Abstract
The white stork (Ciconia ciconia) is being increasingly used in biomonitoring programmes of environmental contaminants due to its growing population in Europe; however, studies on inorganic elements are scarce. The blood of 70 white storks was collected and analysed by inductively coupled plasma mass spectroscopy (ICP-MS) to determine the presence of the following elements: lead (Pb), mercury (Hg), arsenic (As), nickel (Ni), iron (Fe), zinc (Zn), copper (Cu), selenium (Se), manganese (Mn), chromium (Cr), cobalt (Co), and cadmium (Cd). Our main goals were to determine the mean concentrations of these elements in the blood and to study its association with age and gender. Mean concentrations were highest for Fe, followed by Zn, and lowest for Co and Cd. The metal levels were similar to the values referred in the literature for the same species from different locations. No statistically significant differences were found between males and females. Regarding age, statistically significant differences were observed for Ni, Cu, Se, Hg, and Pb between young and adult animals (except for Pb, values in adults were higher than in fledglings). Many element concentrations were correlated, with the strongest correlations between the pairs Hg–Se, Hg–As, and Fe–Zn, mainly in adults. This study provides the baseline data for a monitoring program based on white stork blood as a nondestructive sample.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Birds have been playing a prominent role in the monitoring of different types of pollutants that are released into the environment (Furness 1993). Although most biomonitoring studies using birds have been conducted on internal tissues, the number of studies using nondestructive methods, such as measuring concentrations in unhatched eggs, faeces, feathers, and/or blood, is increasing (Blanco et al. 2003; Berglund et al. 2011; Burger and Gochfeld 2009; De la Casa-Resino et al. 2015a, b). Blood is the tissue of choice for nondestructive biomonitoring when determining the recent exposure to inorganic contaminants, because it is obtained quickly and easily with minimal risk and can be obtained repeatedly from the same individual if required, without sacrifice (Fossi and Leonzio 1993). However, to assess an ecosystem’s health adequately by means of biomonitoring, representative species must be selected, as some species have biological habits that increase their likelihood of exposure to contaminants and thus provide relevant information (Carneiro et al. 2014). The white stork (Ciconia ciconia) has been considered a good indicator of the quality of the natural environment (Tkachenko and Kurhaluk 2012), and the blood of white stork chicks has been used as a bioindicator of environmental contamination by metals (Baos et al. 2006a, b; Benito et al. 1999; Cabo et al. 2012; De la Casa-Resino et al. 2014, 2015b; Kamiński et al. 2006, 2008; Pérez-López et al. 2016). The blood of white storks also has been used, although scarcely in biomonitoring projects on organic contaminants (Blázquez et al. 2006; De la Casa-Resino et al. 2015a, b; Pérez-López et al. 2016).
Metals and metalloids are natural components of the environment, and many are essential to metabolic activities and indispensable to life, such as cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), and selenium (Se) (Benito et al. 1999); however, in high doses, they may be toxic (Benito et al. 1999; Carneiro et al. 2014; Lucia et al. 2010). Moreover, the variability in the levels of these elements found in bird bodies is influenced by background environmental levels (Blanco et al. 2003), which will be reflected in the amounts accumulated. Other elements, such as lead (Pb), mercury (Hg), nickel (Ni), cadmium (Cd), and arsenic (As), are generally not required for the metabolic activity, are highly toxic to wildlife, and even in low doses can have harmful effects on animals (Carneiro et al. 2014; Florea and Büsselberg 2006; Merian 1991). In fact, pollution by metals and metalloids represents a serious threat to ecosystems and is responsible for numerous pathologies in wildlife (Lucia et al. 2010). These elements are commonly found in the environment because of natural occurrence and/or human activities, such as industries and agriculture (Lucia et al. 2010), and their concentration might be increased through food webs (Hernández et al. 1999; Koivula and Eeva 2010).
It is known that even closely related bird species may show different metal accumulation and excretion (Beyer et al. 1988; Berglund et al. 2011; Burger and Gochfeld 2009; Eeva et al. 2009; Hofer et al. 2010). This fact could be related to different trophic levels, diets, or physiological and ecological species-specific trace-element requirements (Berglund et al. 2011). The levels of trace elements and their effects on organisms are in fact influenced by numerous factors related to habitat, physiology, and life history (Peakall and Burger 2003). Also, interactions between elements sometimes occur, including essential elements that can have an active role in the detoxification of toxic elements (e.g., Se and Hg, respectively) (Yang et al. 2008).
When interpreting metal concentrations in blood, the birds’ age also should be considered (García-Fernández et al. 1996; Burger and Gochfeld 1997a). The sampling of nestlings has the benefit of providing data from a limited time-period and territory as they are fed entirely on food resources obtained locally (De la Casa-Resino et al. 2014). On the other hand, adults, as already mentioned, might accumulate metals over a longer period and during migration (Furness 1993; Berglund et al. 2011). Most of the studies on metals presence in the blood of white storks have focused on nestlings (Alvarez et al. 2013; Baos et al. 2006a, b; Benito et al. 1999; Cabo et al. 2012; De la Casa-Resino et al. 2014, 2015b; Meharg et al. 2002; Pérez-López et al. 2016), and only one study reported an increase in the concentration of Cd, Pb, and Co in the blood of white stork chicks during the nestling development (Tkachenko and Kurhaluk 2012). Information on metal concentrations in adult white storks is almost inexistent and only related to internal tissues, such as the kidney, liver, or muscle (Gómez et al. 2004). So, data about potential differences in metal levels in white storks according to age are needed.
Sex has been indicated as a significant factor for the accumulation of Cd in the liver of several bird species, including the white stork, with males showing higher concentrations than females (Gómez et al. 2004). Laying females may pass some metals to their eggs (e.g., Hg, Pb, and Ni) (Blanco et al. 2003; Mansouri and Hoshyari 2012) but usually a smaller amount than that excreted during moulting (Furness 1993; Honda et al. 1986). Although several studies have shown significant differences in metal levels between male and female birds, these differences can be considered small compared with the high levels of individual variation (Furness 1993). Peakall and Burger (2003) also referred gender-related differences in the susceptibility of birds to metals, because the size and type of the captured prey they eat (and, consequently, the amount of contaminants that are ingested) is conditioned by the size differences between males and females. The white stork is a monomorphic bird species, and its size is similar in both genders (Kamiński et al. 2015). So, differences in feeding related to different sizes and types of prey are not expected, neither are important differences in the concentrations of chemical elements in their blood. However, to the best of our knowledge, the influence of gender on blood metal levels in white storks has not been previously studied.
Blood samples from white stork fledglings and adults from Extremadura (a region in western Spain) were collected, and 12 elements [Fe, zinc (Zn), Cu, Se, Pb, Hg, Mn, As, Ni, chromium (Cr), Co, and Cd] were analysed. The study was designed to evaluate the white storks’ degree of exposure to those elements and to examine whether the levels of the elements in their blood are affected by age and gender, as happens in other bird species. Of the 12 elements, some were selected for being nonessential elements considered highly toxic (As, Cd, Pb, and Hg) and the others for being essential trace elements whose metabolism can be altered by the nonessential ones.
Materials and Methods
Selection of Animals and Collection of Samples
The blood samples were collected from a total of 70 white storks living in the “Los Hornos” Wildlife Recovery Centre, in Extremadura, Spain, during 2006 (11 fledglings), 2007 (26 fledglings and 19 adults), and 2008 (14 adults). The birds were classified into two groups: adults, which had more than 2 years of age and a bright red beak and red legs (n = 33), and young birds, which were fledglings aged 6–9 weeks (n = 37). In the case of fledglings, the cause of entry at the Recovery Centre was having fallen to the ground when practising their flight, without suffering wounds. The adults from our study were admitted after suffering electrocution from overhead power lines or wing injuries that prevented them from flying; in both cases, there had been no significant loss of blood or life-threatening injuries. The blood samples were only collected after the animals had recovered from inflammatory processes and when their health status allowed drawing blood (5–10 days). Every bird was physically and clinically assessed by the veterinary staff of the Recovery Centre. They were kept outdoor in a flight cage (20 m × 20 m × 5 m), with water ad libitum. Their diet was based on cut up domestic chicken and 1-day-old dead domestic chicks (Gallus gallus domesticus) throughout the recovery period. We were authorised by the local government (Consejería de Medio Ambiente of Junta de Extremadura) to perform blood sampling.
The manipulation of birds was performed carefully to generate minimal stress to the animals, which were gently restrained by hand during blood collection. The blood was sampled in the morning, to avoid errors due to circadian variations, in the spring season. Each sample of blood (3–5 ml) was obtained from the brachial vein using a 0.8 × 25-mm needle and a 5-ml heparinised syringe (lithium heparin). Following clinical practice guidelines, the blood collected did not correspond to more than 1% of each bird’s body weight (Gaunt et al. 1997). The sample was transferred into 1.5-ml Eppendorf® tubes that had been previously washed with 2% HNO3 for elemental analysis. Additionally, a drop of blood was preserved in alcohol in an Eppendorf® tube for further bird gender identification. All samples were individually labelled, refrigerated and transported to the laboratory where they were stored at −80 °C until the elemental determination by inductively coupled plasma mass spectroscopy (ICP-MS).
Determination of Elements in Blood
A detailed description of the methodology and analytical conditions of this study can be found in De la Casa-Resino et al. (2014). In summary, 200 µL of blood were added to 50 µL of isopropanol and 25 µL of the internal standard solution (a solution of yttrium, rhenium, rhodium, and tellurium at 10 mg/L purchased from PerkinElmer Inc., Shelton, CT). An aqueous solution containing NH4OH (0.7 mM), Triton X-100 (0.07% v/v), and EDTA (0.01 mM) was added to the previous mix until reaching a total of 5 mL; the final mixture was vortexed and analysed. The analysis was performed in the Elemental and Molecular Analysis Laboratory of the Research Support Service (SAIUEX, accredited by ISO 9001:2008) of the University of Extremadura (Caceres, Spain), using the NexION 300D ICP-MS equipped with an S10 autosampler (PerkinElmer, Inc.). The method chosen was ICP-MS, because it provides adequate low detection limits and allows the simultaneous determination of several elements. The limit of detection (LOD) of the elements in the blood were 1.40 µg/L for Cr, 0.92 µg/L for Ni, 41.18 µg/L for Fe, 3.46 µg/L for Cu, 0.59 µg/L for Mn, 0.42 µg/L for Co, 0.25 µg/L for Cd, 0.21 µg/L for Pb, 3.01 µg/L for Hg, 1.55 µg/L for Se, 13.45 µg/L for Zn, and 0.50 µg/L for As. Calibrating solutions were prepared every day using a 10-mg/L Multi-element Calibration Standard 3 (PerkinElmer, Inc., Shelton, CT) and were surveyed the same way as the samples. A whole-blood certified reference sample (Seronorm® Trace Elements Whole Blood) was used for elemental precision. The values obtained for every element were consistent with the certified reference values. The obtained recoveries ranged from 92% (Hg) to 107% (Se), and the coefficients of variation for replicate samples (n = 5) were lower than 6.5%. The syringes and tubes used for blood sampling, as well as all the material used in this analysis, were prewashed with 10% HNO3, and blanks (including lithium heparin) were included in every batch of analysis.
Gender Determination
Because the white stork is a monomorphic species, a PCR technique based on the CDH-W-related sequence on the W chromosome was used to determine the gender of the sampled animals, as described by Griffiths et al. (1996). Female birds have heterogametic (ZW) chromosomes, whereas male birds have two identical chromosomes (ZZ). The Genetic Unit of the Faculty of Veterinary Medicine of the University of Extremadura (Caceres, Spain) applied this methodology and safely determined the gender of 23 fledgling and 19 adult white storks. Therefore, the influence of gender was only studied in these 42 animals.
Statistical Analysis
The obtained results were presented based on mean ± standard deviation, 25 and 75 percentiles, median, and minimum and maximum values for the whole samples. The results also were grouped according to age (fledglings or adults).
The data were analysed using the statistical software Prism 6, version for Windows (GraphPad software, Inc., CA) and the LibreOffice spreadsheet. The data were tested for normality (test of Kolmogorov–Smirnov, D’Agostino–Pearson and Shapiro–Wilk) and homoscedasticity (Levene’s test), but the distributions of the data significantly differed from the normal. After log transformation, not all variables showed a normal distribution, and the assumptions of the parametric tests were not fulfilled, so nonparametric tests were used in every analysis. The Mann–Whitney test for two independent samples was used when comparing two groups (fledglings 2006 vs. fledglings 2007; adults 2006 vs. adults 2007; fledglings vs. adults; females vs. males). The Kruskal–Wallis test (followed by post hoc Dunn’s test) was applied to compare four groups with samples smaller than 30 (fledgling males; fledgling females; adult females; adult males) when studying the influence of both factors (age and gender) for each element. To assess the correlation between the different element concentrations in the blood, the Spearman correlation test and its statistical significance were used. Data were considered statistically significant at p < 0.05.
A value of one-half the detection limit was assigned to every blood sample below the LOD and included in the data set for statistical treatment; this technique minimises nominal type I error rates (Clarke 1998).
Results
Most of the samples analysed exhibited values above the LOD for the elements evaluated, except for Cr, Ni, Cd, As, Co, and Hg (with 57, 35, 30, 11, 1.4, and 1.4% of samples below the LOD, respectively). Co and Cd were the elements with the lowest concentrations in the blood, whereas Fe, Zn, and Cu were the elements with the highest concentrations (Table 1).
When comparing the data of different years of sampling for each age group, no statistical differences were observed (p > 0.05 in the Mann–Whitney test for fledglings 2006 vs. fledglings 2007 and adults 2006 vs. adults 2007, in all variables studied). So, the years of sampling were not considered when grouping the specimens by age, and only two groups were considered: fledglings and adults.
Influence of Age and Gender
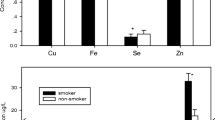
A statistical influence of age was observed in the concentrations of five elements: Ni, Cu, Se, Hg, and Pb (Table 2). For Ni, Cu, Se, and Hg, values in adults were higher than in fledglings. For Pb, the opposite was observed. No statistically relevant differences were detected in the concentrations when only gender was considered. However, when age and gender were considered simultaneously (Table S1, Supplementary Material), the differences related to age that had been previously observed for Se and Pb were directly associated with males (Fig. 1). It is noteworthy that, when assessing age and gender simultaneously, a statistically significant difference in Cr was found between the four groups (p < 0.05), mainly due to a statistically significant difference between female fledglings (all the animals in this group had values < LOD) and male adults (the highest mean).
Correlations
With respect to the correlation analysis, several significant inter-element correlations were detected (Table 3). When all data were considered together, a total of 20 significant Spearman correlations were obtained, most of which were positive and only two (Mn–Zn and Ni–Pb) were negative. As, Mn, and Cu were the elements with the highest number of significant correlations (6, 6, and 5, respectively). However, the most significant correlations were found in pairs Hg–Se, Hg–As, and Fe–Zn (Fig. 2). When the correlation study was performed considering age, the adult group showed a higher number of significant correlations (n = 25) compared with only nine in fledglings (Table S2, Table S3, Supplementary Material). With respect to gender, no correlations were observed when all data were used.
When both factors were considered simultaneously (age and gender), these same correlations were detected, especially associated with adults and particularly with males: 19 pairs of elements were significantly correlated in male adults and 15 in female adults. On the other hand, female fledglings represented the group with the smallest number of significant correlations—only five, and male fledglings had eight.
Discussion
In our study, Fe was the metal with the highest concentration, followed by Zn, Cu, Se, Pb, Hg, Mn, and As; Ni, Cr, Co, and Cd had the lowest concentrations. A similar order of levels of concentration—Fe with the highest concentrations, followed by Zn, Cu, Cr, Ni, Pb, and Cd—was observed in mallards (Anas platyrhynchos) from urban areas in Poland. Se, Hg, Mn, As, and Co were not measured in those animals (Binkowski and Meissner 2013). Fe, Zn, Cu, and Se were expected to have the highest concentrations since all of them are essential elements and are always at higher levels than nonessential elements, such as As, Cd, and Hg, which showed much lower concentrations in this study. The very high level of Fe (which had to be expressed in mg/L instead of µg/L) results from the fact that most of the Fe in the body is bound to haemoglobin, and we used total blood (including erythrocytes loaded with haemoglobin) in our analysis, instead of plasma or serum, as done in other studies (Kamiński et al. 2008).
The values obtained for Se were similar to those quantified in the blood of white stork chicks from Southern Spain (382 µg/L) (Alvarez et al. 2013) but markedly higher than the values of white stork chicks sampled in the same region of Spain—Extremadura (De la Casa-Resino et al. 2014). These differences are not unusual because blood concentrations of Se reflect recent exposure due to its short half-life in the blood—37 days for wild spectacled eiders (Somateria fischeri) and ten days for captive mallards (Grand et al. 2002; Wilson et al. 2004). If the background levels of Se (100–400 µg/L) proposed by the U.S. Department of the Interior (Interior 1998) were applied, almost 30% of the samples (most of them from adults) would exceed those levels.
Cu levels in the blood of white storks were, overall, similar to or even lower than those found in conspecifics from other natural areas around Spain (i.e., Doñana National Park, Southwestern Spain) (Baos et al. 2006b; Benito et al. 1999) and, in all cases, lower than those reported for birds sampled near potential sources of contamination by metals (Kamiński et al. 2008; Van Eeden and Schoonbee 1996). Concentrations of Cu in tissues may vary highly during the year and also may be associated with pollution of the environment (Kalisinska et al. 2004; Parslow et al. 1982), mainly when caused by a farming activity where herbicides and fungicides with Cu are being used (Binkowski and Sawicka-Kapusta 2015). The maximum value of Cu obtained in this study (934.7 µg/L) was lower than the minimum value (≈1880 µg/L) observed in the blood of sacred ibises (Threskiornis aethiopicus) from highly contaminated areas, with no apparent chronic or negative effects on survival (Van Eeden and Schoonbee 1996).
As observed in Cu, the values of Zn were similar to or even lower than those obtained from white storks sampled at different locations in Spain (Baos et al. 2006b; Benito et al. 1999; De la Casa-Resino et al. 2014) and markedly lower than those in animals living in polluted areas of Poland (Kamiński et al. 2008). Zn concentrations in tissues are influenced by the month of collection (directly related to the moult cycle) and the amount of plants in the animal’s diet (Parslow et al. 1982). Zn poisoning is observed quite often in nature, and, in most cases, it is related to the ingestion of Zn compounds (Binkowski and Meissner 2013), with plasma concentrations in poisoned birds reaching more than 15,000 µg/L (Eisler 1993). Moreover, values of Se > 7500 µg/L have been observed in birds in contaminated areas (Falandysz et al. 1988), and those are far from the values quantified in the present study.
Only a few studies conducted on white storks have determined the blood levels of Co, Fe, and Mn, and they have all been conducted in Spain (Benito et al. 1999; De la Casa-Resino et al. 2014) and Poland (Kamiński et al. 2008, 2009). Levels of Fe in white storks in the present study were ten times higher than those from Poland, but concentrations were lower than those observed in all the other studies.
Cr and Ni were the elements with the highest nondetectable concentrations: 57 and 35% were <LOD, respectively. This fact also has been found in mallards from urban areas in Poland with similar percentages (Binkowski and Meissner 2013), and that article is the only one we have found that reports Cr in the blood of birds (mean values < 100 µg/L). Other authors, such as Burger and Gochfeld (2009), found that Cr levels in the feathers of some bird species are higher than As and Cd levels. The mean levels of Ni in blood in the present study were much lower than those reported in sacred ibises from a highly metal-contaminated area (≈1780–17,120 µg/L) (Van Eeden and Schoonbee 1996). Despite Ni and Cr playing physiological roles in animals (Binkowski and Meissner 2013), references in the literature about their effects and their basal concentrations in birds are almost nonexistent. Also, Cr in experimental studies on birds is considered teratogenic, mutagenic and carcinogenic (Eisler 1986). Ni, in high levels, can cause adverse health effects (Mansouri and Hoshyari 2012), such as growth depression in broiler chicks (Weber and Reid 1968).
Concentrations of As were similar to those found in ciconiiformes in Spain (Baos et al. 2006b; Benito et al. 1999; De la Casa-Resino et al. 2014). It must be noted that the available information about the threshold value of this metalloid is limited in nestling or fledgling blood, but some authors have reported As value of 20 µg/L as the reference value for uncontaminated areas (Burger and Gochfeld 1997a). If this threshold value is considered, as many as 26% of the samples in the present study exceeded it, indicating a possible situation of risk for the population under study; however, the previously mentioned authors stated that there is great variability between specimens of the same species.
With respect to Hg, its concentrations were higher than those previously reported by De la Casa-Resino et al. (2014) in nestlings of the same species, with mean blood values of Hg ranging from 8.89 µg/L in an uncontaminated area to 53.1 µg/L in an area close to a landfill and intensive agriculture. However, Hg values in the present study were always below 1000 µg/L, which has been reported as the threshold value for a situation of risk to birds (Alvarez et al. 2013; Evers et al. 2008), thus indicating that Hg levels were low enough to not pose a risk to any of these birds. Moreover, considering that Hg is transported via blood to the other tissues, the blood concentrations probably reflect recent Hg exposure, and the low levels quantified could indicate the absence of high concentrations of this contaminant in the considered ecosystem (Gariboldi et al. 2001).
Of all elements, Cd levels (1.05 ± 2.41 µg/L) were those with the highest coefficient of variation (229.5%), and a high percentage (30%) of samples were below the LOD (0.25 µg/L). These levels were near to those reported in the blood of white storks from the Doñana National Park in 1999 and from the region of Murcia in 1996, both in Spain (García-Fernández et al. 1996; Benito et al. 1999), and from uncontaminated areas of Poland (Kamiński et al. 2008, 2009). However, these values were higher than those observed in the Doñana National Park from 1999 to 2002 (Baos et al. 2006a, b). When the individual values were compared with the reference values found in the literature for uncontaminated areas (1 µg/L) (García Fernández et al. 1995), only 12 of the animals exceeded this reference value (of which only three were >5 µg/L).
Pb is one of the most toxic metals and one of the most studied in several bird species. Pb can affect all body systems, reduces growth and reproduction success, causes haemolytic anaemia and low packed cell volume, and has pathological effects on the immune system and behaviour of the affected animal (Franson and Pain 2011). Pb was identified in all the analysed samples, but the mean average of Pb in the blood of fledglings and adults was below 200 µg/L, which is considered a background level for falconiformes (Franson and Pain 2011). Accordingly, it must be noted that three samples (4.5% of the total samples) were above this threshold limit associated with nestling animals. Moreover, Scheuhammer (1987) established that Pb concentrations of 150 µg/L in blood could indicate the absence of an abnormal Pb exposure (Scheuhammer 1987), and only 18% of the animals in the present study exceeded this limit. However, it must be noted that there are not many data on specific Pb toxicity thresholds in ciconiiformes (Franson 1996). The Pb concentrations obtained in this study are similar, or only slightly higher, than those obtained by other authors in different works conducted in Spain and Poland (Benito et al. 1999; De la Casa-Resino et al. 2014, 2015a; Kamiński et al. 2008, 2009). Baos et al. (2006b) found mean blood Pb levels of 90.7 µg/L (quite similar to those obtained in the present study) in white stork chicks from a reference colony also located in the province of Caceres in a natural area far from urban environments and other apparent sources of pollution (the exact place was not indicated). Higher values were detected in white stork chicks from different areas of the province of Madrid (Spain) (Cabo et al. 2012), with blood Pb levels in the range of 105–222.6 µg/L, corresponding to the highest levels in an area characterised by the presence of industries and several rubbish dumps. Higher levels were detected in white stork nestlings from southwestern Spain after an important accidental spill (median of 168 µg/L) (Meharg et al. 2002), which may result from Pb being transferred from the mother to the egg and/or being present in food collected from the contaminated zones and fed to the chicks.
Finally, it must be noted that blood concentrations of the toxic elements Cd, Hg, and Pb are good indicators of contamination and may be appropriate indicators of recent exposure (Carneiro et al. 2015), even though no commonly accepted toxicity thresholds exist for many species (for example, the white stork). However, all the inorganic elements exert toxic effects on birds when involved in unusual biochemical reactions. The threshold concentration at which toxic effects occur is usually higher for essential elements than for nonessential ones; however, some essential elements only need a slight increase in their concentration to become toxic, and many chemicals cannot be broken down into less-toxic compounds (Kamiński et al. 2009).
Influence of Age and Gender
Statistically significant differences related to age were detected in the blood concentrations of five elements. Fledglings showed lower values than adults for Ni, Cu, Se, and Hg (all with p < 0.001) and higher values than adults for Pb (p < 0.01). This last finding is not common, because blood Pb levels are expected to be higher in adults than in juveniles, which often is reported (Carneiro et al. 2014; Eskildsen and Grandjean 1984; Furness 1993; Sebastiano et al. 2016). Because blood Pb values reflect immediate dietary intake, the differences we observed between age groups could be explained by the feeding behaviour (Burger and Gochfeld 1997a).
Age differences regarding metal residues have been documented for a variety of organisms and contaminants (Furness 1993; Honda et al. 1990). More specifically, age differences in birds exist, because certain inorganic pollutants accumulate in the internal tissues of vertebrates, namely, bone, organs, or fat (i.e., birds tend to bioaccumulate heavy metals) (Furness 1993). The other possible explanation for age-related differences in birds from the same colony is that adult and young birds eat different foods, eat different proportions of the same food, and their bodies release the metals differently (Burger and Gochfeld 1997a). Moreover, for some specific metals (e.g., Hg), apart from the dietary intake, their concentration in blood reflects physiological influences, such as mobilisation and accumulation in feathers and eggs (Furness 1993; Burger and Gochfeld 1997b). In fact, during the period of feather production, Hg is incorporated in the keratin structure of feathers, thus reducing the Hg level in the blood (Dauwe et al. 2000). This could explain the low levels detected in chicks compared with adults, as has been reported in raptors, such as the common buzzard (Buteo buteo) (Carneiro et al. 2014), and in seabirds, such as the franklin’s gull (Larus pipixcan) and the magnificent frigatebird (Fregata magnificens) (Sebastiano et al. 2016). The influence of age also has been detected in similar studies with different bird species but with other elements: Cd, Ni, and Fe (García-Fernández et al.1996; Kamiński et al. 2006; Lucia et al. 2010; Carneiro et al. 2014); all cases indicated a potential relevance of this endogenous factor for future biomonitoring studies.
When the effect of gender was analysed, none of the metal concentrations was significantly influenced by this factor. However, it is noteworthy that females in both groups (fledglings and adults) showed lower Cr concentrations, which also has been reported in mallards, by Binkowski and Meissner (2013). In previous studies, As concentrations were influenced by gender in greylag gooses (Anser anser) (Lucia et al. 2010), with higher concentrations reported in females. On the other hand, in another study, Hg blood levels were markedly higher in females than in males of the common buzzard (Carneiro et al. 2014), whereas As and Pb were not affected by this factor. A study developed in Poland with white storks determined no influence of gender on blood Cd concentrations but a slight effect on blood Pb concentrations, with females showing higher blood concentrations than males; however, these differences were not statistically significant (Kamiński et al. 2015). Similar results of Pb were observed in adult swans (Cygnus olor) (Eskildsen and Grandjean 1984).
Correlations
It must be indicated that the accumulation of certain essential elements may be affected by elevated exposure to other elements, either essential or not. Accordingly, in the present study, highly statistically significant correlations (p < 0.001) between Fe–Zn (r = 0.573), Hg–Se (r = 0.707) and Hg–As (r = 0.571) were found. The accumulation of Se in tissues exposed to Hg (Cuvin-Aralar and Furness 1991; Yang et al. 2008) is a well-known phenomenon. One of the explanations for this relation is that Se has a protective action against Hg toxicity, and the formation of a stable and inert complex between selenite and Hg2+ has been described in mammals (Yang et al. 2008). Correlations like this one between essential and nonessential metals might indicate an implication of essential metals in the detoxification of nonessential metals (Blanco et al. 2003; De la Casa-Resino et al. 2014; Kamiński et al. 2006). We did not find any references in the literature regarding the correlation or interaction between Hg–As and Fe–Zn.
Most of the significant correlations were observed in the male-adult group and fewest in the female-fledglings group. This finding is consistent with the fact that most of the elements are accumulative, and adults accumulate metals over a longer period (Berglund et al. 2011; Furness 1993), thus increasing the likelihood of interactions between accumulated elements, such as Hg and Se. The correlations of most of the elements in the blood of adult white storks were higher than in fledglings, and the highest correlation was observed for the pair Hg–Se.
Conclusions
The present study adds to a growing body of scientific literature indicating blood levels of different inorganic elements, which are useful for future biomonitoring programmes, by using the white stork as a good and sensitive species. Our study provides the first report on the levels of 12 elements in the blood of white storks in Europe. Several elements (Cu, Ni, Pb, Se, and Hg) showed different accumulations in adults and fledglings and, thus, special care must be taken when analysing those elements to distinguish between young and older birds during biomonitoring studies. With respect to gender, it had no clear influence on the obtained results.
References
Alvarez CR, Moreno MJ, Alonso LL, Gómara B, Bernardo FJG, Martín-Doimeadios RCR et al (2013) Mercury, methylmercury, and selenium in blood of bird species from Doñana national park (Southwestern Spain) after a mining accident. Environ Sci Pollut Res 20(8):5361–5372
Baos R, Bias J, Bortolotti GR, Marchant TA, Hiraldo F (2006a) Adrenocortical response to stress and thyroid hormone status in free-living nestling white storks (Ciconia ciconia) exposed to heavy metal and arsenic contamination. Environ Health Perspect 114(10):1497–1501
Baos R, Jovani R, Pastor N, Tella JL, Jiménez B, Gómez G et al (2006b) Evaluation of genotoxic effects of heavy metals and arsenic in wild nestling white storks (Ciconia ciconia) and black kites (Milvus migrans) from southwestern Spain after a mining accident. Environ Toxicol Chem 25(10):2794–2803
Benito V, Devesa V, Muñoz O, Suñer MA, Montoro R, Baos R et al (1999) Trace elements in blood collected from birds feeding in the area around Doñana National Park affected by the toxic spill from the Aznalcollar mine. Sci Total Environ 242(1–3):309–323
Berglund ÅM, Koivula M, Eeva T (2011) Species-and age-related variation in metal exposure and accumulation of two passerine bird species. Environ Pollut 159(10):2368–2374
Beyer WN, Spann JW, Sileo L, Franson JC (1988) Lead poisoning in six captive avian species. Arch Environ Contam Toxicol 17(1):121–130
Binkowski LJ, Meissner W (2013) Levels of metals in blood samples from mallards (Anas platyrhynchos) from urban areas in Poland. Environ Pollut 178:336–342
Binkowski TJ, Sawicka-Kapusta K (2015) Lead poisoning and its in vivo biomarkers in Mallard and Coot from two hunting activity areas in Poland. Chemosphere 127:101–108
Blanco G, Frias O, Jimenez B, Gomez G (2003) Factors influencing variability and potential uptake routes of heavy metals in black kites exposed to emissions from a solid-waste incinerator. Environ Toxicol Chem 22(11):2711–2718
Blázquez E, Aguirre J, Martínez-Haro M, Mateo R, Jiménez B (2006) The use of white stork (Ciconia ciconia) nestlings in a biomonitoring programme for organochlorines through the region of Madrid (Spain). Organohalogen Compd 68:2081–2084
Burger J, Gochfeld M (1997a) Age differences in metals in the blood of herring (Larus argentatus) and franklin’s (Larus pipixcan) gulls. Arch Environ Contam Toxicol 33(4):436–440
Burger J, Gochfeld M (1997b) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75(2):160–172
Burger J, Gochfeld M (2009) Comparison of arsenic, cadmium, chromium, lead, manganese, mercury and selenium in feathers in bald eagle (Haliaeetus leucocephalus), and comparison with common eider (Somateria mollissima), glaucous-winged gull (Larus glaucescens), pigeon guillemot (Cepphus columba), and tufted puffin (Fratercula cirrhata) from the aleutian chain of Alaska. Environ Monit Assess 152(1–4):357–367
Cabo P, Espín S, Martínez-López E, Roscales JL, Jiménez B, García-Fernández AJ (2012) Metales pesados en sangre de pollos de cigüeña blanca (Ciconia ciconia) de Madrid y Aragón. Revista de Toxicología 29(1):72
Carneiro M, Colaco B, Brandao R, Ferreira C, Santos N, Soeiro V et al (2014) Biomonitoring of heavy metals (Cd, Hg, and Pb) and metalloid (As) with the portuguese common buzzard (Buteo buteo). Environ Monitor Assess 186(11):7011–7021
Carneiro M, Colaço B, Brandão R, Azorín B, Nicolas O, Colaço J et al (2015) Assessment of the exposure to heavy metals in griffon vultures (Gyps fulvus) from the Iberian Peninsula. Ecotox Environ Safe 113:295–301
Clarke JU (1998) Evaluation of censored data methods to allow statistical comparisons among very small samples with below detection limit observations. Environ Sci Technol 32(1):177–183
Cuvin-Aralar MLA, Furness RW (1991) Mercury and selenium interaction: a review. Ecotox Environ Safe 21(3):348–364
Dauwe T, Bervoets L, Blust R, Pinxten R, Eens M (2000) Can excrement and feathers of nestling songbirds be used as biomonitors for heavy metal pollution? Arch Environ Contam Toxicol 39(4):541–546
De la Casa-Resino I, Hernández-Moreno D, Castellano A, Pérez-López M, Soler F (2014) Breeding near a landfill may influence blood metals (Cd, Pb, Hg, Fe, Zn) and metalloids (Se, As) in white stork (Ciconia ciconia) nestlings. Ecotoxicology 23(8):1377–1386
De la Casa-Resino I, Hernández-Moreno D, Castellano A, Pérez-López M, Soler F (2015a) Chlorinated pollutants in blood of white stork nestlings (Ciconia ciconia) in different colonies in Spain. Chemosphere 118:367–372
De la Casa-Resino I, Hernández-Moreno D, Castellano A, Soler Rodríguez F, Pérez-López M (2015b) Biomarkers of oxidative status associated with metal pollution in the blood of the white stork (Ciconia ciconia) in Spain. Toxicol Environ Chem 97(5):588–598
Eeva T, Ahola M, Lehikoinen E (2009) Breeding performance of blue tits (Cyanistes caeruleus) and great tits (Parus major) in a heavy metal polluted area. Environ Pollut 157(11):3126–3131
Eisler R (1986) Chromium hazards to fish, wildlife, and invertebrates: a synoptic review. Fish and Wildlife Service, US Department of the Interior
Eisler R (1993) Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. Biol Rep 10:33–47
Eskildsen J, Grandjean P (1984) Lead exposure from lead pellets: age-related accumulation in mute swans. Toxicol Lett 21(2):225–229
Evers DC, Savoy LJ, DeSorbo CR, Yates DE, Hanson W, Taylor KM et al (2008) Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology 17(2):69–81
Falandysz J, Jakuczun B, Mizera T (1988) Metals and organochlorines in four female white-tailed eagles. Mar Pollut Bull 19(10):521–526
Florea A, Büsselberg D (2006) Occurrence, use and potential toxic effects of metals and metal compounds. Biometals 19(4):419–427
Fossi C, Leonzio C (1993) Nondestructive biomarkers in vertebrates. CRC Press, Boca Raton
Franson JC (1996) Interpretation of tissue lead residues in birds other than waterfowl. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife. Interpreting tissue concentrations. Lewis Publishers, Boca Raton, pp 265–279
Franson JC, Pain DJ (2011) Lead in birds. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota. Interpreting tissue concentrations. Taylor and Francis Group, Boca Raton, pp 563–593
Furness R (1993) Birds as monitors of pollutants. Birds as monitors of environmental change. Springer, Berlin, pp 86–143
García Fernández AJ, Sánchez-García JA, Jiménez Montalbán P, Luna A (1995) Lead and cadmium in wild birds in southeastern Spain. Environ Toxicol Chem 14(12):2049–2058
García-Fernández A, Sánchez-García J, Gómez-Zapata M, Luna A (1996) Distribution of cadmium in blood and tissues of wild birds. Arch Environ Contam Toxicol 30(2):252–258
Gariboldi JC, Bryan AL, Jagoe CH (2001) Annual and regional variation in mercury concentrations in wood stork nestlings. Environ Toxicol Chem 20(7):1551–1556
Gaunt AS, Oring LW, Council O (1997) Guidelines to the use of wild birds in research. Ornithological Council, Washington
Gómez G, Baos R, Gómara B et al (2004) Influence of a mine tailing accident near Donana National Park (Spain) on heavy metals and arsenic accumulation in 14 species of waterfowl (1998 to 2004). Arch Environ Contam Toxicol 47:521–529
Grand JB, Franson JC, Flint PL, Petersen MR (2002) Concentrations of trace elements in eggs and blood of spectacled and common eiders on the Yukon-Kuskokwim delta, Alaska, USA. Environ Toxicol Chem 21(8):1673–1678
Griffiths R, Daan S, Dijkstra C (1996) Sex identification in birds using two CHD genes. Proc Biol Sci R Soc 263(1374):1251–1256
Hernández LM, Gómara B, Fernández M, Jiménez B, González M, Baos R et al (1999) Accumulation of heavy metals and As in wetland birds in the area around Doñana National park affected by the Aznalcollar toxic spill. Sci Total Environ 242(1):293–308
Hofer C, Gallagher FJ, Holzapfel C (2010) Metal accumulation and performance of nestlings of passerine bird species at an urban brownfield site. Environ Pollut 158(5):1207–1213
Honda K, Min BY, Tatsukawa R (1986) Distribution of heavy metals and their age-related changes in the eastern great white egret, Egretta alba modesta. Korea. Arch Environ Contam Toxicol 15(2):185–197
Honda K, Marcovecchio JE, Kan S, Tatsukawa R, Ogi H (1990) Metal concentrations in pelagic seabirds from the North Pacific Ocean. Arch Environ Contam Toxicol 19(5):704–711
Interior USD. o. t. (1998) Guidelines for interpretation of the biological effects of selected constituents in biota, water, and sediment. Selenium. In: National Irrigation Water Quality Program Information Report No. 3 United States Department of the Interior. GRA and I
Kalisińska E, Salicki W, Mysłek P, Kavetska KM, Jackowski A (2004) Using the mallard to biomonitor heavy metal contamination of wetlands in north-western Poland. Sci Total Environ 320(2):145–161
Kamiński P, Kurhalyuk N, Kasprzak M, Szady-Grad M, Jerzak L (2006) Dynamics of chemical elements in the blood of white stork Ciconia ciconia chicks from polluted environments in W Poland. In: Tryjanowski P, Sparks TH, Jerzak L (eds) The white stork in Poland: Studies in biology, ecology and conservation. Bogucki Wydawnictwo Naukowe, Poznań, pp 201–211
Kamiński P, Kurhalyuk N, Szady-Grad M, Tkachenko H, Kasprzak M, Jerzak L (2008) Chemical elements in the blood of white stork Ciconia ciconia chicks in differential Poland regions. Med Biol Sci 22(4):31–37
Kamiński P, Kurhalyuk N, Jerzak L, Kasprzak M, Tkachenko H, Klawe JJ et al (2009) Ecophysiological determinations of antioxidant enzymes and lipoperoxidation in the blood of white stork Ciconia ciconia from Poland. Environ Res 109(1):29–39
Kamiński P, Grochowska E, Mroczkowski S, Jerzak L, Kasprzak M, Koim-Puchowska B et al (2015) Sex ratio of white stork Ciconia ciconia in different environments of Poland. Environ Sci Pollut R 22(17):13194–13203
Koivula MJ, Eeva T (2010) Metal-related oxidative stress in birds. Environ Pollut 158(7):2359–2370
Lucia M, André J, Gontier K, Diot N, Veiga J, Davail S (2010) Trace element concentrations (mercury, cadmium, copper, zinc, lead, aluminium, nickel, arsenic, and selenium) in some aquatic birds of the southwest Atlantic coast of France. Arch Environ Contam Toxicol 58(3):844–853
Mansouri B, Hoshyari E (2012) Nickel concentration in two bird species from Hara biosphere reserve of southern Iran. Chin Birds 3(1):54–59
Meharg AA, Pain DJ, Ellam RM, Baos R, Olive V, Joyson A et al (2002) Isotopic identification of the sources of lead contamination for white storks (Ciconia ciconia) in a marshland ecosystem (Doñana, SW Spain). Sci Total Environ 300(1):81–86
Merian E (1991) Metals and their compounds in the environment: Occurrence, analysis and biological relevance. VCH Verlagsgesellschaft mbH, Hoboken
Parslow J, Thomas G, Williams T (1982) Heavy metals in the livers of waterfowl from the Ouse Washes, England. Environ Pollut A 29(4):317–327
Peakall D, Burger J (2003) Methodologies for assessing exposure to metals: speciation, bioavailability of metals, and ecological host factors. Ecotox Environ Safe 56(1):110–121
Pérez-López M, De la Casa-Resino I, Hernández-Moreno D, Galeano J, Míguez-Santiyán MP, de Castro-Lorenzo A, Soler F (2016) Concentrations of metals, metalloids, and chlorinated pollutants in blood and plasma of white stork (Ciconia ciconia) nestlings from Spain. Arch Environ Contam Toxicol 71:313–321
Scheuhammer A (1987) The chronic toxicity of aluminium, cadmium, mercury, and lead in birds: a review. Environ Pollut 46(4):263–295
Sebastiano M, Bustamante P, Costantini D, Eulaers I, Malarvannan G, Mendez-Fernandez P et al (2016) High levels of mercury and low levels of persistent organic pollutants in a tropical seabird in French Guiana, the magnificent frigatebird, Fregata magnificens. Environ Pollut 214:384–393
Tkachenko H, Kurhaluk N (2012) Pollution-induced oxidative stress and biochemical parameter alterations in the blood of white stork nestlings Ciconia ciconia from regions with different degrees of contamination in Poland. J Environ Monitor 14(12):3182–3191
Van Eeden P, Schoonbee H (1996) Metal concentrations in liver, kidney, bone and blood of three species of birds from a metal-polluted wetland. Water Sa Pretoria 22:351–358
Weber C, Reid B (1968) Nickel toxicity in growing chicks. J Nutr 95:612–616
Wilson HM, Petersen MR, Troy D (2004) Concentrations of metals and trace elements in blood of spectacled and king eiders in northern Alaska, USA. Environ Tox Chem 23(2):408–414
Yang D, Chen Y, Gunn JM, Belzile N (2008) Selenium and mercury in organisms: Interactions and mechanisms. Environ Rev 16:71–92
Acknowledgements
The authors thank Dr. Jose Luis Rodríguez from the Laboratory of Genetics of the Faculty of Veterinary Medicine of the University of Extremadura (Caceres, Spain), for the assistance in gender determination, and to the staff of the “Los Hornos” Wildlife Recovery Centre (Junta de Extremadura, Caceres, Spain) for the management and sampling of blood. This research was partially supported by the Consejería de Economía e Infraestructuras of the Junta de Extremadura (Ayuda GR15114, Spain) and by FEDER Funds through the Programa Operativo FEDER (2014–2020) of Extremadura.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maia, A.R., Soler-Rodriguez, F. & Pérez-López, M. Concentration of 12 Metals and Metalloids in the Blood of White Stork (Ciconia ciconia): Basal Values and Influence of Age and Gender. Arch Environ Contam Toxicol 73, 522–532 (2017). https://doi.org/10.1007/s00244-017-0431-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-017-0431-8