perfluorododecanoate
- PFHpA:
-
Perfluoroheptanoate
- PFHxA:
-
Perfluorohexanoate
- PFHxS:
-
Perfluorohexane sulfonate
- PFNA:
-
Perfluorononanoate
- PFOA:
-
Perfluorooctanoic acid or perfluorooctanoate
- PFOS:
-
Perfluorooctane sulfonate
- PFOSA:
-
Perfluorooctane sulfonamide
- PFPeA:
-
Perfluoropentanoate
- PFUnA:
-
Perfluoroundecanoate
- QA/QC:
-
Quality assurance/quality control
- SD:
-
Standard deviation
- SQT:
-
Sediment quality target
- SRV:
-
Soil reference value
- SVOCs:
-
Semivolatile organic compounds
- TCEP:
-
Tris(2-chloroethyl)phosphate
- TDCPP:
-
Tris(1,3-dichloro-2-propyl)phosphate
- TEB:
-
Threshold effect benchmark
- TEQ:
-
Toxic equivalent
- TOC:
-
Total organic carbon
- TU:
-
Toxic unit
- TX:
-
Texas
- USEPA:
-
United States Environmental Protection Agency
- USGS:
-
United States Geological Survey
- UTL95-95:
-
The 95% upper tolerance limit with 95% coverage
- WWTP:
-
Wastewater treatment plant
References
Ahrens L, Yamashita N, Yeung LWY, Taniyasu S, Horii Y, Lam PKS, Ebinghaus R (2009) Partitioning behavior of per- and polyfluoroalkyl compounds between pore water and sediment in two sediment cores from Tokyo Bay, Japan. Environ Sci Technol 43:6969–6975
Alimi OS, Budarz JF, Hernandez LM, Tufenkji N (2018) Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ Sci Technol 52:1704–1724
Amweg EL, Weston DP, You J, Lydy MJ (2006) Pyrethroid insecticides and sediment toxicity in urban creeks from California and Tennessee. Environ Sci Technol 40:1700–1706
Anderson PD (2011) Pyrethroids in freshwater sediments of King County. Publication No. 11-03-061. Washington State Department of Ecology, Olympia, WA. https://fortress.wa.gov/ecy/publications/documents/1103061.pdf. Accessed 15 Aug 2018
ATSDR (2003) Public health statement: pyrethrins and pyrethroids. U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA. https://www.atsdr.cdc.gov/toxprofiles/tp155.pdf. Accessed 21 Sept 2018
Baldwin AK, Corsi SR, Mason SA (2016) Plastic debris in 29 Great Lakes tributaries: relations to watershed attributes and hydrology. Environ Sci Technol 50:10377–10385
Bartlett AJ, Rochfort Q, Brown LR, Marsalek J (2012) Causes of toxicity to Hyalella azteca in a stormwater management facility receiving highway runoff and snowmelt. Part II: salts, nutrients, and water quality. Sci Total Environ 414:238–247
Berger D, Brooks B, Crane J, Fairbairn D, McDonald S (2017) Managing stormwater sediment best management practices guidance. wq-strm4-16. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/wq-strm4-16.pdf. Accessed 16 Mar 2018
Björklund K, Li L (2018) Sorption of organic pollutants frequently detected in stormwater: evaluation of five potential sorbents. Environ Technol 39:2335–2345
Björklund K, Cousins AP, Strömvall A-M, Malmqvist P-A (2009) Phthalates and nonylphenols in urban runoff: occurrence, distribution and area emission factors. Sci Total Environ 407:4665–4672
Blaszczak JR, Steele MK, Badgley BD, Heffernan JB, Hobbie SE, Morse JL, Rivers EN, Hall SJ, Neill C, Pataki DE, Groffman PM, Bernhardt ES (2018) Sediment chemistry of urban stormwater ponds and controls on denitrification. Ecosphere 9:e02318. https://doi.org/10.1002/ecs2.2318
Boonyatumanond R, Murakami M, Wattayakorn G, Togo A, Takada H (2007) Sources of polycyclic aromatic hydrocarbons (PAHs) in street dust in a tropical Asian mega-city, Bangkok, Thailand. Sci Total Environ 384:420–432
Brand AB, Snodgrass JW, Gallagher MT, Casey RE, Van Meter R (2010) Lethal and sublethal effects of embryonic and larval exposure of Hyla versicolor to stormwater pond sediments. Arch Environ Contam Toxciol 58:325–331
Brander SM, Gabler MK, Fowler NL, Connon RE, Schlenk D (2016) Pyrethroid pesticides as endocrine disruptors: molecular mechanisms in vertebrates with a focus on fishes. Environ Sci Technol 50:8977–8992
Braun JM (2017) Early life exposure to endocrine disrupting chemicals and childhood obesity and neurodevelopment. Nat Rev Endocrinol 13:161–173
Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7:513–541
Burgess RM, Ahrens MJ, Hickey CW, Den Besten PJ, Ten Hulscher D, Van Hattum B, Meador JP, Douben PET (2003) An overview of the partitioning and bioavailability of PAHs in sediments and soils. In: Douben PET (ed) PAHs: an ecological perspective. Wiley, West Sussex, pp 99–126
California Stormwater Quality Association (2016) Estimated urban runoff copper reductions resulting from brake pad copper restrictions. Prepared by TDC Environment, LLC for California Stormwater Quality Association. https://www.casqa.org/sites/default/files/library/technical-reports/estimated_urban_runoff_copper_reductions_resulting_from_brake_pad_copper_use_restrictions_casqa_4-13.pdf. Accessed 6 Sept 2018
Carlisle J, Henkel S, Li L-H, Painter P, Qiao D (2009) Toxicological profile for di-(2-ethylhexyl) phthalate (DEHP). California Environmental Protection Agency. http://www.opc.ca.gov/webmaster/ftp/project_pages/MarineDebris_OEHHA_ToxProfiles/DEHP%20Final.pdf. Accessed 14 May 2018
CCME (2002) Canadian sediment quality guidelines for the protection of aquatic life. Nonylphenol and its ethoxylates. Canadian Council of Ministers of the Environment, Ottawa, ON. http://ceqg-rcqe.ccme.ca/download/en/242. Accessed 29 Mar 2018
Chokwe TB, Okonkwo JO, Sibali LL (2017) Distribution, exposure pathways, sources and toxicity of nonylphenol and nonylphenol ethoxylates in the environment. Water SA 43:529–542
Codling G, Vogt A, Jones PD, Wang T, Wang P, Lu Y-L, Corcoran M, Bonina S, Li A, Sturchio NC, Rockne KJ, Ji K, Khim J-S, Naile JE, Giesy JP (2014) Historical trends of inorganic and organic fluorine in sediments of Lake Michigan. Chemosphere 114:203–209
Codling G, Hosseini S, Corcoran MB, Bonina S, Lin T, Li A, Sturchio NC, Rockne KJ, Ji K, Peng H, Giesy JP (2018a) Current and historical concentrations of poly and perfluorinated compounds in sediments of the northern Great Lakes—Superior, Huron, and Michigan. Environ Pollut 236:373–381
Codling G, Sturchio NC, Rockne KJ, Li A, Peng H, Tse TJ, Jones PD, Giesy JP (2018b) Spatial and temporal trends in poly- and per-fluorinated compounds in the Laurentian Great Lakes Erie, Ontario and St. Clair. Environ Pollut 237:396–405
Cordner A, Mulcahy M, Brown P (2013) Chemical regulation on fire: rapid policy advances on flame retardants. Environ Sci Technol 47:7067–7076
Crane JL (2014) Source apportionment and distribution of polycyclic aromatic hydrocarbons, risk considerations, and management implications for urban stormwater pond sediments in Minnesota, USA. Arch Environ Contam Toxicol 66:176–200
Crane JL (2017) Ambient sediment quality conditions in Minnesota lakes, USA: effects of watershed parameters and aquatic health implications. Sci Total Environ 607–608:1320–1338
Crane JL, Hennes S (2007) Guidance for the use and application of sediment quality targets for the protection of sediment-dwelling organisms in Minnesota. tdr-g1-04. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/tdr-gl-04.pdf. Accessed 16 Mar 2018
Crane JL, Hennes S (2016) Ambient sediment quality conditions in Minnesota. tdr-g1-19. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/tdr-g1-19.pdf. Accessed 21 Sept 2018
Crane JL, MacDonald DD, Ingersoll CG, Smorong DE, Lindskoog RA, Severn CG, Berger TA, Field LJ (2000) Development of a framework for evaluating numerical sediment quality targets and sediment contamination in the St. Louis River Area of Concern. EPA 905-R-00-008. Great Lakes National Program Office, U.S. Environmental Protection Agency, Chicago, IL. https://www.pca.state.mn.us/sites/default/files/sqt-slraoc.pdf. Accessed 24 Aug 2018
Crane JL, MacDonald DD, Ingersoll CG, Smorong DE, Lindskoog RA, Severn CG, Berger TA, Field LJ (2002) Evaluation of numerical sediment quality targets for the St. Louis River Area of Concern. Arch Environ Contam Toxicol 43:1–10
Crane JL, Grosenheider K, Wilson CB (2010) Contamination of stormwater pond sediments by polycyclic aromatic hydrocarbons (PAHs) in Minnesota—the role of coal tar-based sealcoat products as a source of PAHs. tdr-g1-07. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/tdr-g1-07.pdf. Accessed 11 Sept 2018
Crawford KD, Weinstein JE, Hemingway RE, Garner TR, Globensky G (2010) A survey of metal and pesticide levels in stormwater retention pond sediments in coastal South Carolina. Arch Environ Contam Toxicol 58:9–23
Davies TGE, Field LM, Usherwood PNR, Williamson MS (2007) DDT, pyrethrins, pyrethroids and insect sodium channels. Life 59:151–162
Davis AP, Shokouhian M, Ni S (2001) Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere 44:997–1009
de Souza Machado AA, Lau CW, Till J, Kloas W, Lehmann A, Becker R, Rillig MC (2018) Impacts of microplastics on the soil biophysical environment. Environ Sci Technol 52:9656–9665
Depaolini AR, Bianchi G, Fornai D, Cardelli A, Badalassi M, Cardelli C, Davoli E (2017) Physical and chemical characterization of representative samples of recycled rubber from end-of-life tires. Chemosphere 184:1320–1326
Diamond JM, Latimer HA II, Munkittrick KR, Thornton KW, Bartell SM, Kidd KA (2011) Prioritizing contaminants of emerging concern for ecological screening assessments. Environ Toxicol Chem 30:2385–2394
Ding Y, Harwood AD, Foslund HM, Lydy MJ (2010) Distribution and toxicity of sediment-associated pesticides in urban and agricultural waterways from Illinois, USA. Environ Toxicol Chem 29:149–157
Driedger AGJ, Dürr HH, Mitchell K, Van Cappellen P (2015) Plastic debris in the Laurentian Great Lakes: a review. J Great Lakes Res 41:9–19
Egemose S, Sønderup MJ, Grudinina A, Hansen AS, Flindt MR (2015) Heavy metal composition in stormwater and retention in ponds dependent on pond age, design and catchment type. Environ Technol 36:959–969
EPA (2014) Certain nonylphenols and nonylphenol ethoxylates; Significant new use rule. Fed Reg 79:59186–59195
Fairbairn DJ, Elliott SM, Kiesling RL, Schoenfuss HL, Ferrey ML, Westerhoff BM (2018) Contaminants of emerging concern in urban stormwater: spatiotemporal patterns and removal by iron-enhanced sand filters (IESFs). Water Res 145:332–345
Ferrey M (2011) Wastewater treatment plant endocrine disrupting chemical monitoring study. lrp-ei-1sy11. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/lrp-ei-1sy11.pdf. Accessed 21 Sept 2018
Gan J, Lee SJ, Liu WP, Haver DL, Kabashima JN (2005) Distribution and persistence of pyrethroids in runoff sediments. J Environ Qual 34:836–841
Gandhi N, Gewurtz SB, Drouillard KG, Kolic T, MacPherson K, Reiner EJ, Bhavsar SP (2017) Polybrominated diphenyl ethers (PBDEs) in Great Lakes fish: levels, patterns, trends and implications for human exposure. Sci Total Environ 576:907–916
Harrad S, Mohamed A-EA (2011) Brominated flame retardants in dust from UK cars—within-vehicle spatial variability, evidence for degradation and exposure implications. Chemosphere 82:1240–1245
Helsel DR (2010) Summing nondetects: incorporating low-level contaminants in risk assessment. Integr Environ Assess Manag 6:361–366
Helsel DR (2012) Statistics for censored environmental data using Minitab® and R, 2nd edn. Wiley, Hoboken
Hintzen EP, Lydy MJ, Belden JB (2009) Occurrence and potential toxicity of pyrethroids and other insecticides in bed sediments of urban streams in central Texas. Environ Pollut 157:110–116
Hladik ML, Kuivila KM (2012) Pyrethroid insecticides in bed sediments from urban and agricultural streams across the United States. J Environ Monit 14:1838–1845
Holmes RW, Anderson BS, Phillips BM, Hunt JW, Crane DB, Mekebri A, Connor V (2008) Statewide investigation of the role of pyrethroid pesticides in sediment toxicity in California’s urban waterways. Environ Sci Technol 42:7003–7009
Hooper K, McDonald TA (2000) The PBDEs: an emerging environmental challenge and another reason for breast-milk monitoring programs. Environ Health Perspect 108:387–392
Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM (2016) Detection of poly- and perfluoralkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3:344–350
Huber M, Welker A, Helmreich B (2016) Critical review of heavy metal pollution of traffic area runoff: occurrence, influencing factors, and partitioning. Sci Total Environ 541:895–919
Hurley RR, Lushe AL, Olsen M, Nizzetto L (2018) Validation of a method for extracting microplastics from complex, organic-rich, environmental matrices. Environ Sci Technol 52:7409–7417
Hwang H-M, Fiala MJ, Park D, Wade TL (2016) Review of pollutants in urban road dust and stormwater runoff: part 1. Heavy metals released from vehicles. Int J Urban Sci 20:334–360
Identification Task Team (2015) Draft binational summary report: nonylphenol and its ethoxylates. Chemicals of Mutual Concern Identification Task Team. https://binational.net//wp-content/uploads/2015/05/EN-NP-NPEs-Binational-Summary-Report-Final-Draft.pdf. Accessed 7 June 2018
Johnson GW, Ehrlich R, Full W (2004) Principal components analysis and receptor models in environmental forensics. In: Murphy BL, Morrison RD (eds) Introduction to environmental forensics. Academic Press, Burlington, pp 461–515
Kalmykova Y, Björklund K, Strömvall A-M, Blom L (2013) Partitioning of polycyclic aromatic hydrocarbons, alkylphenols, bisphenol A and phthalates in landfill leachates and stormwater. Water Res 47:1317–1328
Khairy MA, Lohmann R (2017) Using polyethylene passive samplers to study the partitioning and fluxes of polybrominated diphenyl ethers in an urban river. Environ Sci Technol 51:9062–9071
Kolic TM, MacPherson KA, Reiner EJ, Ho T, Kleywegt S, Dove A, Marvin C (2004) Brominated diphenyl ether levels: a comparison of tributary sediments versus biosolid material. Organohalogen Compd 66:3830–3835
Kuivila KM, Hladik ML, Ingersoll CG, Kemble NE, Moran PW, Calhoun DL, Nowell LH, Gilliom RJ (2012) Occurrence and potential sources of pyrethroid insecticides in stream sediments from seven U.S. metropolitan areas. Environ Sci Technol 46:4297–4303
Lagalante AF, Oswald TD, Calvosa FC (2009) Polybrominated diphenyl ether (PBDE) levels in dust from previously owned automobiles at United States dealerships. Environ Int 35:539–544
Landers DH, Simonich SL, Jaffe DA et al (2008) The fate, transport, and ecological impacts of airborne contaminants in Western National Parks (USA). EPA/600/R-07/138. U.S. Environmental Protection Agency, Corvallis, OR. https://water.usgs.gov/nrp/proj.bib/Publications/2008/landers_simonich_etal_2008a.pdf. Accessed 21 Sept 2018
Lassen C, Jensen AA, Crookes M, Christensen F, Jeppesen CN, Clausen AJ, Mikkelsen SH (2014) Survey of brominated flame retardants. ISBN 978-87-93026-90-2. The Danish Environmental Protection Agency, Copenhagen, Denmark. https://www2.mst.dk/Udgiv/publications/2014/01/978-87-93026-90-2.pdf. Accessed 21 Sept 2018
Lee KE, Sanocki CA, Montz GR (2005) Physical, chemical, and biological characteristics of Sturgeon Lake, Goodhue County, Minnesota, 2003–2004. Scientific Investigations Report 2005-5182. U.S. Geological Survey, Mounds View, MN
Lee H, Lee H-J, Kwon J-H (2019) Estimating microplastic-bound intake of hydrophobic organic chemicals by fish using measured desorption rates to artificial gut. Sci Total Environ 651:162–170
LeFevre GH, Novak PJ, Hazalski RM (2012) Fate of naphthalene in laboratory-scale bioretention cells: implications for sustainable stormwater management. Environ Sci Technol 46:995–1002
Lerman A, Weiler RR (1970) Diffusion and accumulation of chloride and sodium in Lake Ontario sediments. Earth Planet Sci Lett 10:150–156
Li A, Jang J-K, Scheff PA (2003) Application of EPA CMB8.2 model for source apportionment of sediment PAHs in Lake Calumet, Chicago. Environ Sci Technol 37:2958–2965
Li H, Cheng F, Wei Y, Lydy MJ, You J (2017) Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview. J Hazard Mater 324:258–271
Liebens J (2001) Heavy metal contamination of sediments in stormwater management systems: the effect of land use, particle size, and age. Environ Geol 41:341–351
Lindstrom AB, Strynar MJ, Libelo EL (2011) Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45:7954–7961
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Mahler BJ, Van Metre PC, Callender E (2006) Trends in metals in urban and reference lake sediments across the United States, 1970 to 2001. Environ Toxicol Chem 7:1698–1709
Mahler BJ, Van Metre PC, Crane JL, Watts AW, Scoggins M, Williams ES (2012) Coal-tar-based pavement sealcoat and PAHs: implications for the environment, human health, and stormwater management. Environ Sci Technol 46:3039–3045
Markiewicz A, Björklund K, Eriksson E, Kalmykova Y, Strömvall A-M, Siopi A (2017) Emissions of organic pollutants from traffic and roads: priority pollutants selection and substance flow analysis. Sci Total Environ 580:1162–1174
Mayer T, Rochfort Q, Borgmann U, Snodgrass W (2008) Geochemistry and toxicity of sediment porewater in a salt-impacted urban stormwater detention pond. Environ Pollut 156:143–151
McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ (2014) Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol 48:11863–11871
McCormick AR, Hoellein TJ, London MG, Hittie J, Scott JW, Kelly JJ (2016) Microplastic in surface waters of urban rivers: concentration, sources, and associated bacterial assemblages. Ecosphere 7:e01556
McKenzie ER, Money JE, Green PG, Young TM (2009) Metals associated with stormwater-relevant brake and tire samples. Sci Total Environ 407:5855–5860
MDA (2013) Non-agricultural pesticide sales 2008–2009. Minnesota Department of Agriculture, St. Paul, MN. http://www.mda.state.mn.us/chemicals/pesticides/pesticideuse/~/media/Files/chemicals/pesticides/200809nonagpestsales.pdf. Accessed 25 June 2018
MDH (2011) G. Phthalates. Minnesota Department of Health, St. Paul, MN. http://www.health.state.mn.us/divs/eh/hazardous/topics/toxfreekids/pclist/phthalates.pdf. Accessed 20 Sept 2018
Minnesota Stormwater Manual contributors (2017) Operation and maintenance of stormwater ponds. Minnesota Stormwater Manual. https://stormwater.pca.state.mn.us/index.php/Operation_and_maintenance_of_stormwater_ponds. Accessed 11 Sept 2018
MMCD (2010) 2009 Operational review and plans for 2010. Annual report to the Technical Advisory Board. Metropolitan Mosquito Control District, St. Paul, MN. https://www.leg.state.mn.us/docs/2010/other/100716.pdf. Accessed 25 June 2018
Monson B (2013) Perfluorochemicals in Mississippi River Pool 2: 2012 Update. c-pfc1-21. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/c-pfc1-21.pdf. Accessed 21 Sept 2018
Moon H-B, Kannan K, Choi M, Choi H-G (2007) Polybrominated diphenyl ethers (PBDEs) in marine sediments from industrialized bays of Korea. Mar Pollut Bull 54:1402–1412
MPCA (2016a) Twin Cities metropolitan area chloride management plan. wq-iw11-06ff. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/wq-iw11-06ff.pdf. Accessed 30 Apr 2018
MPCA (2016b) Soil reference value (SRV) technical support document. c-r1-05. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/c-r1-05.pdf. Accessed 28 Aug 2018
MPCA (2016c) SRV spreadsheet. c-r1-06. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/waste/risk-based-site-evaluation-guidance. Accessed 28 Aug 2018
Newsted JL, Holem R, Hohenstein G, Lange C, Ellefson M, Reagen W (2017) Spatial and temporal trends of poly- and perfluoroalkyl substances in fish fillets and water collected from Pool 2 of the Upper Mississippi River. Environ Toxicol Chem 36:3138–3147
Nowell LH, Moran PW, Gilliom RJ, Calhoun DL, Ingersoll CG, Kemble NE, Kuivila KM, Phillips PJ (2013) Contaminants in stream sediments from seven United States metropolitan areas. Part I: distribution in relation to urbanization. Arch Environ Contam Toxicol 64:32–51
Nowell LH, Norman JE, Ingersoll CG, Moran PW (2016) Development and application of freshwater sediment-toxicity benchmarks for currently used pesticides. Sci Total Environ 550:835–850
NYSDEC (2014) Screening and assessment of contaminated sediment. New York State Department of Environmental Conservation. https://www.dec.ny.gov/docs/fish_marine_pdf/screenasssedfin.pdf. Accessed 15 May 2018
OECD (2002) Co-operation on existing chemicals, hazard assessment of perfluorooctane sulfonate (PFOS) and its salts. JT00135607. Organisation for Economic Co-operation and Development, Paris, France. https://www.oecd.org/chemicalsafety/risk-assessment/2382880.pdf. Accessed 16 May 2018
OECD (2013) OECD/UNEP Global PFC Group, synthesis paper on per- and polyfluorinated chemicals (PFCs). Environment Directorate, OECD, Paris, France. https://www.oecd.org/env/ehs/risk-management/PFC_FINAL-Web.pdf. Accessed 16 May 2018
Oliaei F, Kriens D, Kessler K (2006) Investigation of perfluorochemical (PFC) contamination in Minnesota. Phase one. Report to Senate Environment Committee, St. Paul, MN. https://www.peer.org/assets/docs/mn/06_27_2_pfc_report.pdf. Accessed 8 May 2018
Oliaei F, Kriens D, Weber R, Watson A (2013) PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ Sci Pollut Res 20:1977–1992
Orihel DM, Bisbicos T, Darling CTR, Dupuis AP, Williamson M, Muir DCG (2016) Probing the debromination of the flame retardant decabromodiphenyl ether in sediments of a boreal lake. Environ Toxicol Chem 35:573–583
Osborne JW, Costello AB (2004) Sample size and subject to item ratio in principal components analysis. Pract Assess Res Eval 9(11). http://PAREonline.net/getvn.asp?v=9&n=11. Accessed 15 Mar 2018
Pacyna JM, Pacyna EG (2001) An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ Rev 9:269–298
Palmquist K, Salatas J, Fairbrother A (2012) Pyrethroid insecticides: use, environmental fate, and ecotoxicology. In: Perveen F (ed) Insecticides—advances in integrated pest management. InTech, pp 251–278. https://www.intechopen.com/books/insecticides-advances-in-integrated-pest-management/pyrethroid-insecticides-use-environmental-fate-and-ecotoxicology. Accessed 17 Sept 2018
Paul AG, Jones KC, Sweetman AJ (2009) A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol 43:386–392
Pavlowsky RT (2012) Baseline study of PAH sources and concentrations in pond and stream sediments, Springfield, Missouri. OEWRI EDR-12-002. Ozarks Environmental and Water Resources Institute, Missouri State University, Springfield, MO. https://oewri.missouristate.edu/assets/OEWRI/PAHReport.pdf. Accessed 12 Sept 2018
Pivnenko K, Eriksen MK, Martin-Fernández JA, Eriksson E, Astrup TF (2016) Recycling of plastic waste: presence of phthalates in plastics from households and industry. Waste Manag 54:44–52
Polta R (2004) A survey of regulations used to control the use and disposal of stormwater pond sediments in the United States and Canada. EQA report 04-542. Environmental Quality Assurance Department, Metropolitan Council Environmental Services, St. Paul, MN
Richardson SD (2007) Water analysis: emerging contaminants and current issues. Anal Chem 79:4295–4324
Rogers HA, Schmidt TS, Dabney BL, Hladik ML, Mahler BJ, Van Metre PC (2016) Bifenthrin causes trophic cascade and altered insect emergence in mesocosms: implications for small streams. Environ Sci Technol 50:11974–11983
Rotander A, Kärrman A, Toms L-ML, Kay M, Mueller JF, Gómez Ramos MJ (2015) Novel fluorinated surfactants tentatively identified in firefighters using liquid chromatography quadrupole time-of-flight tandem mass spectrometry and a case-control approach. Environ Sci Technol 49:2434–2442
Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG (2003) Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37:4543–4553
Sauvé S, Desrosiers M (2014) A review of what is an emerging contaminant. Chem Cent J 8:15. https://doi.org/10.1186/1752-153x-8-15
Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR, Peaslee GF (2017) Fluorinated compounds in U.S. fast food packaging. Environ Sci Technol Lett 4:105–111
Scholes L, Revitt M, Gasperi J, Donner E (2008) Priority pollutant behavior in stormwater best management practices (BMPs). Middlesex University, Queensway, UK. http://documents.env.dtu.dk/Projects/ScorePP/ScorePP%20D5.1%20PP%20behaviour%20in%20stormwater%20BMPs,%202008-02-15.pdf. Accessed 7 June 2018
Sébastian C, Becouze-Lareure C, Lipeme Kouyi G, Barraud S (2015) Event-based quantification of emerging pollutant removal for an open stormwater retention basin—loads, efficiency and importance of uncertainties. Water Res 72:239–250
Sharley DJ, Sharp SM, Marshall S, Jeppe K, Pettigrove VJ (2017) Linking urban land use to pollutants in constructed wetlands: implications for stormwater and urban planning. Landsc Urban Plan 162:80–91
Shedd KB (2017) 2015 Minerals yearbook. Cobalt (advanced release). U.S. Geological Survey. https://minerals.usgs.gov/minerals/pubs/commodity/cobalt/. Accessed 21 Sept 2018
Snodgrass JW, Moore J, Lev SM, Casey RE, Ownby DR, Flora RF, Izzo G (2017) Influence of modern stormwater management practices on transport of road salt to surface waters. Environ Sci Technol 51:4165–4172
Song W, Ford JC, Li A, Mills WJ, Buckley DR, Rockne KJ (2004) Polybrominated diphenyl ethers in the sediments of the Great Lakes. 1. Lake superior. Environ Sci Technol 38:3286–3293
Song W, Ford JC, Li A, Sturchio NC, Rockne KJ, Buckley DR, Mills WJ (2005a) Polybrominated diphenyl ethers in the sediments of the Great Lakes. 3. Lakes Ontario and Erie. Environ Sci Technol 39:5600–5605
Song W, Li A, Ford JC, Sturchio NC, Rockne KJ, Buckley DR, Mills WJ (2005b) Polybrominated diphenyl ethers in the sediments of the Great Lakes. 2. Lakes Michigan and Huron. Environ Sci Technol 39:3474–3479
Stollenwerk J, Smith J, Ballavance B, Rantala J, Thompson D, McDonald S, Schnick E (2014) Managing dredge materials in the State of Minnesota. wq-gen2-01. Minnesota Pollution Control Agency, St. Paul, MN. https://www.pca.state.mn.us/sites/default/files/wq-gen2-01.pdf. Accessed 20 Sept 2018
Sutton R, Sedlak MD, Yee D, Davis JA, Crane D, Grace R, Arsem N (2015) Declines in polybrominated diphenyl ether contamination of San Francisco Bay following production phase-outs and bans. Environ Sci Technol 49:777–784
Sutton R, Sedlak M, Sun J, Lin D (2017) Contaminants of emerging concern in San Francisco Bay. A strategy for future investigations. 2017 revision. RMP Contribution #815. San Francisco Estuary Institute, Richmond, CA. https://www.sfei.org/sites/default/files/biblio_files/RMP%20CEC%20Strategy%20Nov%202017_1.pdf. Accessed 13 Sept 2018
USEPA (1988) Integrated Risk Information System (IRIS) chemical assessment summary. Di(2-ethylhexyl)phthalate (DEHP); CASRN 117-81-7. U.S. Environmental Protection Agency, Washington, DC. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0014_summary.pdf. Accessed 20 Sept 2018
USEPA (2009) Polybrominated diphenyl ethers (PBDEs) action plan. U.S. Environmental Protection Agency. https://www.epa.gov/sites/production/files/2015-09/documents/pbdes_ap_2009_1230_final.pdf. Accessed 20 Sept 2018
USEPA (2010) Nonylphenol (NP) and nonylphenol ethoxylates (NPEs) action plan. RIN 2070-ZA09. U.S. Environmental Protection Agency. https://www.epa.gov/sites/production/files/2015-09/documents/rin2070-za09_np-npes_action_plan_final_2010-08-09.pdf. Accessed 31 May 2018
USEPA (2012) Significant new use and test rules: certain polybrominated diphenylethers. EPA-HQ-OPPT-2010-1039-0001. U.S. Environmental Protection Agency. https://www.regulations.gov/document?D=EPA-HQ-OPPT-2010-1039-0001. Accessed 21 Aug 2018
USEPA (2014) An alternatives assessment for the flame retardant decabromodiphenyl ether (decaBDE). U.S. Environmental Protection Agency. https://www.epa.gov/sites/production/files/2014-05/documents/decabde_final.pdf. Accessed 18 Sept 2018
USEPA (2018) Summary of the Clean Water Act Web page. U.S. Environmental Protection Agency. https://www.epa.gov/laws-regulations/summary-clean-water-act. Accessed 11 Sept 2018
USEPA Science Advisory Board (1992) An SAB report: Review of sediment criteria development methodology for non-ionic organic contaminants. EPA-SAB-EPEC-93-002. Prepared by the Sediment Quality Subcommittee of the Ecological Processes and Effects Committee, USEPA Science Advisory Board. https://nepis.epa.gov/Exe/ZyPDF.cgi/9100RVL6.PDF?Dockey=9100RVL6.PDF. Accessed 29 Mar 2018
Van Meter RJ, Swan CM (2014) Road salts as environmental constraints in urban pond food webs. PLoS ONE 9:e90168. https://doi.org/10.1371/journal.pone.0090168
Van Metre PC, Mahler BJ (2014) PAH concentrations in lake sediment decline following ban on coal-tar-based pavement sealants in Austin, Texas. Environ Sci Technol 48:7222–7228
Verreault J, Letcher RJ, Gentes M-L, Braune BM (2018) Unusually high Deca-BDE concentrations and new flame retardants in a Canadian Arctic top predator, the glaucous gull. Sci Total Environ 639:977–987
Wang J, Bo L, Li L, Wang D, Chen G, Christie P, Teng Y (2014) Occurrence of phthalate esters in river sediments in areas with different land use patterns. Sci Total Environ 500–501:113–119
Weinstein JE, Crawford KD, Garner TR (2010) Polycyclic aromatic hydrocarbon contamination in stormwater detention pond sediments in coastal South Carolina. Environ Monit Assess 162:21–35
Weiss PT, LeFevre G, Gulliver JS (2008) Contamination of soil and groundwater due to stormwater infiltration practices. A literature review. Project Report No. 515. St. Anthony Falls Laboratory, University of Minnesota, Minneapolis, MN. https://www.pca.state.mn.us/sites/default/files/stormwater-r-weiss0608.pdf. Accessed 21 Sept 2018
Weston DP, Lydy MJ (2010) Urban and agricultural sources of pyrethroid insecticides to the Sacramento-San Joaquin Delta of California. Environ Sci Technol 44:1833–1840
Weston Solutions, Inc. (2010) City of San Diego targeted aggressive street sweeping pilot study effectiveness assessment. Final report. Prepared by Weston Solutions, Inc., Carlsbad, CA for City of San Diego, San Diego, CA. https://www.sandiego.gov/sites/default/files/legacy/thinkblue/pdf/streetsweeppilotfinalreport.pdf. Accessed 25 June 2018
Weston DP, Holmes R, Lydy M (2009) Residential runoff as a source of pyrethroid pesticides to urban creeks. Environ Pollut 157:287–294
Weston DP, Poynton HC, Wellborn GA, Lydy MJ, Blalock BJ, Sepulveda MS, Colbourne JK (2013) Multiple origins of pyrethroid insecticide resistance across the species complex of a nontarget aquatic crustacean, Hyalella azteca. Proc Natl Acad Sci 110:16532–16537
Xiao F, Halbach TR, Simcik MF, Gulliver JS (2012a) Input characterization of perfluoroalkyl substances in wastewater treatment plants: source discrimination by exploratory data analysis. Water Res 46:3101–3109
Xiao F, Simcik MF, Gulliver JS (2012b) Perfluoroalkyl acids in urban stormwater runoff: influence of land use. Water Res 46:6601–6608
Yeung LWY, De Silva AO, Loi EIH, Marvin CH, Taniyasu S, Yamashita N, Mabury SA, Muir DCG, Lam PKS (2013) Perfluoroalkyl substances and extractable organic fluorine in surface sediments and cores from Lake Ontario. Environ Int 59:389–397
Ying G-G, Williams B, Kookana R (2002) Environmental fate of alkylphenols and alkylphenol ethoxylates—a review. Environ Int 28:215–226
Zgheib S, Moilleron R, Saad M, Chebbo G (2011) Partition of pollution between dissolved and particulate phases: what about emerging substances in urban stormwater catchments? Water Res 45:913–925
Zgheib S, Moilleron R, Chebbo G (2012) Priority pollutants in urban stormwater. Part 1—case of separate storm sewers. Water Res 46:6683–6692
Zhao L, Zhu L, Yang L, Liu Z, Zhang Y (2012) Distribution and desorption of perfluorinated compounds in fractionated sediments. Chemosphere 88:1390–1397
Zhou W, Guo L (2015) Iron triad (Fe, Co, Ni) nanomaterials: structural design, functionalization and their applications. Chem Soc Rev 44:6697–6707
Acknowledgements
Staff from ten Minnesota cities proposed stormwater pond monitoring sites and provided ancillary data. Field work assistance was provided by the following MPCA staff: Harold Wiegner, Alexander Levchuk, Steven Hennes, Mike Walerak, Mike Anderson, Mary Jean Fenske, Dale Thompson, Anna Kerr, Louise Hotka, and Steven Weiss. Other MPCA assistance was provided by William Scruton (QA/QC), Kris Parson and Shawn Nelson (GIS maps), and Bonnie Brooks (SRV applications). Sections of the manuscript were reviewed internally by Bill Cole, David Fairbairn, and Mike Trojan (MPCA). Angie Whetung coordinated the analytical work at AXYS Analytical Services Ltd. for all CECs, Diane Anderson (Pace Analytical Services Inc.) did so for SVOCs, and Jeff Brenner (Minnesota Department of Health) managed the analysis of metal(loid)s and chloride. The anonymous reviewer comments were very helpful and appreciated. This project was funded by Minnesota Legislature House File 1231.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crane, J.L. Distribution, Toxic Potential, and Influence of Land Use on Conventional and Emerging Contaminants in Urban Stormwater Pond Sediments. Arch Environ Contam Toxicol 76, 265–294 (2019). https://doi.org/10.1007/s00244-019-00598-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-019-00598-w