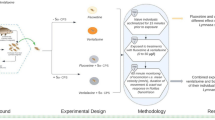
Abstract
Chemical pollutants enter aquatic systems through numerous pathways (e.g., surface runoff and ground water contamination), thus associating these contaminant sources with varying hydrodynamic environments. The hydrodynamic environment shapes the temporal and spatial distribution of chemical contaminants through turbulent mixing. The differential dispersal of contaminants is not commonly addressed in ecotoxicological studies and may have varying implications for organism health. The purpose of this study is to understand how differing routes of exposure to atrazine alter social behaviors and physiological responses of aquatic organisms. This study used agonistic encounters in crayfish Orconectes virilis as a behavioral assay to investigate impact of sublethal concentrations of atrazine (0, 40, 80, and 160 µg/L) delivered by methods mimicking ground water and surface runoff influx into flow-through exposure arenas for a total of 23 h. Each experimental animal participated in a dyadic fight trial with an unexposed opponent. Fight duration and intensity were analyzed. Experimental crayfish hepatopancreas and abdominal muscle tissue samples were analyzed for cytochrome P450 and acetylcholinesterase levels to discern mechanism of detoxification and mode of action of atrazine. Atrazine delivered via runoff decreased crayfish overall fight intensity and contrastingly ground water delivery increased overall fight intensity. The behavioral differences were mirrored by increases in cytochrome P450 activity, whereas no differences were found in acetylcholinesterase activity. This study demonstrates that method of delivery into fluvial systems has differential effects on both behavior and physiology of organisms and emphasizes the need for the consideration of delivery pathway in ecotoxicological studies and water-impairment standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Anthropogenic contaminants are introduced to aquatic systems via multiple pathways or modes of delivery, including ground water contamination, overland runoff, release from sediments, and spray drift following application (Davies et al. 2003; Long et al. 1995; Schulz 2001; Zoumis et al. 2001). Ground water contamination occurs due to leaching or percolation of pollutants vertically downward through the soil until the water table is reached (Doležal and Kvi´tek 2004; Reichenberger et al. 2007). Ground water not only often sustains river flow and depth but also is an important source of drinking water, making contamination of these sources of growing concern (Lapworth et al. 2012). Ground water mainly supplies baseflow to streams, baseflow being water that persistently enters river systems. This flow characterization differs from event flow or water that enters systems swiftly in response to a water input event (e.g., rainfall). Runoff serves as an example of event flow (Sophocleous 2002). Runoff, specifically infiltration runoff, occurs when the infiltration or soil saturation capacity is exceeded by precipitation events, causing water to immediately runoff the soil into surface waters of aquatic systems (Garen and Moore 2005). When applied to agricultural fields, pesticides can adsorb to soil or be dissolved into runoff water (Krutz et al. 2005; Leonard 1990). Contaminants also can be absorbed to substrate particles once within aquatic systems and then be subsequently released back into the system with the occurrence of changes in geochemical parameters, such as pH. Sediments, depending on sorption capabilities, can be considered as major repositories for anthropogenic contaminants and serve as a significant route of exposure for some species (Zoumis et al. 2001). Contaminants can be more directly deposited to aquatic systems through spray drift or the unintentional deposition of application to nontarget areas, such as surface water of aquatic environments (Hilz and Vermeer 2013). Due to the hydrodynamic variation in fluvial environments with these sources of contaminants, the spatial and temporal dynamics of toxicant concentrations will be different according to origin of the contaminant (Edwards and Moore 2014; Nikora 2010; Wolf et al. 2004).
Considerations of exposure paradigms and contaminant distribution should be based on the characteristics of flow an organism encounters in nature. The hydrodynamic character of flow regimes can be described by the Reynolds number, a ratio of inertial to viscous forces (Vogel 1994). At most biologically relevant spatial scales, inertial forces dominate flow regimes and natural flow systems are typically turbulent (Sanford 1997). Similarly, the Péclet number describes the relative contribution of the processes of flow (i.e., advection) and diffusion to the spatial and temporal distribution of toxicants within a fluid medium (Vogel 1994). These two numbers demonstrate that the degree of spatial and temporal distribution of a toxicant plume is shaped by mixing that occurs within turbulent flows. Furthermore, this mixing determines the magnitude (intensity or concentration of exposure), frequency (rate or number of exposure events), and duration (total time of exposure) of the pulsatile nature of exposure in flowing systems (Gordon et al. 2012). Thus, characteristics of the hydrodynamic environment shape the characteristics of exposure that organisms experience.
The dependency of toxicant distribution on hydrodynamic regimes is demonstrated further when considering smaller scale organisms (e.g., caddisflies, stoneflies, algae, and fungi) that are either attached to or reside on benthic substrates in aquatic habitats. Aside from turbulence, a specific aspect of the hydrodynamic environment that may be particularly influential for the dispersion of chemical contaminants is the boundary layer. A boundary layer exists against any solid surface in a flowing fluid medium. The boundary layer occurs between the free stream velocity (at some distance away from the surface) and a stagnant layer of flow directly in contact with this surface (Nikora 2010). Because the velocity within the boundary layer decreases, the Reynolds number also decreases indicating that viscous forces become more important near the solid surface (Jørgensen and Des Marais 1990; Moore et al. 1994; Vogel 1994). Therefore, as the relative contributions of inertial and viscous forces change, so too does the amount of mixing that occurs (Nowell and Jumars 1984; Sanford 1997). Chemicals released within an area where a boundary layer has formed, such as from a ground water source, will result in lower intensity fluctuations in magnitude, frequency, and duration (Moore and Crimaldi 2004). A more detailed discussion of the dispersal of chemicals in turbulence and flow has been reported by Moore and Crimaldi (2004) and Webster and Weissburg (2009). Therefore, while seldom considered within ecotoxicological studies, the mode of delivery of contaminants into an aquatic ecosystem may have profound effects on the influence of toxicant exposure on the behavior and physiology of animals within a habitat.
The dynamics of plume structures and contaminant distribution in flowing systems has been addressed by few ecotoxicological studies (Edwards and Moore 2014; Neal and Moore 2017; Pedersen and Friberg 2009; Rasmussen et al. 2012; Thorp et al. 2006). Instead, time-average static models (Ehrsam et al. 2016; Glusczak et al. 2007; Williams and Dusenbery 1990) or pulsed exposures to fixed contaminant concentrations (Ashauer et al. 2006; Handy 1994) often are used to expose organisms in a laboratory setting. These studies are important in understanding how different toxicants can influence the health and physiology of organisms. However, these exposure models and resultant definitions of toxicity fail to reflect the dynamic exposure conditions experienced under realistic ecological conditions (Gordon et al. 2012). Furthermore, ecotoxicological research has yet to address the effect of the differential distribution of contaminants due to their mode of delivery on organisms. To reduce the gap in knowledge that currently exists in understanding the implications of dynamic exposure paradigms, the mode of delivery of contaminants is specifically addressed in this research.
The purpose of this study was to understand how differing modes of delivery of a contaminant alter multilevel responses in organisms. Previous work on sublethal exposure to atrazine, a surface water and ground water contaminant, has shown physiological and behavioral changes in aquatic organisms as a result of exposure (Belanger et al. 2015, 2016; Diana et al. 2000; Graymore et al. 2001; Hayes et al. 2002; Jablonowski et al. 2011; Lesan and Bhandari 2003; Pionke and Glotfelty 1990; Steinberg et al. 1995). In this study, organismal responses examined included both behavioral, in the form of agonistic encounters (i.e., fighting behaviors) displayed by crayfish, and physiological responses, in the form of cytochrome P450 expression and activity of acetylcholinesterase (AChE) within crayfish tissues. Crayfish have been studied as a model organism with assays used ranging from behavioral to physiological biomarkers (e.g., cytochrome P450 and AChE) that have been shown to be sensitive to a variety of pollutants (review by Belanger et al. 2017). Additionally, crayfish serve as a keystone species in aquatic systems due their role as a link for energy transfer between terrestrial and aquatic food chains as well as their multitrophic role in aquatic food web dynamics (Hill and Lodge 1999). This ecological role of crayfish species creates an imperative to understand factors that may influence the health of these organisms. Preceding research has shown that agonistic behavior is important in the ecology and allocation resources for these animals and thus serves as a significant behavior to examine in relation to contaminant effects (Bovbjerg 1953; Martin and Moore 2010; Wofford et al. 2015). Previously studied physiological reactions range from inspecting variables within the removal of contaminants from the body to alterations in neurological function. Cytochrome P450 is an enzyme involved in the first phase of detoxification within animals. An additional enzymatic biomarker used is the activity of AChE, an important enzyme in the nervous systems of both vertebrates and invertebrates (Aksu et al. 2015). These informative biomarkers were examined along with agnostic behaviors to determine the effects of mode of delivery and differential contaminant distribution on aquatic organisms. Due to variation in hydrodynamic environments applied to each contaminant source, mid-water contaminant sources will have highly variant chemical plume structure compared to boundary layer ground water sources. Therefore, we predicted that mid-water delivery would significantly alter crayfish behavior and physiology due to increased peaks surpassing the mean concentration as a result of increased turbulence.
Methods
Animal Collection and Holding
Nonreproductive, female Orconectes virilis (3.3 ± 0.03 cm, mean postorbital carapace length ± SEM; 2.8 ± 0.04 cm, chelae length ± SEM) were hand collected from Maple Bay of Burt Lake located in Cheboygan County, Michigan (45.4873°N, 84.7065°W). Only crayfish with intact appendages were used in this study. Animals were mechanically and visually isolated in plastic containers (15 × 10 × 10 cm) for at least 7 days before the trial to minimize the effect of previous social status (Bergman et al. 2003; Guiasu and Dunham 1997; Karavanich and Atema 1998; Zulandt Schneider et al. 2001). Isolation containers were held in artificial streams constructed of cinder blocks (20.3 × 20.3 × 40.6 cm) and 4-mil polyethylene sheeting and located at the University of Michigan Biological Station Stream Research facility, Pellston, Michigan. Unfiltered water from the East Branch of the Maple River was pumped into the holding streams and crayfish fed on naturally occurring detritus. During the holding period, crayfish were exposed to ambient water temperature (~ 19 °C) and a natural light:dark cycle (15:9 h).
Experimental Design
A 2 × 4 fully factorial experiment was designed to investigate the differential effects of ground water and surface runoff (i.e., mid-water column delivery) of the contaminant atrazine on freshwater crustaceans. The first factor being mode of exposure (mid-water column exposure or ground water exposure) and the second factor being concentration of atrazine at the animal (control: 0, low: 40, medium: 80, and high: 160 µg/L). A total of 120 trials were analyzed in this study. Each animal was used only once during trials (Table 1).
Exposure Arenas
An artificial stream system consisting of six flow-through streams (160 × 40.6 × 40.6 cm, interior L × W × H) was constructed (Fig. 1). Streams were created using cinder blocks lined with 4-mil polyethylene sheeting and utilized as exposure arenas to subject crayfish to various atrazine exposure regimes. Minimally filtered water was pumped from the East Branch Maple River into 208-L reservoir tanks and distributed to each stream using 1-cm inner diameter garden hosing at a constant flow of 0.19 ± 0.01 L/s (mean flow ± SEM). Water was minimally filtered through nylon mesh (0.01 cm2 holes) to remove large debris that may impact water flow into artificial streams. Collimators constructed of 1.7 cm2 acrylic egg crating covered with fiberglass window screening were placed 30.5 cm downstream to regulate flow pattern of water entering the exposure arenas. The bottom of each stream was filled with sand substrate (~ 4.2 × 10−2 cm diameter) to a depth of approximately 3 cm. The boundary layer thickness at the location of the crayfish was approximately 1.3 cm as calculated from the roughness Reynolds number. Water depth and outflow of water from the artificial streams were controlled through the placement of outflow blocks at the downstream end. Outflow blocks consisted of a cinder block with window screening attached to outflow holes to control volume of water leaving the stream. Water level in each arena was held at a depth of 26 ± 2 cm (mean water depth ± SEM) throughout the data collection period. Atrazine or control solution was held in 22.7-L reservoir buckets located at the upstream end of each exposure stream and was delivered to the streams via 0.4-cm interior diameter aquarium tubing. Tubing was attached to a 22.7-L reservoir via a plastic connector adhered to bottom of reservoir. Opaque bucket lids were fastening on reservoirs to prevent dilution due to precipitation, contamination of atrazine solution, and to reduce the possibility of photolytic degradation of atrazine. Flow rate of chemical input was controlled by 1.9-cm open Hoffman compressor clamps placed on aquarium tubing input. Input tubing of atrazine was placed 53.3-cm upstream of animal location. Placement of input tubing within water column was dependent upon treatment (see Exposure Paradigm section). Animals were restricted to one location in each stream by use of tethers consisting of a 0.64 cm2 Velcro® square attached to a tile weight via fishing line. The opposite Velcro® piece was fastened to the carapace of each animal using superglue to secure the animal’s position in exposure arenas (Ludington and Moore 2017; Neal and Moore 2017). During the 23-h exposure period, crayfish were subject to a natural light:dark cycle of approximately 15:9 h and water temperature of ~ 19 °C.
Diagram of artificial stream exposure arenas (160 × 40.6 × 40.6 cm, interior L × W × H) constructed at University of Michigan Biological Station Stream Research Facility. Crayfish (solid black star) were placed 53.3 cm from atrazine source input (solid black circle). Black arrows indicate flow direction. Flow pattern of water entering stream was regulated by a collimator
Electrochemical Measurement of Dilution
An Epsilon electrochemical detection system (Epsilon; Bioanalytical Systems, West Lafayette, IN) was used to calculate dilution based on the hydrodynamic characteristics of the artificial stream system. A microelectrode consisting of three, 30-µm carbon fibers was mounted to the system to measure oxidation–reduction reactions in the water column 5 cm above the sand substrate (Edwards and Moore 2014; Harrigan and Moore 2017; Ludington and Moore 2017). The oxidative reaction of chemical tracer, dopamine, was used to measure dilution. Dopamine is an appropriate tracer to demonstrate the distribution of the toxicant within the stream system due to the similar diffusion coefficient of dopamine and atrazine in water (6.0 × 10−6 and 5.579 × 10−6 cm2/s respectively) and the relative ratio of advection and diffusion, i.e., Péclet number (Gerhardt and Adams 1982). The Péclet number for this flowing system shows that advection is more important in the dispersal of the chemical rather than diffusion (Denny 1993). This system has been repeatedly used to model toxicant (and odorant) movement in different flowing systems and, given the relative contributions of diffusion and advection to the movement of toxicants, can accurately measure these dilutions at the scale of the organisms under consideration here (Moore et al. 1994, 2000; Moore and Crimaldi 2004).
The Epsilon system was set to record at a sample interval of 0.05 s with a 100-Hz noise filter for a total of 300 s. The applied potential of the system was set at 500 mV. The microelectrode used to calculate dilution factor was calibrated using 5 known concentrations of dopamine (2, 4, 6, 8, and 10 µM). The concentration of the stock solution delivered to the artificial streams during Epsilon recordings was 31.16 µM. The dilution factor was calculated as 25 at the location 53.3-cm away from the chemical source and used to create appropriate stock solution concentrations of atrazine. Each experimental animal was placed in this location in each of the following trials. Fine-scale distribution of a toxicant in a flowing system may differ in frequency and magnitude of the chemical signal due to the hydrodynamic characteristics of the flowing environment. Overall average concentration in each control, low, medium, and high treatment remained constant; however, the fine-scale distribution of atrazine throughout the stream may vary depending on the route of entry of the toxicant, i.e., differing hydrodynamic environments experienced by a ground water and mid-water column chemical source (Finelli et al. 1999; Hart et al. 1996; Lahman and Moore 2015; Moore et al. 2000; Rahman and Webster 2005; Webster and Weissburg 2009).
Exposure Paradigm
Environmentally relevant concentrations of atrazine (0, 40, 80, 160 µg/L) were used throughout this experiment (Graymore et al. 2001; USEPA 2014). The herbicide product Hi-Yield Atrazine Weed Killer was used to create stock solutions for all experimental trials. Hi-Yield Atrazine Weed Killer contains 4.00% w/w of atrazine. This w/w ratio was considered in calculating the concentration of atrazine in stock solutions and that experienced by the animal. The nominal concentrations of stock solutions created were 0, 1000, 2000, or 4000 µg/L, in accordance with the dilution factor measured as 25 for the stream systems, to allow the test animals to experience an average concentration of 0, 40, 80, or 160 µg/L of atrazine solution depending on the treatment the crayfish was assigned. Stock solutions were prepared in reservoir buckets by diluting appropriate amount of Hi-Yield Atrazine Weed Killer with 20 L of river water. For mid-water column exposures, input tubing was suspended in the center of the water column, 13 cm below surface of water. Tubing was attached via cable ties to hard wire cloth braces to ensure opening of tubing remained parallel to stream substrate and at fixed height in water column. At this water depth, the mid-water column chemical output experienced free stream velocity and correspondingly, was within an area with a greater Reynolds number relative to the ground water chemical source. Therefore, atrazine released from this mid-water column source was subject to a higher degree of turbulent mixing, creating fluctuating chemical plume structures. For the ground water exposure regime, tubing was buried 2.5 cm beneath the substrate (Edwards and Moore 2014). The introduction of ground water to a stream system occurs by multiple pathways and is thereby complex (Brunke and Gonser 1997; Doležal and Kvi´tek 2004). The methodology chosen represents one possible model of ground water introduction. Being introduced at the substrate-fluid interface, the ground water chemical source interacted with the boundary layer and therefore atrazine released was more greatly influenced by viscous forces, relative to inertial forces, compared with the mid-water column source. The ground water contaminant source was thereby subject to a lesser degree of turbulent mixing, influencing the degree of fluctuations within the resulting chemical plume structure. Each experimental crayfish was placed in an exposure arena for 23 h before removal for behavioral assay. Total volume of atrazine or control solution entering streams during the 23-h exposure period was recorded. Experimental streams were allowed to flush for a total of 1 h between trials to rid streams of remaining atrazine or odors from previous trials.
Behavioral Analysis
Immediately following removal from the exposure arena, fight trials with experimental crayfish and unexposed, socially naïve crayfish occurred for each exposure treatment (Cook and Moore 2008; Neal and Moore 2017). Opponents were sized matched within a maximum of 10% difference in post orbital carapace length. Fights occurred in an opaque Plexiglas fight arena (39 × 39 × 14 cm: L × W × H) filled with 13.8 L of water from the East Branch Maple river and divided into four equal quadrants by retractable Plexiglas walls. The fight tank design allowed for animals to be mechanically, visually, and chemically isolated until start of fight trial. Crayfish were initially separated in each quadrant and allowed to acclimate for 15 min. After the acclimation period, the retractable wall of two quadrants was removed and crayfish were allowed to interact for a total of 15 min (Wofford et al. 2015). Each fight trial was video recorded using a Panasonic (Model # HDC-HS250) or Sony (Model # HDR-CX405) handheld camera. Duration, intensity (escalated or nonescalated), time to reach each intensity level, and time spent at each intensity level were analyzed. Intensity levels were determined using a pre-established ethogram (Table 2; adapted from Wofford et al. 2017). Initiation of a fight was defined as the time at which a crayfish aggressive contest begins (i.e., when one animal approaches the other within one body length). End of a fight was defined as opponents remaining approximately two body lengths away for a minimum of 10 s. Duration was defined as difference in seconds between the time at which a fight ended and time at which a fight was initiated. Agonistic behavior was compared between treatments to determine if route of exposure to toxicants differentially affects fighting behavior in crayfish.
Enzyme-Linked Immunosorbent Assays (ELISA)
After dyadic fight trials, five crayfish from each treatment were immediately dissected. Mass of total crayfish was taken before dissections (20.4 ± 0.9 g, mean mass ± SEM). Hepatopancreas and abdominal muscle tissue were harvested from each experimental animal. Tissue mass was recorded, and each tissue sample was frozen in liquid nitrogen (0.04 ± 0.020 g, mean hepatopancreas mass/total mass ± SEM; 0.08 ± 0.003 g, mean abdominal muscle mass/total mass ± SEM). Frozen tissues were placed in a − 80 °C freezer until further tissue preparation. Tissues were prepared for analysis using methods described in Cusabio ELISA Fish Acetylcholinesterase and Cytochrome P450 1A1 kits (Cusabio Technology LLC, College Park, MD). Tissue (100 mg) was extracted from each sample, rinsed with 0.1 M of phosphate-buffered saline (PBS) solution (pH 7.4), and then homogenized on ice in 1 mL of PBS solution. Samples were frozen overnight at − 20 °C. Homogenized samples were subject to two freeze–thaw cycles and were then centrifuged in a refrigerated centrifuge (Axygen Axyspin R Refrigerated Centrifuge, Thermo Fisher Scientific, Waltham, MA) at 4 °C for 5 min at 5000 RPMs. Supernatant was removed and stored at − 80 °C until ELISA testing. Supernatant samples were thawed, centrifuged, and then subject to a Pierce BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA) to control for total protein content among samples. The lowest concentration of total protein was determined, and all samples were diluted to this concentration using 0.1 M of PBS solution before the ELISA. Optical density for each sample was obtained using a Versa max microplate reader (Molecular Devices LLC, Sunnyvale, CA) with Softmax Pro 5 software measuring absorbance at 450 nm. All standards and samples were analyzed in duplicate, and the average optical density reading for each trial was calculated. The average optical density of the blank wells was subtracted from each trial reading. This final optical density was then converted to enzyme concentration using the line of best fit equation obtained from plotting standard readings. Line of best fit equations were generated using a four-parameter logistic curve-fit with XLStat software.
Statistical Analysis
Measurements of start time, intensity, and end time of first fight were extracted from the video footage of each trial by a single observer blind to treatment. Dependent variables were calculated from these measurements including: time to initiate, time to each intensity level (see ethogram Table 2), time at each intensity level (see ethogram Table 2), and duration of fight. Measurements of cytochrome P450 concentrations (pg/mL) and acetylcholinesterase (ng/mL) were extracted from ELISA results. Agonistic behaviors and enzymatic responses were analyzed using R statistical software (version 3.3.0) (R Core Development Team 2016). All dependent variables were assessed for outliers and collinearity through the implementation of methods described in Zuur et al. (2009). A total of 11 agonistic behavior trials were consistently identified as outliers across multiple dependent variables and consequently removed from analysis (Table 1). A total of four pairs of variables showed collinearity thus dependent variables time at intensity − 1, time at intensity 4, time at intensity 8, and time to intensity 6 were removed from analysis. Regression analysis was implemented to examine the relationship of liters of solution (atrazine or control solution) entering stream during exposure period and dependent variables. Effect of independent variables were compared using a multivariate analysis of covariance (MANCOVA) to determine differences in adjusted means for variables between treatments with liters of solution entering the stream defined as the covariate. A Tukey Multiple Proportions test was used to determine differences in winner/loser outcome of agonistic fight trials (Hothorn et al. 2008).
Results
Fight Intensity and Dynamics
The interaction of atrazine concentration and mode of delivery significantly altered the dynamics of dyadic fights among crayfish. Specifically, time at intensity level 2 (MANCOVA: F8, 111, 0.05 = 6.46, p < 0.001) and time at intensity level 3 (MANCOVA: F8, 111, 0.05 = 3.53, p = 0.016) were significantly impacted by the interaction of atrazine concentration and mode of delivery.
Crayfish, exposed to 40-µg/L concentration of atrazine through mid-water column delivery, spent a significantly longer time at intensity level 2 compared with control, 80, and 160 µg/L mid-water delivery treatments (Tukey-HSD: Table 3; Fig. 2). Additionally, in this 40-µg/L concentration mid-water delivery treatment, crayfish also spent a longer time at intensity level 2 compared with 40 µg/L ground water delivery treatment (Table 3). Crayfish, exposed to 80-µg/L concentration via ground water delivery, had shorter times at intensity 3 compared with controls (Tukey-HSD: Table 4; Fig. 3).
Mean (± SEM) time spent at intensity level 2 (s) (see ethogram, Table 2) for ground water (solid black squares) and mid-water column (hollow red circles) modes of delivery for crayfish exposed to varying concentrations of atrazine (0, 40, 80, 160 µg/L). A MANCOVA with Tukey-HSD post hoc test was used to assess significant differences (p < 0.05) between exposure treatments
Mean (± SEM) time spent at intensity level 3 (s) (see ethogram, Table 2) for ground water (solid black squares) and mid-water column (hollow red circles) modes of delivery for crayfish exposed to varying concentrations of atrazine (0, 40, 80, 160 µg/L). A MANCOVA with Tukey-HSD post hoc test was used to assess significant differences (p < 0.05) between exposure treatments
Fight Initiation
The main effect of atrazine concentration delayed the time at which dyadic fights were initiated (MANCOVA: F8, 111, 0.05 = 3.17, p = 0.018). Crayfish, exposed to 40-µg/L concentration of atrazine, took longer to initiate in a fight compared with crayfish subjected to the control concentration (Tukey-HSD: p = 0.014; Fig. 4).
Fight Outcome and Duration
The concentration of atrazine and mode of delivery did not significantly impact the winner/loser outcome of agonistic interactions (Tukey Multiple Proportions: Concentration, \(\chi_{4,114}^{2}\) = 0.72, p > 0.05; Mode of delivery, \(\chi_{1,115}^{2}\) = 0.30, p > 0.05). Furthermore, total duration of agonistic contests was not altered due to concentration of atrazine (MANCOVA: F3, 111, 0.05 = 0.238, p > 0.05) or mode of delivery (MANCOVA: F1, 111, 0.05 = 0.026, p > 0.05).
Enzymatic Response
The interaction of atrazine concentration and mode of entry significantly affected the levels of cytochrome P450 detected in crayfish hepatopancreas samples (MANCOVA: F7, 24, 0.05 = 11.00, p < 0.001). Tissue from crayfish exposed to 40-µg/L concentration of atrazine through mid-water column delivery exhibited elevated cytochrome P450 levels relative to other treatments (Fig. 5). Specifically, cytochrome P450 levels were increased in 40-µg/L mid-water column crayfish compared with crayfish exposed to control, 80-, and 160-µg/L concentration via mid-water delivery exposure (Tables 5 and 6). Acetylcholinesterase activity was not significantly impacted by atrazine concentration or exposure type (MANCOVA: Concentration, F3, 24, 0.05 = 0.345, p > 0.05; Mode of delivery, F1, 24, 0.05 = 2.527, p > 0.05; Table 6).
Mean (± SEM) cytochrome P450 concentration (pg/mL) in hepatopancreas tissue for ground water (solid black squares) and mid-water column (hollow red circles) modes of delivery for crayfish exposed to varying concentrations of atrazine (0, 40, 80, 160 µg/L). A MANCOVA with Tukey-HSD post hoc test was used to assess significant differences (p < 0.05) between exposure treatments
Discussion
This study demonstrated three main findings regarding dynamic exposure to atrazine to benthic crustaceans. First, the intensity of fighting behaviors displayed by the crayfish is significantly affected by the interaction between the mode of delivery and concentration of atrazine. Specifically, 40 µg/L of atrazine through mid-water column delivery resulted in increased time spent at nonescalated behaviors, whereas 80 µg/L of atrazine through ground water delivery led to decreased time spent at nonescalated behaviors. Second, the temporal dynamics of crayfish agonistic interactions were significantly altered by concentration of atrazine, with the 40-µg/L exposed crayfish displaying a delay in time to initiate a fight. Third, alterations of fighting intensity, specifically that of crayfish displaying lower intensity fights, is mirrored in the physiological enzymatic response displayed. Crayfish exposed 40-µg/L concentration of atrazine through mid-water delivery also had increased cytochrome P450 expression within the hepatopancreas tissue; however, these exposures did not alter AChE activity. These results demonstrated both behavioral and physiological alterations in crayfish that are dependent on both source and concentration.
These findings show that exposure to atrazine does impact the social behaviors displayed by crayfish. Social interactions have been shown to be impacted by atrazine in other aquatic organisms subject to static exposure regimes (Saglio and Trijasse 1998; Schmidel et al. 2014; Shenoy 2012). It is important to point out that in this study exposure was dynamic in nature to mimic the fluctuating spatial and temporal distributions of chemicals in flowing systems. Moreover, exposure alters the temporal dynamics and overall intensity of agonistic interactions between animals. Delivery of low concentrations of atrazine via contaminated run-off cause more time spent at lower intensity behaviors, thus driving down the overall intensity of the interaction. Contrastingly, higher concentrations delivered through contaminated ground water cause the overall intensity of fights to increase, with less time dedicated to low intensity behaviors. These results do not exhibit a clear, linear dose–response to the contaminant. Furthermore, due the complexity of turbulent dispersion of chemicals within fluid systems, resulting toxicant plume structures are variant in magnitude, frequency, and duration (Edwards and Moore 2014; Harrigan and Moore 2017). These dynamic chemical structures result in complex responses in exposed animals. In the current study, dynamic exposure to toxicants alter behaviors, but the precise nature of that dynamic exposure (ground water vs. mid-water) determines the type and extent of the impairments seen.
Previous work on dynamic chemical dispersion has established that exposure pattern of contaminants differs depending on the method on habitat characteristics and contaminant source (Edwards and Moore 2014; Ludington and Moore 2017; Sanford 1997; Wolf et al. 2004). This is also seen by different distribution patterns of pollutants delivered to stream systems from point and non-point sources (Lahman and Moore 2015). The variation of chemical plume structure as a result of mode of delivery causes differing exposure regimes to a contaminant instigating varying effects to responses of aquatic organisms. The differing hydrodynamic environments and resulting chemical signal patterns referenced by this research aid in creating a clear and applicable definition for exposure for future ecotoxicological studies not currently captured by static exposure studies. The commonly used time-average static models do not appropriately capture the variations and episodic nature of natural exposure conditions in which create variable responses in exposed animals as exemplified in this study.
The dynamic nature of variation of chemical plume structure is highly dependent upon the presence or absence of turbulent mixing. The presence of turbulent eddies increases the temporal and spatial variation of the chemical plume as the chemical is carried, mixed, and dispersed by the eddy structures (Moore et al. 1994). Meanwhile, while approaching the substrate solid surface, water velocity decreases reaching near zero at the surface where the boundary layer formed (Hart et al. 1996; Hart and Finelli 1999; Nikora 2010). This boundary layer contains sublayers in which turbulence is dampened or nearly not present as the power of viscous forces outweigh the inertial forces thus mixing of the chemical plume is reduced (Moore et al. 1994; Nikora 2010). Contrastingly, the mid-depth in the water column offers a more chaotic environment with increasing velocities, where turbulence occurs thus causing swirling eddies and mixing of water molecules (Nikora 2010). These differences in hydrodynamic characteristics influence contaminant dispersion and change the expected paradigm of exposure resultant from ground water versus runoff sources. Thus, the differences found in exposure effects in this study are directly tied to differences in the physical forces associated with the different toxic plume dispersion.
Exposure has been shown to differentially impact agonistic behaviors of organisms by a variety of studies (Alkahem 1994; Cook and Moore 2008; Saglio et al. 1996, 2001; Sloman 2007; Sopinka et al. 2010). These varying effects could be explained by differences in exposure paradigms (e.g., turbulent dispersion) or even within the concept of exposure itself (e.g., pulse lengths or intermittency). The present study offers insight into the importance of considering chemical dispersion dynamics in relation to toxic effects on organisms. Currently, definitions of toxicant exposure focus on static concentration when measuring physiological (Aksu et al. 2015; Glusczak et al. 2007; Gluth and Hanke 1984) developmental (Choung et al. 2011; Hayes et al. 2002; Johnson et al. 2007), and behavioral effects of toxicant exposure (Ehrsam et al. 2016; Gaworecki and Klaine 2008; Sherba et al. 2000). These applications define toxicity based on a time-averaged concentration, which is a chemically based measure of concentration (e.g., molarity, ppt/ppb) over a measured time period (e.g., 24, 48, 72 h) within a static testing environment. Yet, the fluctuations of the fine scale chemical plume dispersion that occur in the environment are not captured in these commonly utilized time-averaged static exposure models. Toxicant plumes, whether terrestrial or aquatic, are dynamic in nature (Edwards and Moore 2014; Harrigan and Moore 2017). Concentrations of toxicants are time-dependent and are really fluxes moving past an organism (Finelli et al. 1999; Reinert et al. 2002). Moreover, subtle changes in environmental variables (e.g., flow speed, roughness elements) change the nature of those fluxes such that organisms residing in a similar region but experiencing hydrodynamically different regimes (such as in this study) will be exposed to different fluctuations of toxicants (Harrigan and Moore 2017). These results point to the need for an increased understanding of in situ chemical distribution and differences in this distribution due to contaminant source. The results of this study infer that the mode of delivery of the toxicant may impact the execution of ecologically important behaviors in various ways.
From an ecological perspective, agonistic behaviors delegate the allocation of important resources, such as shelter, food, and mates between competitors (Earley and Hsu 2013; Fero et al. 2007; Martin and Moore 2010; Parker 1974; Smith 1974). In addition, the behaviors displayed by animals serve to link physiological processes to ecological consequences; therefore, the alterations to physiology likely result in important behavioral and ecological process changes (Scott and Sloman 2004). Toxicant exposure can result in increased maintenance cost of which, given the requirement of an energy budget by every animal, energy is then pulled from reserves used to fuel other processes such as behavior (Jager et al. 2014). The physiological response of detoxification, i.e., levels of cytochrome P450, present within exposed animals, with an increase in the low concentration runoff treatment, is mirrored in the agonistic behavioral responses observed. Induction of detoxification mechanisms in crayfish has been a documented response to a range of differing environmental contaminants (Ashley et al. 1996; Snyder 2000). Differences in agonistic interactions may be influenced by the energetic cost of detoxification, rather than loss of motor control as displayed in crayfish exposed to other contaminants, because acetylcholinesterase levels did not differ across treatments due to atrazine exposure (Aksu et al. 2015, Fornstrom et al. 1997) (Table 6). Increased energy allocation in the process of detoxification may also impact the ability to maintain and/or manipulate resources obtained proceeding an agonistic interaction, such as a mating opportunity or defending a habitat/shelter used to protect oneself against predators. The evaluation of these survival and fitness impacts should include mode of delivery of contaminant, because these consequences may differ for various pollutant sources that create differing distribution patterns of contaminants.
The alarming increase of human impact on freshwater environments and the resulting effects of contaminant exposure create an imperative to understand the distribution of chemical pollutants introduced to aquatic systems. Additionally, regulatory target levels of a contamination within an environment rely on static-models addressing lethal concentrations (USEPA 2017). This study elucidates the importance of considering dynamic exposure, incorporating the pathway (i.e., ground water contamination or surface water run-off) that a contaminant enters an aquatic system, in future ecotoxicological studies as well as water-impairment standards.
References
Aksu O, Yildirim NC, Yildirim N, Danabas D, Danabas S (2015) Biochemical response of crayfish Astacus leptodactylus exposed to textile wastewater treated by indigenous white rot fungus Coriolus versicolor. Environ Sci Pollut Res 22:2987–2993. https://doi.org/10.1007/s11356-014-3550-z
Alkahem HF (1994) The toxicity of nickel and the effects of sublethal levels on haematological parameters and behaviour of the fish, Oreochromis niloticus. Kuwait J Sci 21:243–251
Ashauer R, Boxall A, Brown C (2006) Predicting effects on aquatic organisms from fluctuating or pulsed exposure to pesticides. Environ Toxicol Chem 25:1899–1912. https://doi.org/10.1897/05-393R.1
Ashley CM, Simpson MG, Holdich DM, Bell DR (1996) 2, 3, 7, 8-tetrachloro-dibenzo-p-dioxin is a potent toxin and induces cytochrome P450 in the crayfish, Pacifastacus leniusculus. Aquat Toxicol 35:157–169. https://doi.org/10.1016/0166-445X(96)00014-8
Belanger RM, Peters TJ, Sabhapathy GS, Khan S, Katta J, Abraham NK (2015) Atrazine exposure affects the ability of crayfish (Orconectes rusticus) to localize a food odor source. Arch Environ Contam Toxicol 68:636–645. https://doi.org/10.1007/s00244-015-0142-y
Belanger RM, Mooney LN, Nguyen HM, Abraham NK, Peters TJ, Kana MA, May LA (2016) Acute atrazine exposure has lasting effects on chemosensory responses to food odors in crayfish (Orconectes virilis). Arch Environ Contam Toxicol 70:289–300. https://doi.org/10.1007/s00244-015-0234-8
Belanger RM, Lahman SE, Moore PA (2017) Crayfish: an experimental model for examining exposure to environmental contamination. In: Larramendy ML (ed) Ecotoxicology and genotoxicology: non-traditional aquatic models. RSC Publishing, Philadelphia, pp 124–156
Bergman DA, Kozlowski CP, McIntyre JC, Huber R, Daws AG, Moore PA (2003) Temporal dynamics and communication of winner-effects in the crayfish, Orconectes rusticus. Behaviour 140:805–825. https://doi.org/10.1163/156853903322370689
Bovbjerg RV (1953) Dominance order in the crayfish Orconectes virilism (Hagen). Physiol Zool 26:173–178. https://doi.org/10.1086/physzool.26.2.30154514
Brunke M, Gonser T (1997) The ecological significance of exchange processes between rivers and groundwater. Freshw Biol 37:1–33. https://doi.org/10.1046/j.1365-2427.1997.00143.x
Choung CB, Hyne RV, Mann RM, Stevens MM, Hose GC (2011) Developmental toxicity of two common corn pesticides to the endangered southern bell frog (Litoria raniformis). Environ Pollut 159:2648–2655. https://doi.org/10.1016/j.envpol.2011.05.037
Cook ME, Moore PA (2008) The effects of the herbicide metolachlor on agonistic behavior in the crayfish, Orconectes rusticus. Arch Environ Contam Toxicol 55:94–102. https://doi.org/10.1007/s00244-007-9088-z
Davies J, Honegger JL, Tencalla FG, Meregalli G, Brain P, Newman JR, Pitchford HF (2003) Herbicide risk assessment for non-target aquatic plants: sulfosulfuron–a case study. Pest Manag Sci 59:231–237. https://doi.org/10.1002/ps.625
Denny MW (1993) Air and water: the biology and physics of life’s media. Princeton University Press, Princeton
Diana SG, Resetarits WJ, Schaeffer DJ, Beckmen KB, Beasley VR (2000) Effects of atrazine on amphibian growth and survival in artificial aquatic communities. Environ Toxicol Chem 19:2961–2967. https://doi.org/10.1002/etc.5620191217
Doležal F, Kvı́tek T (2004) The role of recharge zones, discharge zones, springs and tile drainage systems in peneplains of Central European highlands with regard to water quality generation processes. Phys Chem Earth Parts A/B/C 29:775–785. https://doi.org/10.1016/j.pce.2004.05.005
Earley RL, Hsu Y (2013) Contest behaviour in fishes. In: Hardy IC, Briffa M (eds) Animal contests. Cambridge University Press, Cambridge, pp 199–227
Edwards DD, Moore PA (2014) Real exposure: field measurement of chemical plumes in headwater streams. Arch Environ Contam Toxicol 67:413–425. https://doi.org/10.1007/s00244-014-0055-1
Ehrsam M, Knutie SA, Rohr JR (2016) The herbicide atrazine induces hyperactivity and compromises tadpole detection of predator chemical cues. Environ Toxicol Chem 35:2239–2244. https://doi.org/10.1002/etc.3377
Fero K, Simon JL, Jourdie V, Moore PA (2007) Consequences of social dominance on crayfish resource use. Behaviour 144:61–82. https://doi.org/10.1163/156853907779947418
Finelli CM, Pentcheff ND, Zimmer-Faust RK, Wethey DS (1999) Odor transport in turbulent flows: constraints on animal navigation. Limnol Oceanogr 44:1056–1071. https://doi.org/10.4319/lo.1999.44.4.1056
Fornstrom CB, Landrum PF, Weisskopf CP, La Point TW (1997) Effects of terbufos on juvenile red swamp crayfish (Procambarus clarkii): differential routes of exposure. Environ Toxicol Chem 16:2514–2520. https://doi.org/10.1002/etc.5620161212
Garen DC, Moore DS (2005) Curve number hydrology in water quality modeling: uses, abuses, and future directions. JAWRA J Am Water Resour Assoc 41:377–388. https://doi.org/10.1111/j.1752-1688.2005.tb03742.x
Gaworecki KM, Klaine SJ (2008) Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat Toxicol 88:207–213. https://doi.org/10.1016/j.aquatox.2008.04.011
Gerhardt G, Adams RN (1982) Determination of diffusion coefficient by flow injection analysis. Anal Chem 54:2618–2620
Glusczak L, Santos Miron D, Moraes BS, Simões RR, Schetinger MRC, Morsch VM, Loro VL (2007) Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen). Comp Biochem Physiol Part C Toxicol Pharmacol 146:519–524. https://doi.org/10.1016/j.cbpc.2007.06.004
Gluth G, Hanke W (1984) A comparison of physiological changes in carp, Cyprinus carpio, induced by several pollutants at sublethal concentration—II. The dependency on the temperature. Comp Biochem Physiol Part C Comp Pharmacol 79:39–45. https://doi.org/10.1016/0742-8413(84)90160-9
Gordon AK, Mantel SK, Muller NW (2012) Review of toxicological effects caused by episodic stressor exposure. Environ Toxicol Chem 31:1169–1174. https://doi.org/10.1002/etc.1781
Graymore M, Stagnitti F, Allinson G (2001) Impacts of atrazine in aquatic ecosystems. Environ Int 26:483–495. https://doi.org/10.1016/S0160-4120(01)00031-9
Guiasu RC, Dunham DW (1997) Initiation and outcome of agonistic contests in male form I Cambarus robustus Girard, 1852 crayfish (Decapoda, Cambaridae). Crustaceana 70:480–496. https://doi.org/10.1163/156854097X00069
Handy RD (1994) Intermittent exposure to aquatic pollutants: assessment, toxicity and sublethal responses in fish and invertebrates. Comp Biochem Physiol Part C Pharmacol Toxicol Endocrinol 107:171–184. https://doi.org/10.1016/1367-8280(94)90039-6
Harrigan KM, Moore PA (2017) Scaling to the organism: an innovative model of dynamic exposure hotspots in stream systems. Arch Environ Contam Toxicol. https://doi.org/10.1007/s00244-017-0444-3
Hart DD, Finelli CM (1999) Physical-biological coupling in streams: the pervasive effects of flow on benthic organisms. Annu Rev Ecol Syst 30:363–395. https://doi.org/10.1146/annurev.ecolsys.30.1.363
Hart DD, Clark BD, Jasentuliyana A (1996) Fine-scale field measurement of benthic flow environments inhabited by stream invertebrates. Limnol Oceanogr 41:297–308. https://doi.org/10.4319/lo.1996.41.2.0297
Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A (2002) Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci 99:5476–5480. https://doi.org/10.1073/pnas.082121499
Hill AM, Lodge DM (1999) Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecol Soc Amer 9:678–690. https://doi.org/10.1890/1051-0761(1999)009[0678:RORCBA]2.0.CO;2
Hilz E, Vermeer AW (2013) Spray drift review: the extent to which a formulation can contribute to spray drift reduction. Crop Prot 44:75–83. https://doi.org/10.1016/j.cropro.2012.10.020
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biomet J 50:346–363. https://doi.org/10.1002/bimj.200810425
Jablonowski ND, Schäffer A, Burauel P (2011) Still present after all these years: persistence plus potential toxicity raise questions about the use of atrazine. Environ Sci Pollut Res 18:328–331. https://doi.org/10.1007/s11356-010-0431-y
Jager T, Barsi A, Hamda NT, Martin BT, Zimmer EI, Ducrot V (2014) Dynamic energy budgets in population ecotoxicology: applications and outlook. Ecol Model 280:140–147. https://doi.org/10.1016/j.ecolmodel.2013.06.024
Johnson A, Carew E, Sloman KA (2007) The effects of copper on the morphological and functional development of zebrafish embryos. Aquat Toxicol 84:431–438. https://doi.org/10.1016/j.aquatox.2007.07.003
Jørgensen BB, Des Marais DJ (1990) The diffusive boundary layer of sediments: oxygen microgradients over a microbial mat. Limnol Oceanogr 35:1343–1355. https://doi.org/10.4319/lo.1990.35.6.1343
Karavanich C, Atema J (1998) Olfactory recognition of urine signals in dominance fights between male lobster, Homarus americanus. Behaviour 135:719–730. https://doi.org/10.1163/156853998792640440
Krutz LJ, Senseman SA, Zablotowicz RM, Matocha MA (2005) Reducing herbicide runoff from agricultural fields with vegetative filter strips: a review. Weed Sci 53:353–367. https://doi.org/10.1614/WS-03-079R2
Lahman SE, Moore PA (2015) Fine-scale chemical exposure differs in point and nonpoint source plumes. Arch Environ Contam Toxicol 68:729–744. https://doi.org/10.1007/s00244-014-0116-5
Lapworth DJ, Baran N, Stuart ME, Ward RS (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut 163:287–303. https://doi.org/10.1016/j.envpol.2011.12.034
Leonard RA (1990) Movement of pesticides into surface waters. In: Cheng HH (ed) Pesticides in the soil environment: processes, impacts, and modeling. Social Science Society of America Inc, Madison, pp 303–349
Lesan HM, Bhandari A (2003) Atrazine sorption on surface soils: time-dependent phase distribution and apparent desorption hysteresis. Water Res 37:1644–1654. https://doi.org/10.1016/S0043-1354(02)00497-9
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97. https://doi.org/10.1007/BF02472006
Ludington TS, Moore PA (2017) The degree of impairment of foraging in crayfish (Orconectes virilis) due to insecticide exposure is dependent upon turbulence dispersion. Arch Environ Contam Toxicol 72:281–293. https://doi.org/10.1007/s00244-016-0341-1
Martin AL III, Moore PA (2010) The influence of reproductive state on the agonistic interactions between male and female crayfish (Orconectes rusticus). Behav 147:1309–1325. https://doi.org/10.1163/000579510X520989
Moore P, Crimaldi J (2004) Odor landscapes and animal behavior: tracking odor plumes in different physical worlds. J Mar Syst 49:55–64. https://doi.org/10.1016/j.jmarsys.2003.05.005
Moore PA, Weissburg MJ, Parrish JM, Zimmer-Faust RK, Gerhardt GA (1994) Spatial distribution of odors in simulated benthic boundary layer flows. J Chem Ecol 20:255–279. https://doi.org/10.1007/BF02064435
Moore PA, Grills JL, Schneider RW (2000) Habitat-specific signal structure for olfaction: an example from artificial streams. J Chem Ecol 26:565–584. https://doi.org/10.1023/A:1005482027152
Neal AE, Moore PA (2017) Mimicking natural systems: changes in behavior as a result of dynamic exposure to naproxen. Ecotoxicol Environ Saf 135:347–357. https://doi.org/10.1016/j.ecoenv.2016.10.015
Nikora V (2010) Hydrodynamics of aquatic ecosystems: an interface between ecology, biomechanics and environmental fluid mechanics. River Res Appl 26:367–384. https://doi.org/10.1002/rra.1291
Nowell ARM, Jumars PA (1984) Flow environments of aquatic benthos. Annu Rev Ecol Syst 15:303–328. https://doi.org/10.1146/annurev.es.15.110184.001511
Parker GA (1974) Assessment strategy and the evolution of fighting behaviour. J Theor Biol 47:223–243
Pedersen ML, Friberg N (2009) Influence of disturbance on habitats and biological communities in lowland streams. Fund Appl Limnol 174:27–41. https://doi.org/10.1016/0022-5193(74)90111-8
Pionke HB, Glotfelty DW (1990) Contamination of groundwater by atrazine and selected metabolites. Chemosphere 21:813–822. https://doi.org/10.1016/0045-6535(90)90268-X
R Core Development Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at: https://www.R-project.org/
Rahman S, Webster DR (2005) The effect of bed roughness on scalar fluctuations in turbulent boundary layers. Exp Fluids 38:372–384. https://doi.org/10.1007/s00348-004-0919-7
Rasmussen JJ, Wiberg-Larsen P, Baattrup-Pedersen A, Friberg N, Kronvang B (2012) Stream habitat structure influences macroinvertebrate response to pesticides. Environ Pollut 164:142–149. https://doi.org/10.1016/j.envpol.2012.01.007
Reichenberger S, Bach M, Skitschak A, Frede HG (2007) Mitigation strategies to reduce pesticide inputs into ground and surface water and their effectiveness; a review. Sci Total Environ 384:1–35. https://doi.org/10.1016/j.scitotenv.2007.04.046
Reinert KH, Giddings JM, Judd L (2002) Effects analysis of time-varying or repeated exposures in aquatic ecological risk assessment of agrochemicals. Environ Toxicol Chem 21:1977–1992. https://doi.org/10.1002/etc.5620210928
Saglio P, Trijasse S (1998) Behavioral responses to atrazine and diuron in goldfish. Arch Environ Contam Toxicol 35:484–491. https://doi.org/10.1007/s002449900406
Saglio P, Trijasse S, Azam D (1996) Behavioral effects of waterborne carbofuran in goldfish. Arch Environ Contam Toxicol 31:232–238. https://doi.org/10.1007/BF00212371
Saglio P, Olsén KH, Bretaud S (2001) Behavioral and olfactory responses to prochloraz, bentazone, and nicosulfuron-contaminated flows in goldfish. Arch Environ Contam Toxicol 41:192–200. https://doi.org/10.1007/s002440010237
Sanford LP (1997) Turbulent mixing in experimental ecosystem studies. Mar Ecol Prog Ser 161:265–293
Schmidel AJ, Assmann KL, Werlang CC, Bertoncello KT, Francescon F, Rambo CL, Beltrame GM, Calegari D, Batista CB, Blaser RD, Júnior WAR, Conterato GMM, Piato AL, Zanatta L, Magro JD, Rosemberg DB (2014) Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol Teratol 44:62–69. https://doi.org/10.1016/j.ntt.2014.05.006
Schulz R (2001) Comparison of spray drift-and runoff-related input of azinphos-methyl and endosulfan from fruit orchards into the Lourens River, South Africa. Chemosphere 45:543–551. https://doi.org/10.1016/S0045-6535(00)00601-9
Scott GR, Sloman KA (2004) The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68:369–392. https://doi.org/10.1016/j.aquatox.2004.03.016
Shenoy K (2012) Environmentally realistic exposure to the herbicide atrazine alters some sexually selected traits in male guppies. PLoS ONE 7:e30611. https://doi.org/10.1371/journal.pone.0030611
Sherba M, Dunham DW, Harvey HH (2000) Sublethal copper toxicity and food response in the freshwater crayfish Cambarus bartonii (Cambaridae, Decapoda, Crustacea). Ecotoxicol Environ Saf 46:329–333. https://doi.org/10.1006/eesa.1999.1910
Sloman KA (2007) Effects of trace metals on salmonid fish: the role of social hierarchies. Appl Anim Behav Sci 104:326–345. https://doi.org/10.1016/j.applanim.2006.09.003
Smith JM (1974) The theory of games and the evolution of animal conflicts. J Theor Biol 47:209–221. https://doi.org/10.1016/0022-5193(74)90110-6
Snyder MJ (2000) Cytochrome P450 enzymes in aquatic invertebrates: recent advances and future directions. Aquat Toxicol 48:529–547. https://doi.org/10.1016/S0166-445X(00)00085-0
Sophocleous M (2002) Interactions between groundwater and surface water: the state of the science. Hydrogeol J 10:52–67. https://doi.org/10.1007/s10040-001-0170-8
Sopinka NM, Marentette JR, Balshine S (2010) Impact of contaminant exposure on resource contests in an invasive fish. Behav Ecol Sociobiol 64:1947–1958. https://doi.org/10.1007/s00265-010-1005-1
Steinberg CE, Lorenz R, Spieser OH (1995) Effects of atrazine on swimming behavior of zebrafish, Brachydanio rerio. Water Res 29:981–985. https://doi.org/10.1016/0043-1354(94)00217-U
Thorp JH, Thoms MC, Delong MD (2006) The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Res Appl 22:123–147. https://doi.org/10.1002/rra.901
United State Environmental Protection Agency (2014) Atrazine updates: 2014 atrazine ecological exposure monitoring program data. https://www.regulations.gov/document?D=EPA-HQ-OPP-2003-0367-0303. Accessed 26 Jan 2018
United States Environmental Protection Agency (2017) ECOTOX Database Release 4.0. http://www.epa.gov/ecotox. Accessed 20 Dec 2017
Vogel S (1994) Life in moving fluids. The physical biology of flow, 2nd edn. Princeton University Press, Princeton
Webster DR, Weissburg MJ (2009) The hydrodynamics of chemical cues among aquatic organisms. Annu Rev Fluid Mech 41:73–90. https://doi.org/10.1146/annurev.fluid.010908.165240
Williams PL, Dusenbery DB (1990) Aquatic toxicity testing using the nematode, Caenorhabditis elegans. Environ Toxicol Chem 9:285–1290. https://doi.org/10.1002/etc.5620091007
Wofford SJ, Earley RL, Moore PA (2015) Evidence for assessment disappears in mixed-sex contests of the crayfish, Orconectes virilis. Behaviour 152:995–1018. https://doi.org/10.1163/1568539X-00003265
Wofford SJ, LaPlante PM, Moore PA (2017) Information depends on context: behavioural response to chemical signals depends on sex and size in crayfish contests. Behaviour 154:287–312. https://doi.org/10.1163/1568539X-00003422
Wolf MC, Voigt R, Moore PA (2004) Spatial arrangement of odor sources modifies the temporal aspects of crayfish search strategies. J Chem Ecol 30:501–517. https://doi.org/10.1023/B:JOEC.0000018625.83906.95
Zoumis T, Schmidt A, Grigorova L, Calmano W (2001) Contaminants in sediments: remobilisation and demobilisation. Sci Total Environ 266:195–202. https://doi.org/10.1016/S0048-9697(00)00740-3
Zulandt Schneider RA, Huber R, Moore PA (2001) Individual and status recognition in the crayfish Orconectes rusticus: the effects of urine release on fight dynamics. Behaviour 138:137–154. https://doi.org/10.1163/15685390151074348
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York City
Acknowledgements
The authors thank the Laboratory for Sensory Ecology for their continued support, comments, and help throughout this project. Specific thanks to LSE member Cassidy Florey for her help with the data collection process. Thanks also goes to the University of Michigan Biological Station for funding through the Marian P. and David M. Gates Graduate Student Endowment Fund to A. N. S. and also for the use of facilities. Lastly, thanks to the Crustacean Society for a Fellowship in Graduate Studies to A.N.S., University of Detroit Mercy for a Faculty Research Award to R.M.B., and Bowling Green State University Faculty Research Committee for a Building Strength Award and a Fulbright Fellowship to P.A.M. for help in funding this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steele, A.N., Belanger, R.M. & Moore, P.A. Exposure Through Runoff and Ground Water Contamination Differentially Impact Behavior and Physiology of Crustaceans in Fluvial Systems. Arch Environ Contam Toxicol 75, 436–448 (2018). https://doi.org/10.1007/s00244-018-0542-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-0542-x