Abstract
This study was undertaken to determine the level of 16 polycyclic aromatic hydrocarbon (PAH), listed as priority pollutants by the United States Environmental Protection Agency (USEPA), in surface soils around a coal-transporting facility in the western part of South Kalimantan, Indonesia. Three composite soil samples were collected from a coal stockpile, coal-hauling road, and coal port. Identification and quantification of PAH was performed by gas chromatography–mass spectrometry. The total content of 16 USEPA-PAH ranged from 11.79 to 55.30 mg/kg with arithmetic mean value of 33.14 mg/kg and median of 32.33 mg/kg. The 16 USEPA-PAH measured levels were found to be greater compared with most of the literature values. The levels of high molecular-weight PAH (5- and 6-ring) were dominant and formed 67.77–80.69 % of the total 16 USEPA-PAH The most abundant of individual PAH are indeno[1,2,3-cd] pyrene and benzo[a]pyrene with concentration ranges of 2.11–20.56 and 1.59–17.84 mg/kg, respectively. The degree of PAH contamination and subsequent toxicity assessment suggest that the soils of the study area are highly contaminated and pose a potential health risk to humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
South Kalimantan is one of the five Indonesian provinces in Borneo Island. It lies between 114º19′13″ and 116º33′28″ east longitudes, and 1º21′49″ and 4º10′14″ south latitudes. South Kalimantan is the Indonesian top coal producer with total production of 0.12 and 0.15 billion tons in 2011 and 2012, respectively (ESDM 2012). The coal-mining area in South Kalimantan can be divided into western and eastern parts, which are separated by the Meratus Mountains. There are 688 coal mine sites, 813 coal stockpiles, 97 coal ports, and several hundred kilometers of coal-hauling road or conveyors in South Kalimantan (ESDM 2012). The coal-mining area potentially has significant polycyclic aromatic hydrocarbon (PAH) contamination as reported by Liu et al. (2012), Wang et al. (2010a), and Ribeiro et al. (2012) in their study areas in China and Portugal.
PAH are aromatic hydrocarbons made up from interconnection of two or more benzene rings in variety of structural configurations (Gan et al. 2009; Haritash and Kaushik 2009). They are group of organic pollutants with strong toxic, carcinogenic, and mutagenic characteristics (Wang et al. 2010a). From several hundred different combinations of PAH, 16 compounds have been listed as priority pollutants by the United States Environmental Protection Agency (16 USEPA-PAH) (Achten and Hofmann 2009; Gan et al. 2009, 2012). Due to their ubiquity and persistence, PAH are present in most soils where they accumulate because of their low solubility in water (Gan et al. 2009; Wang et al. 2010a). The existence of PAH in the environment is usually associated with incomplete combustion at high temperature (500–800 °C), or subjection of organic materials at low temperature (100–300 °C) for long periods (Ene et al. 2012; Haritash and Kaushik 2009). It has been reported in several sources that PAH have been widely researched in fossil fuels utilization including coal combustion and coal pyrolysis (Achten and Hofmann 2009).
Surprisingly, unburnt coal has rarely been considered as a source of PAH pollution (Ahrens and Morrisey 2005). PAH in coal are usually present as complex mixtures with varying concentrations and patterns due to their differences in physicochemical properties and composition (Laumann et al. 2011). PAH concentrations in soils vary by coal rank (35–11,000 mg/kg coal), and reaching a maximum within the high volatile bituminous rank (Stout and Emsbo-Mattingly 2008; Wang et al. 2010b). PAH in sub-bituminous coals were dominated by medium molecular-weight (MMW) and high molecular-weight (HMW) PAH (Achten and Hofmann 2009; Puettmann and Schaefer 1990). Meanwhile, the low molecular-weight (LMW) PAH was the most dominant PAH type in bituminous and anthracite coals (Ahrens and Morrisey 2005; Chen et al. 2004). PAH release to the environment during mining operations, transport, and storage activities could pose a risk to the quality of soils, sediments, groundwater, surface water, and biodiversity (Ahrens and Morrisey 2005; Pies et al. 2007; Wang et al. 2010a).
Although earlier publications have reported that concentrations of PAH in soil around coal-mining areas greatly exceed the level of natural PAH concentrations in soil, investigations on PAH in soil around coal-transporting facilities is very limited. The objective of the present study was to determine the pattern and distribution of PAH in surface soils around a coal-transporting facility located in the western part of South Kalimantan in the Tapin district of Indonesia. The coal-haul road of 28.60 km distance connects several coal mine sites in Tapin district with approximately 30 intermediate coal-stockpile sites and a coal port in Nagara river side. The total transported coal through this coal haul road amounts to 2.5 million tons annually (BPS-Tapin 2013). The coals from Tapin coal-mining district are mostly sub-bituminous in rank (Nas and Hidartan 2010; Riswandi 2008). Data from the present study will be beneficial for the provision of pollution abatement and proper land-use planning around coal-mining areas.
Materials and Methods
Sample Collection and Preparation
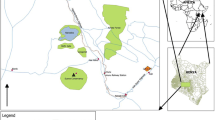
Surface soil samples (5–20 cm) were collected on May 2014 from three different sites surrounding the coal-transporting facility. Site 1 (SP) was selected near the coal stockpile; site 2 (HR) was located at side of the coal-haul road; and site 3 (PR) was situated near the coal port. Location of the soil-sampling sites is depicted in Fig. 1. Eight to 10 soil samples were collected at each sampling sites by grab sampling. Soil samples containing oily materials were not used and were replaced with new samples. After removal of stones and large particles, the grab samples were mixed to make three composite samples. Samples were placed in polyethylene bags and transported to the laboratory.
PAH Analysis
Before analysis, the soil samples were air-dried and homogenized by passing through a 2-mm sieve and stored in the laboratory at room temperature. Forty grams of soil samples were extracted by the Soxhlet extraction method with 120 mL of dichloromethane (Merck, Germany) for 8 h. The extract was transferred to a Micro Snyder column in a water bath (1–15 °C) and reconcentrated to a volume of 1 mL. The concentrated extract was cleaned up using alumina column (Sigma-Aldrich type WB-5, USA). PAH in the extracts were fractionated by a silica column (silica gel 60, 70 to 230 mesh; Merck, Germany). PAH were eluted with methylene dichloride–hexane mixture (proportion 1:1, 5 mL; Merck, Germany) and then evaporated to 1 mL in a water bath. Before analysis by gas chromatography-mass spectrometry, 1 mL of the solution of Semivolatile Internal Standards Mix (naphthalene-d8, acenaphthalene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12; Sigma-Aldrich, USA) was added to the sample.
Sample analysis was performed with a HP 5890 Series II gas chromatograph and a HP 5989 mass spectrometer (Hewlett-Packard, USA) operated in selected ion monitoring of molecular ion peaks (Table 1). Chromatographic resolution is achieved with a 30 m × 250–μm DB-5 capillary column with a 0.25-μm film thickness. The carrier gas was helium at a flow rate of 1 mL/min. The temperature of the injection port was 300 °C, and the transfer line was 290 °C. The quantitative analysis was performed by internal calibration method, and PAH identification was performed by comparison of their retention time with standards.
Analytical Quality Control
To ensure precision and accuracy, the analytical methods were checked. All of the samples were analyzed in triplicate. Replicate analyses yielded an error rate between 10 and 15 %. The recovery efficiency was checked by analyzing soil samples spiked with known amounts of PAH standard. Recoveries ranged from 80 to 95 % for the reported PAH in soil samples. The procedural blank was determined by going through the extraction and clean-up procedures using glass beads instead of soil sample. Results indicated that they were generally low and posed no problem to the analytical quantification.
Results and Discussion
Concentration and Distribution of PAH in Soils
The measured concentrations of 16 USEPA-PAH are listed in Table 2. The total content of 16 USEPA-PAH in SP soil is 11.79 mg/kg. In contrast, 16 USEPA-PAH in HR and PR soils are 32.33 and 55.30 mg/kg, respectively. These concentrations greatly exceed the level of natural PAH concentrations in soil (0.001–0.010 mg/kg) (Edwards 1983). The sum of 7 PAH indicated as carcinogens by the International Agency for Research on Cancer (7 CPAH) varies among the sampling sites. The sum of these 7 CPAH formed 71.76, 84.16, and 86.58 % of total 16 USEPA-PAH in SP, HR, and PR soils, respectively.
The measured levels were also significantly greater than those measured in several coal mine sites. For example, Liu et al. (2012) reported the sum of 16 USEPA-PAH of 0.05–5.64 mg/kg of soil in the Tiefa coal mine district at Diaobingshan city, northeast Liaoning province, China. Lower concentrations of 0.13 and 3.54 mg/kg soil were observed by Wang et al. (2010a) in three coal mines located in Huainan and Huaibei city, northern Anhui Province, China. Meanwhile, levels of 0.002–0.07 mg/kg have been reported from coal waste pile in the Douro coal field (northwest of Portugal) (Ribeiro et al. 2012). Conversely, compared with those previous studies in industrial sites, relatively low PAH concentrations were found in the present work. Literature data are listed in Table 3.
Being one of the leading coal producers, the Indonesian Government has not established national standards concerning allowable PAH concentrations in soils. Therefore, the present data were compared with the Netherland standards (0.02–0.05 mg/kg), the Mexican standards (0–6 mg/kg), and the Poland standards (0.02–10 mg/kg) (Ray et al. 2008). The comparison showed that the PAH concentration in studied samples exceeded the maximum value standards of those three countries. According to the Polish standard (Skrbic et al. 2005), all soil samples can be classified under the pollution class of V and assessed as “very heavily polluted” soil with respect to PAH contamination.
PAH Pattern in Soils
The distributions of PAH molecular-weight groups in all soil samples were found to be similar (Fig. 2). The levels of HMW-PAH (5- and 6-rings) were dominant and formed 67.77 to 80.69 % of the total 16 USEPA-PAH. Meanwhile, LMW-PAH (2- and 3- ring) and MMW-PAH (4-ring) formed 9.58 to 17.13 % and 9.73 to 15.10 % of the total 16 USEPA-PAH, respectively. In addition to the variation in the total content of PAH groups, the individual PAH also show variations. The most abundant PAH in the samples are indeno[1,2,3-cd] pyrene and benzo[a]pyrene with concentrations ranging from 2.11 to 20.56 and 1.59 to 17.84 mg/kg, respectively, and arithmetic means of 11.28 mg/kg and 9.05 mg/kg, respectively. Only in the SP soil sample was a predominance of benzo[b]fluoranthene observed, while Indeno[1,2,3-cd] pyrene was most dominant PAH in both the HR and PR soil samples.
Although it is difficult to identify PAH contamination derived from coal particulate, several previous studies have shown that PAH in soil and sediment around coal mine areas are associated with native PAH in raw coal. For example, Pies et al. (2007), Hofmann et al. (2007), and Yang et al. (2008) reported that the bituminous coal in the Saar coal district (Germany) was the major reason for high naphthalene and methylnaphthalene concentrations in Saar and Mosel River floodplain soils downstream from the mining activity. In addition, the predominance of naphthalene and acenaphthene in soils around Luling, Liuer, and Zhangji coal mines (China) might be due to the bituminous and anthracite coals in those coal mine sites (Wang et al. 2010a; Xue et al. 2007). Based on the findings of those previous studies, the high content of HMW-PAH in the studied samples might be associated with the sub-bituminous coals in Tapin coal district, which were transported by this coal-transporting facility. This predominance of HMW-PAH might also be due to the occurrence of thermal transformations derived from natural coal fires (Emsbo-Mattingly and Stout 2011; Readman et al. 2002).
PAH of molecular mass of 178 and 202 are commonly used to distinguish between petrogenic and pyrogenic sources (Liu et al. 2012; Soclo et al. 2000). For PAH with a mass of 178, a ratio of anthracene to anthracene plus phenanthrene (Anth/178) of 0.10 is defined as the petrogenic/pyrogenic transition point (Yunker et al. 2002). Meanwhile, for PAH with a mass of 202, a ratio of fluoranthene to fluoranthene plus pyrene (Flan/Flan + Pyr) <0.50 is taken as an indication of petrogenic origin, whereas a ratio >0.50 indicates a pyrogenic origin (Yunker et al. 2002). The Anth/178 ratio in SP, HR, and PR soils were 0.55, 0.50, and 0.43, respectively. Meanwhile, Flan/Flan + Pyr ratios of 0.45, 0.49, and 0.82 were detected in SP, HR, and PR samples, respectively. It could be inferred that the primary source of PAH in SP and HR soil was of petrogenic origin, whereas in PR soil sample the source was derived from both petrogenic and pyrogenic sources.
Potential Toxicity Risk Assessment
Information on PAH-contaminated soil is important for determining the extent of carcinogenic exposure to the residents residing near the coal-transporting facility. Because the occupational exposure limit for total PAH has not been established, the carcinogenic toxic equivalent quantity (B[a]Peq) was used to evaluate the toxicity of PAH. The B[a]Peq was calculated as the sum of multiplying PAH concentration by toxic equivalent factors (TEFs) (Ruwei et al. 2013). The list of TEFs compiled by Tsai et al. (2004) was adopted in this study (Table 2). This method has the main advantage of being relatively easy to apply in the affected human environment. However, underestimation of the risk may occur because only limited PAH compounds are considered (World Health Organization/International Programme on Chemical Safety 1998). As listed in Table 1, total B[a]Peq in PR soil (21.56 mg/kg) was found to be much greater than that in HR soil (10.41 mg/kg) and SP soil (2.54 mg/g). This indicates that the higher concentration of PAH species has greater carcinogenic potential.
Conclusion
The total contents of 16 USEPA-PAH in SP, HR, and CP soil samples were 11.79, 32.33, and 55.30 mg/kg, respectively. The measured levels were obviously greater than most of reported PAH concentrations in soil and exceeded the maximum values of the Netherland, Mexican, and Poland standards. All of the soil samples exhibited the presence of predominantly 5- to 6-six ring PAH (67.77–80.69 % of total 16 USEPA-PAH), a finding that is associated with the subbituminous-coals rank as a raw coal in this coal-transporting facility. Evaluations of several diagnostic ratios suggest that the PAH contaminating soils in the coal port was derived both from petrogenic and pyrogenic sources, whereas that in coal stockpile and coal-hauling road was mainly of petrogenic origin.
References
Achten C, Hofmann T (2009) Native polycyclic aromatic hydrocarbons (PAH) in coals—a hardly recognized source of environmental contamination. Sci Total Environ 407:2461–2473
Ahrens MJ, Morrisey DJ (2005) Biological effects of unburnt coal in the marine environment. Oceanography and marine biology—an annual review. CRC Press, Boca Raton, pp 69–122
Antizar-Ladislao B, Lopez-Real J, Beck AJ (2005) Laboratory studies of the remediation of polycyclic aromatic hydrocarbon contaminated soil by in-vessel composting. Waste Manag 25:281–289
Bojakowska I (2005) PAHs spectrum in the soils of the industrial areas. Paper presented at the Valorisation of the Environment in the Areas Exposed to Long Term Industrial and Mining Activities. Warszawa, Poland
BPS-Tapin (2013) Tapin in figures 2013. Agency of Statistics, Rantau
Cajthaml T, Bhatt M, Šašek V, Matějů V (2002) Bioremediation of PAH-contaminated soil by composting: a case study. Folia Microbiol 47:696–700
Canet R, Birnstingl JG, Malcolm DG, Lopez-Real JM, Beck AJ (2001) Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by native microflora and combinations of white-rot fungi in a coal-tar contaminated soil. Bioresour Technol 76:113–117
Chen Y, Bi X, Mai B, Sheng G, Fu J (2004) Emission characterization of particulate/gaseous phases and size association for polycyclic aromatic hydrocarbons from residential coal combustion. Fuel 83:781–790
Edwards NT (1983) Polycyclic aromatic hydrocarbons (PAH’s) in the terrestrial environment—A review. J Environ Qual 12:427–441
Emsbo-Mattingly SD, Stout SA (2011) Semivolatile hydrocarbon residues of coal and coal tar. In: Stracher GB, Prakash A, Sokol EV (eds) Coal and peat fires: a global perspective, vol 1. Elsevier, Amsterdam, pp 173–208
Ene A, Bogdevich O, Sion A, Spanos T (2012) Determination of polycyclic aromatic hydrocarbons by gas chromatography–mass spectrometry in soils from Southeastern Romania. Microchem Jl 100:36–41
ESDM (2012) Indonesia mineral and coal mining statistics 2012. Dirjen Minerba, Kementerian Energi dan Sumber Daya Mineral, Jakarta
Gan S, Lau EV, Ng HK (2009) Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J Hazard Mater 172:532–549
Guo G, Wu F, He H, Zhang R, Li H, Feng C (2012) Distribution characteristics and ecological risk assessment of PAHs in surface waters of China. Sci China Earth Sci 55:914–925
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Hofmann T, Pies C, Yang Y (2007) Elevated polycyclic aromatic hydrocarbons in a river floodplain soil due to coal mining activities. Water Sci Technol 7:69–74
Laumann S, Micic V, Kruge MA, Achten C, Sachsenhofer RF, Schwarzbauer J et al (2011) Variations in concentrations and compositions of polycyclic aromatic hydrocarbons (PAHs) in coals related to the coal rank and origin. Environ Pollut 159:2690–2697
Liu J, Liu G, Zhang J, Yin H, Wang R (2012) Occurrence and risk assessment of polycyclic aromatic hydrocarbons in soil from the Tiefa coal mine district, Liaoning, China. J Environ Monit 14:2634–2642
Nas C, Hidartan (2010) Quality of Kalimantan coking coals, Indonesia. Paper presented at The 37th Symposium of the Geology of the Sydney Basin, Hunter Valley
Pies C, Yang Y, Hofmann T (2007) Distribution of polycyclic aromatic hydrocarbons (PAHs) in floodplain soils of the Mosel and Saar river. J Soil Sed 7:216–222
Puettmann W, Schaefer RG (1990) Assessment of carbonization properties of coals by analysis of trapped hydrocarbons. Energy Fuels 4:339–346
Ray S, Khillare PS, Agarwal T, Shridhar V (2008) Assessment of PAHs in soil around the International Airport in Delhi, India. J Hazard Mater 156:9–16
Readman JW, Fillmann G, Tolosa I, Bartocci J, Villeneuve JP, Catinni C et al (2002) Petroleum and PAH contamination of the Black Sea. Mar Pollut Bull 44:48–62
Ribeiro J, Silva T, Mendonca Filho JG, Flores D (2012) Polycyclic aromatic hydrocarbons (PAHs) in burning and non-burning coal waste piles. J Hazard Mater 199–200:105–110
Riswandi H (2008) Pengaruh lingkungan pengendapan terhadap kualitas batubara daerah tapin kalimantan selatan. Jurnal Ilmiah MTG 2:24–31
Ruwei W, Jiamei Z, Jingjing L, Liu G (2013) Levels and patterns of polycyclic aromatic hydrocarbons in coal-fired power plant bottom ash and fly ash from Huainan, China. Arch Environ Contam Toxicol 65:193–202
Skrbic B, Cvejanov J, Durisic-Mladenovic N (2005) Polycyclic aromatic hydrocarbons in surface soils of Novi Sad and bank sediment of the Danube River. J Environ Sci Health A 40:29–42
Soclo HH, Garrigues P, Ewald M (2000) Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Mar Pollut Bull 40:387–396
Stout SA, Emsbo-Mattingly SD (2008) Concentration and character of PAHs and other hydrocarbons in coals of varying rank—Implications for environmental studies of soils and sediments containing particulate coal. Organic Geochem 39:801–819
Tsai P-J, Shih T-S, Chen H-L, Lee W-J, Lai C-H, Liou S-H (2004) Assessing and predicting the exposures of polycyclic aromatic hydrocarbons (PAHs) and their carcinogenic potencies from vehicle engine exhausts to highway toll station workers. Atmos Environ 38:333–343
Wang R, Liu G, Chou C-L, Liu J, Zhang J (2010a) Environmental assessment of PAHs in soils around the Anhui Coal District, China. Arch Environ Contam Toxicol 59:62–70
Wang R, Liu G, Zhang J, Chou C-L, Liu J (2010b) Abundances of polycyclic aromatic hydrocarbons (PAHs) in 14 Chinese and American coals and their relation to coal rank and weathering. Energy Fuels 24:6061–6066
World Health Organization/International Programme on Chemical Safety (1998) Environmental health criteria 20. Selected non-heterocyclic PAHs. WHO, Geneva
Xue J, Liu G, Niu Z, Chou CL, Qi C, Zheng L (2007) Factors that influence the extraction of polycyclic aromatic hydrocarbons from coal. Energy Fuels 21:881–890
Yang Y, Ligouis B, Pies C, Grathwohl P, Hofmann T (2008) Occurrence of coal and coal-derived particle-bound polycyclic aromatic hydrocarbons (PAHs) in a river floodplain soil. Environ Pollut 151:121–129
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochem 33:489–515
Zhang Y, Zhu YG, Houot S, Qiao M, Nunan N, Garnier P (2011) Remediation of polycyclic aromatic hydrocarbon (PAH) contaminated soil through composting with fresh organic wastes. Environ Sci Pollut Res Int 18:1574–1584
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizwar, A., Trihadiningrum, Y. PAH Contamination in Soils Adjacent to a Coal-Transporting Facility in Tapin District, South Kalimantan, Indonesia. Arch Environ Contam Toxicol 69, 62–68 (2015). https://doi.org/10.1007/s00244-015-0141-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-015-0141-z