Abstract
Concentrations of seven trace elements [arsenic (As), chromium (Cr), nickel (Ni), lead (Pb), copper (Cu), zinc (Zn), and cadmium (Cd)] in the eggshells of Rooks Corvus frugilegus, a focal bird species of Eurasian agricultural environments, are increased above background levels and exceed levels of toxicological concern. The concentrations of Cr, Ni, Pb, Cu, and Zn are greater in eggshells from urban rookeries (large cities) compared with rural areas (small towns and villages) suggesting an urbanisation gradient effect among eggs laid by females. In the present study, the investigators assessed whether the pattern of relationships among the seven trace elements in eggshells change along an urbanisation/pollution gradient. Surprisingly, we found that eggshells with the greatest contaminant burden, i.e., from urban rookeries, showed far fewer significant relationships (n = 4) than eggshells from villages (n = 10), small towns (n = 6), or rural areas (n = 8). In most cases, the relationships were positive. As was an exception: Its concentration was negatively correlated with Ni and Cd levels in eggshells from small town rookeries (where As levels were the highest), whereas eggshells from villages (with a lower As level) showed positive relationships between As and Cd. Our findings suggest that at low to intermediate levels, interactions between the trace elements in Rook eggshells are of a synergistic character and appear to operate as parallel coaccumulation. A habitat-specific excess of some elements (primarily Cr, Ni, Cu, As) suggests their more competitively selective sequestration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Despite the need for better knowledge of elemental interactions about the toxicological assessment of wildlife in the geochemical environment (Möller 1996), avian eggs have rarely been examined or used in such analyses. In view of the indispensable part played by eggs in the reproductive process in birds, the composition and/or interaction of chemical elements present in eggs, i.e., in the egg content and shell, are of critical importance for the proper development of the embryo and body of hatching nestlings (Pinowski et al. 1994; Nys et al. 2004).
The main function of the eggshell is to protect the embryo from external factors, a function that must nevertheless be compatible with ready breakability from the inside to allow the hatchling to emerge. The eggshell structure must permit the exchange of water and gases between the environment and the embryo during its extrauterine development as well as be a source of calcium (Ca) for the growing embryo (Nys et al. 2004). Because eggshell is a ceramic material, some elements or chemical compounds can modify the morphology of calcite crystals and mineralisation and ultimately the properties of whole shells, primarily their thickness and microstructure. This is especially important in the ecotoxicological context because avian eggshells are especially susceptible to the adverse effects of anthropogenic pollutants, with the response to these being the thinning of eggshells (Gonzalez and Hiraldo 1988 [reviewed in Rodriguez-Navarro et al. 2002]). This was one of the first signs of the detrimental effects elicited by high loads of certain organic contaminants, primarily the residues of organochlorine pesticides in birds, observed in the late 1960s, and also by some heavy metals, e.g., mercury (Hg), lead (Pb), cadmium (Cd) and aluminium (Al) (Ratcliffe 1970; for more references see Gonzalez and Hiraldo 1988; Eeva and Lehikoinen 1995; Rodriguez-Navarro et al. 2002; Skalická et al. 2008). More recently, eggshell thinning due to the presence of residues of persistent pesticides has been observed in some seabirds, such as Ivory Gulls Pagophila eburnea from the High Arctic, which may imply a direct link between population decrease and eggshell quality in species from ecosystems remote from industrial areas (Miljeteig et al. 2012).
Despite the growing interest in using avian eggshells to biomonitor environmental contaminants and the small number of studies assessing trace elements and pesticide residues in the eggshells of several bird species (e.g., Burger 1994; Morera et al. 1997; Dauwe et al. 1999; Rodriguez-Navarro et al. 2002; Ayas 2007; Ayas et al. 2008; Hashmi et al. 2013; Orłowski et al. 2010), knowledge of the relationships among the various elements in eggshells remains limited. Some trace elements can interact with one another, primarily essential ones versus nonessential divalent cations, e.g., arsenic (As) versus boron (Bo) (Pendleton et al. 1995). In addition, orally administered dietary Ca or Cr can decrease the negative impact of Cd by increasing the thickness of eggshells and limiting their brittleness (Scheuhammer 1996; Skalická et al. 2008). With detailed information on the direction of interactions between the various chemical elements occurring in eggshells, we can assess potential inhibitory or synergistic effects due to the sequestration of an individual element into this calcified tissue in the presence of others. Moreover, the relationships among various elements in eggshells have been analysed in just a few studies on waterbirds. Because most of these analyses were performed simultaneously on an entire data set with no consideration for habitat-specific differences in the concentrations of eggshell elements (Burger 1994; Morera et al. 1997; Hashmi et al. 2013), they did not reflect the pollution gradient or even the variable intensity or direction of interactions (cf. Rodriguez-Navarro et al. 2002). An exception was a study performed in salt marsh wetlands in coastal Georgia that examined relationships among certain elements, including three trace elements [copper (C), zinc (Zn), and lead (Pb)] in eggshells of Clapper Rails Rallus longirostris across a pollution gradient (Rodriguez-Navarro et al. 2002).
In the present work, we address this issue by attempting to determine the direction of relationships among various trace elements—four essential ones [Zn, Cr, nickel (Ni), and (Cu)] and three nonessential ones (As, Pb, Cd)—in eggshells along a pollution gradient in the context of the results of our extensive ecotoxicological studies of the Rook Corvus frugilegus (Orłowski et al. 2010, submitted), a focal bird species of Eurasian agricultural environments. Because the Rook’s food items are collected on arable land (Kasprzykowski 2003, 2007; Orłowski et al. 2009, 2013), they are especially vulnerable to the impact of agricultural contaminants. Rook tissues and eggs have exceptionally high levels of organic contaminants and toxic metals, and their eggs have displayed signs of thinning (Ratcliffe 1970; Malmberg 1973; Pinowska et al. 1981; Pinowski et al. 1983; Beyerbach et al. 1987; Orłowski et al. 2012a). Recently, we found that eggshells of Rooks in Poland have exceptionally high concentrations of As and Cr (Orłowski et al. 2010; submitted). Five other trace elements (Ni, Pb, Cu, Zn, and Cd) also occurred at levels of ecotoxicological concern (Orłowski et al. 2010, submitted). Furthermore, concentrations of Cr, Ni, Pb, Cu, and Zn were significantly greater in eggshells from urban rookeries compared with those from rural areas, which suggests a clear impact of urbanisation on the bioaccumulation of these elements in eggs laid by female Rooks along a pollution gradient (urban > rural area). This is a consequence of the level of contaminants in dietary items gathered near the rookeries. Here we wanted to test whether the pattern of relationships among these seven trace elements in eggshells changed along an urbanisation/pollution gradient. We hypothesized that along with the growing load of contaminants, there will be a greater number of interactions among these eggshell elements.
Materials and Methods
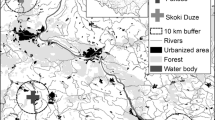
The analytical material, including the levels of elements and the classification of rookeries into urban and rural, was presented in our two earlier articles on the concentrations of seven trace elements (Cr, Ni, Cd, Pb, Cu, Zn, and As) in Rook eggshells in Poland (Orłowski et al. 2010, submitted). The eggshells used for both this and the earlier work were collected in the spring 2005 in 43 rookeries ranging in size from 5 to 480 nests and located in various parts of Poland (Fig. 1). The eggshells were picked up from the ground beneath the nests. Eggshells were sampled in rookeries situated in three different types of built-up area that vary in their densities of buildings and human populations: villages, small towns, and large cities. Rookeries from large cities (hereafter” ‘urban rookeries”; n = 14) and rural areas (villages + small towns, hereafter “rural rookeries”; n = 29) were defined on the basis of the number of inhabitants in each locality, with 50,000 people taken as the threshold for defining a city (after Orłowski and Czapulak 2007). The concentrations of elements in eggshells are presented separately for villages, small towns, and large cities (urban rookeries); villages and small towns (pooled) (rural rookeries); and all three types of colonies (pooled).
Chemical Analysis
Because the amounts of eggshell collected in some rookeries were rather small, metal concentrations for each colony were determined using just two different eggshells or their fragments. In practice, large pieces were used to ensure that the samples had come from different eggs. Remnants of membranes and visible external dirt were removed from the eggshells before these were stored in glass containers for heavy-metal analysis. The chemical analyses were performed at the Department of Aquaculture of the Wrocław University of Environmental and Life Sciences by three of the coauthors of this article (W. D., P. K., R. P.). Before chemical analysis, all eggshells were rinsed twice with water containing detergent and then air-dried. They were then mineralised in a mixture of nitric and hydrochloric acid in a high-pressure microwave digestion system (MARS-5; CEM, Matthews, North Carolina, USA). Atomic absorption spectroscopy (SpectrAA FS220; Varian, Palo Alto, California, USA) was used to determine metal content. The measurement process was validated using DORM-3 (fish protein) reference material, which was provided by the National Research Council of Canada Institute for National Measurement. The precision of the method, taken to be the degree of conformity between the results of multiple analyses performed on the same sample, was 5 % (RSD). The measurements for the reference material were 25.80 ± 1.10 (Cu), 85.80 ± 2.50 (Zn), 16.60 ± 1.10 (As), 1.89 ± 0.17 (Cr), 1.28 ± 0.24 (Ni), 0.290 ± 0.020 (Cd), and 0.395 ± 0.050 (Pb) ppm. In comparison, the average values determined in the eggshells (six measurements on 0.9-g samples) were 25.24 ± 0.25, 87.20 ± 0.35, 17.6 ± 1.0, 1.97 ± 0.09, 1.33 ± 0.12, 0.280 ± 0.013, and 0.381 ± 0.043 ppm for these elements, respectively. All metal concentrations were expressed in milligrams per kilogram (mg kg−1 or parts per million [ppm]) of dry mass (dw) with an accuracy of two decimal points.
Statistical Analyses
In the first step of our analysis, we described the habitat-specific differences in concentrations of the seven trace elements (Cr, Ni, Cd, Pb, Cu, Zn, and As) in eggshells of Rooks breeding along an urbanisation gradient, i.e., villages, small towns and large cities, and between rural rookeries (villages and small towns) and urban ones (large cities), to illustrate the analogous habitat-specific gradient of pollution. Because our earlier work on concentrations of Cu, Zn, and As was focused on the differences between rookeries in large cities and rural areas (i.e. pooled villages and small towns; cf. Orłowski et al. 2010), here we provide new data considering separately villages and small towns. To test habitat-specific differences in the level of concentrations of the elements, we applied analysis of variance (ANOVA) in the general linear model (StatSoft version 7.1., Tulsa, Oklahoma, USA) performed on log-transformed data. Using the post hoc comparison (Least Significant Difference test), we assessed the differences in concentrations of the elements in eggshells among three individual habitat types: villages, small towns, and large cities. In general, the habitat-specific differences in the concentrations of these elements were presented in our two earlier works; however, these data differ from the findings presented in this current work. The earlier presentations were based on averaged data for rookeries, i.e., calculated for two pooled concentrations of two separate eggshells sampled in an individual breeding colony (cf. Orłowski et al. 2010). In the present study, we compiled and compared data for all of these elements simultaneously using a uniform method considering the concentration measured within an individual eggshell as a separate data point.
The primary objective of our analysis was to test whether the pattern of relationships among the seven trace elements along the urbanization/pollution gradient is fixed or changes with the load of contaminants measured in eggshells. To test this hypothesis, we created matrices of relationships of various eggshell elements (Pearson correlation coefficient on log-transformed data) from the three individual habitat types, i.e., villages, small towns, and large cities; rural areas; and all rookeries combined. In view of the large number of statistical tests to control the type I error rate for multiple comparisons, we presented both the actual level of significance (to illustrate in detail the results obtained) and the Bonferroni-adjusted threshold of P ≤ 0.01. Overall, the latter P value agrees with the level of significance recommended for multiple comparisons (Chandler 1995).
Finally, in view of some earlier results, which suggested that it was possible to determine the origins of some eggshell elements (i.e., natural = geochemical; anthropogenic = industrial, agricultural, or traffic related) based on their grouping as a result of the application of principal component analysis (PCA) (cf. Hashmi et al. 2013), we wanted to reassess this approach for Rook eggshells from various habitats. To test and identify the distribution and potential mutual associations of individual eggshell elements resulting from various habitat-specific concentrations, we performed five different PCAs using Statistica 7.0 software (StatSoft) on standardised log-transformed concentrations of eggshell elements across particular habitat types/sets of rookeries: villages, small towns, large cities; rural areas (villages and small towns), and all of the rookeries. In PCA, we applied varimax-normalised factor rotation. Factor loadings were used to interpret PC patterns across different habitats. PCs with eigenvalue >1 were considered to account for a significant contribution to the total variance according to the latent root criterion (Hair et al. 1998).
Results
Habitat-Specific Concentrations of Trace Elements in Eggshells of Rooks
Concentrations of the seven trace elements in the eggshells of Rooks breeding in rookeries in various rural and urban areas of Poland (Fig. 1) are listed in Table 1. There were significant differences in Cr, Ni, Pb, Cu, and Zn concentrations among eggshells from rookeries located in villages, small towns, and large cities [ANOVA (cases degrees of freedom (df) in all cases] = 2 and 83; Cr: F = 11.6, P < 0.0001; Ni F = 5.5, P = 0.005; Pb F = 4.14, P = 0.019; Cu F = 8.4, P = 0.0005; and Zn F = 3.3, P = 0.043). Cd and As levels did not differ between eggshells from these three habitat types (Cd F = 0.4, P = 0.683; As F = 2.8, P = 0.064). Similarly, the same elements differed significantly between eggshells from rural (villages and small towns pooled) and urban areas (large cities). Overall, post hoc comparisons confirmed that Cr, Ni, Pb, Cu, and Zn levels were significantly greater in eggshells from large cities than from small towns and villages, which indicates a clear effect of the urbanisation gradient on the bioaccumulation of these elements in eggs laid by female Rooks along a pollution gradient (urban > rural area). Concentrations of eggshell elements did not differ between small towns and villages, and only the As concentration was significantly greater in small towns than in large cities (P = 0.015) (Table 1).
Relationships Among Eggshell Trace Elements Along the Urbanisation/Pollution Gradient
Overall, we found 10, 6, and 4 significant correlations between the target trace elements in eggshells from rookeries located in villages, small towns, and large cities, respectively. In addition, there were eight and nine such correlations from eggshells from rural areas and all of the rookeries taken together (Table 2). In the majority of cases, the direction of all relationships found was to be positive along the urbanisation/pollution gradient. As was an exception, the concentration of which was negatively correlated with Ni and Cd levels in eggshells from small town rookeries (where the highest As level was found), whereas in eggshells from villages (with a lower As level), a positive relationship was found between As and Cd. The other significant positive relationships with P < 0.05 can be summarised as follows: for eggshells from villages they were between Cr and Ni, Cd, Pb, Cu, and Zn; Ni and Cd; Pb and Cu and Zn; and Cu and Zn. For eggshells from small towns, they were between Cr and Cu; Ni and Cd and Pb; and Cd and Pb. For eggshells from large cities/urban rookeries, they were between Cr and Ni, Cd, and As; and Ni and Cd. For eggshells from rural rookeries (pooled villages and small towns), they were between Cr and Ni, Cd, Cu, and Zn; Ni and Cd; Zn and Pb and Cu; and Pb and Cu. Last, significant relationships for all eggshells were found between Cr and Ni, Cd, Pb, Cu, and Zn; Ni and Cd and Pb; Pb and Cd and Zn (Table 2).
PCA of the seven eggshell elements consistently yielded three components with eigenvalues > 1 across all four specified data sets of rookeries from various habitats, which explained from 16 to 43 % of the variance, even though there are slight differences in the grouping of some elements and the direction of their association (Table 3). For eggshells from villages, the first extracting PC (PC1) consisted of Pb, Cu, and Zn, which were loaded in a positive direction; Ni and Cd were loaded as PC2, which were both positive associations; and As as PC3 in the opposite direction. For eggshells from small towns, the PC1 scores of Ni, Cd, and Pb were loaded in a positive direction but As in the opposite direction; Cr and Cu were loaded as PC2; and only Zn was loaded as PC3 in a positive direction. For eggshells from large cities, Ni and Cd were loaded in the opposite direction as PC1; for PC2 and PC3, only Cr and Cu were, respectively, loaded in a positive direction. Last, for all rookeries jointly, Ni and Cd were loaded in the opposite direction as PC1; Cr and Zn were loaded positively as PC2; and As was loaded in the opposite direction as PC3 (Table 3).
Discussion
Overall, our findings did not confirm our initial hypothesis that there would be an increasing number of elemental correlations in Rook eggshells along an urbanisation/pollution gradient. The nature of interactions between trace eggshell elements is poorly understood. A key channel for the interpretation of our results, however, could be knowledge of the chemical and biochemical associations between various elements in animal tissues, including avian eggs. In most cases, our findings did not agree with the results of some previous studies, mainly research of various animal tissues, which showed that interactions between essential trace elements (Zn, Cr, Ni, and Cu) and nonessential ones (As, Pb, and Cd) were primarily antagonistic; no synergistic effects between them have been found (e.g. Mahaffey et al. 1981; Beckman and Nordenson 1986; Eisler 1998; Kamiński 1998; Kamiński & Warot 2005; Gasparik et al. 2010 (for the interaction of various elements in the internal organs of Rook nestlings [cf. Orłowski et al. 2012b]). Because we found a generally consistent positive direction of relationships for six of the seven elements (As was the exception) across the four eggshell habitats/sets (including three separate habitat types and all samples), the sequestration of all seven trace elements into eggshells could involve their parallel coaccumulation or coprecipitation by female Rooks. This is attributable to specific metalloprotein(s) and/or the same ion transporter(s) binding a few different elements, which are simultaneously deposited in an eggshell (cf. Scheuhammer 1996; Rodriguez-Navarro et al. 2002; Jonchere et al. 2012). During oogenesis in female birds, the absorption of some nonessential Ca-mimetic metals, such as Pb and Cd, increases during Ca sequestration into eggshells (cf. Scheuhammer 1996). All seven elements analysed in our study are divalent cations displaying a negative association with Ca and inhibiting Ca precipitation in calcified tissues (Wada et al. 1995; Parsiegla and Katz 1999). Some of these elements—such as Zn, Ni, and Cu—are known to be bound by phosphate minerals in their structure (cf. Rodriguez-Navarro et al. 2002). Moreover, the part played by biochemical interaction between the different elements, including some negative associations between essential and nonessential ones (such as the negative correlation between Cr and Cd cited in the Introduction; Skalická et al. 2008) may be masked in the case of their intensive sequestration along the Ca binding in eggshells. Because we found a predominantly positive direction of relationships between the seven elements, such a generally superior role of minerals in the precipitation of trace elements in eggshells and in shaping the direction of interactions among them is possible. Interestingly, despite the lack of habitat-specific differences in eggshell levels of Cd, this element exhibited relatively large and significant correlations (as with Pb) loaded with other elements (primarily with Ni) as the principal component. Therefore, concluding our study in a broad ecotoxicological sense, our findings confirm the earlier assumption that for female birds, the laying of eggs is a way of excreting surplus supplies of certain elements, including toxic metals (Burger 1994).
In contrast, because we found that eggshells with the highest load of contaminants (i.e., from urban rookeries) showed fewer significant relationships than eggshells from villages, small towns, or rural areas, this might suggest an inhibitory effect on the part of elements present in greater concentrations, i.e., Cr, Ni, Cu, and As, on the uptake of other elements at lower levels. Such an explanation is in agreement with some other studies, which have shown that the uptake and concentration of an individual element, such as As, Cr, or Cd, could be highly dependent not only on the presence but also on the level of some other trace elements, which may change the direction of their interaction from synergistic to antagonistic at high levels (cf. Bae et al. 2001). In our case, such a possibility exists especially in the case of As, which alone showed divergent findings: A negative correlation with Ni and Cd (found in small towns for the highest As eggshell level). Furthermore, As displayed a variable and opposite direction in PCA loading (cf. Table 3). In conclusion, it seems that our findings could also illustrate a general trend of trace-element interaction from synergism (at lower and intermediate levels) to antagonism as levels increase, which may be due to homeostatic mechanisms in response to low levels of inhibitory challenge (Bae et al. 2001).
Interestingly, the concentration of As in eggshells from small towns was greater than in large cities, a result that could not be obtained in our earlier work because the rookeries from small towns were not been separated in the analyses (cf. Orłowski et al. 2010). The high concentration of As possibly resulted from the high intensity of agriculture around small towns. Intensively managed large-scale arable fields commonly adjoin these towns and are the main foraging grounds of the Rooks during the breeding season. In contrast, Rooks breeding in large cities feed in more differentiated habitats, including crop-free sites (without application of agrochemicals), such as the urban lawns near rookeries (unpublished data). In contrast, the lack of significant differences in concentrations of all of the examined elements in eggshells from small towns and villages (Table 1) suggests a similar level of agriculture and similar sources and intensity of bioaccumulation of contaminants in Rook populations in both of these environments. Finally, it should borne in mind that the varying number of significant correlations in specific habitats or data sets could have resulted partly from the different sample sizes of eggshells from these sites (Table 2). Such a potential constraint, however, did not change the overall positive direction of interactions between the eggshell elements.
Variable or competitive interactions among the eggshell trace elements as an effect of their different levels were also illustrated by PCA. Overall, the majority of extracted PCs (Table 3) agree with the previous correlations between individual elements because subsequent PC scores consisted primarily of highly correlated elements (cf. Table 2). In our opinion, such variable groupings of eggshell elements across different habitats suggest that the potential identification of sources of contaminations by trace metals, e.g., from geochemical, industrial, or agriculture-related processes or origins (cf. Hashmi et al. 2013) is not effective because the results of a statistical analysis (which only deal with concentrations of individual elements) do not always indicate a cause-effect relation.
Relationships Between Various Trace Elements in Eggshells: Comparison With Other Studies and Potential Sources of Variation
To illustrate the potential biochemical or ecotoxicological consequences of high levels of the seven trace elements analysed in our samples, they should be compared with the results of related studies on elemental interaction in avian eggshells. For Herring Gulls Larus argentatus and Roseate Terns Sterna dougallii breeding on the Atlantic Coast of the eastern United States, with an apparently lower eggshell levels of Pb (3 and 11 times lower, respectively, than in all of the eggshells analysed in our study), Cd (10 and 5 times lower), and Cr (4 and 8 times lower), Burger (1994) did not find any significant relationship for these three trace elements.
Similarly, in eggshells of Audouin’s Gulls from the Ebro Delta, southern Spain, with ~2–4 times lower Zn and Cu levels, and Pb and Cd lower than the limit of detection (i.e. <0.05 and <0.005 ppm dw), Morera et al. (1997) did not obtain any significant correlations for these four trace elements. In contrast, the Clapper Rail eggshells examined by Rodriguez-Navarro et al. (2002), with generally lower levels of Cu, Zn, As, Pb, and Cd than in our samples, although with signs of thinning probably due to increased Hg levels, showed only one significant (negative) correlation between Zn and Pb.
More recently, Hashmi et al. (2013) analysed the interactions of six trace elements (Cu, Ni, Pb, Cr, Cd, and Zn) in eggshells of Cattle Egrets Bubulcus ibis and Little Egrets Egretta garzetta from eastern Pakistan. In those eggshells, average levels of Pb, Cd, and Zn was comparable with or even greater than in our samples. That study, however, used different species and site differences linked to local land-use types; moreover, the concentrations of three other elements (Cu, Ni, and Cr) were apparently lower than in our samples. Considering the data pooled across all sites and species, these investigators obtained only two significant interactions: one negative (Cu and Cd) and one positive (Pb and Ni).
Comparing our findings with those presented above, we can state that at lower or baseline levels, interactions between trace elements in eggshells are of a neutral character. In this sense, such a statement stands in agreement with the concept of additivity (i.e., no visible trend in the direction of interaction) of chemical elements, which is operative at low-level exposures to chemical mixtures (Svendsgaard and Hertzberg 1994; cf. Bae et al. 2001 and Discussion). Furthermore, the rather few cases of significant relationships between different eggshell trace elements in published sources can also be explained partly by the pooling of samples with various levels of elements resulting from species or habitat differences. This may imply that relationships between different elements in eggshells should be determined for relatively homogeneous samples/habitats; such an approach would yield biologically/ecologically relevant results.
In conclusion, at lower and intermediate levels, interactions between these trace elements in Rook eggshells are of a synergistic character and appear to operate as their parallel coaccumulation. A habitat-specific excess of some elements (primarily Cr, Ni, Cu, and As) suggests their more competitively selective sequestration. Because the combined effect of a pair or a few trace/toxic metals may be quite different from that of individual pollutants as a result of the interactions between them (e.g., Zhou et al. 2006), further studies are urgently needed of the mechanism of chemical and physiological interactions and the effects of high levels of anthropogenic elements or their mixtures on calcified tissues and bird eggs, including eggshell properties in Rooks and other agricultural birds or species from contaminated environments.
References
Ayas Z (2007) Trace element residues in eggshells of grey heron (Ardea cinerea) and black-crowned night heron (Nycticorax nycticorax) from Nallihan Bird Paradise, Ankara-Turkey. Ecotoxicology 16:1573–3017
Ayas Z, Celikkan H, Aksu ML (2008) Lead (Pb) and copper (Cu) concentration in the eggshells of Audouin’s Gulls (Larus audouinii) in Turkey. Turk J Zool 32:1–6
Bae D, Gennings C, Carter W, Yang R, Campain J (2001) Toxicological interactions among arsenic, cadmium, chromium, and lead in human keratinocytes. Toxicol Sci 63:132–142
Beckman L, Nordenson I (1986) Interaction between some common genotoxic agents. Hum Hered 36:397–401
Beyerbach M, Büthe A, Heidmann WA, Dettmer R, Knüwer H (1987) Chlorierte Kohlenwasserstoffe in Eiern und Lebern von Saatkrähen (Corvus frugilegus) aus niedersächsichen Brutkolonien. J Ornithol 128:277–290
Burger J (1994) Heavy metals in avian eggshells: another excretion method. J Toxicol Environ Health 41:207–220
Chandler CR (1995) Practical considerations in the use of simultaneous inference for multiple tests. Anim Behav 49:524–527
Dauwe T, Bervoets L, Blust R, Pinxten R, Eens M (1999) Are eggshells and egg contents of great and blue tits suitable as indicators of heavy metal pollution? Belg J Zool 129:439–447
Eeva T, Lehikoinen E (1995) Egg shell quality, nest size and hatching success of the great tit (Parus major) and the pied flycatcher (Ficedula hypoleuca) in an air pollution gradient. Oecologia 102:312–323
Eisler R (1998) Nickel hazards to fish, wildlife, and invertebrates: A synoptic review. Contaminant Hazard Reviews. Report No. 34. Patuxent Wildlife Research Center. United States Geological Survey
Gasparik J, Vladarova D, Capcarova M, Smehyl P, Slamecka J, Garaj P et al (2010) Concentration of lead, cadmium, mercury and arsenic in leg skeletal muscles of three species of wild birds. J Environ Sci Health A Tox Hazard Subst Environ Eng 45:818–823
Gonzalez LM, Hiraldo F (1988) Organochloride and heavy metal contamination in the eggs of the Spanish imperial eagle (Aquila (heliaca) adalberti) and accompanying changes in eggshell morphology and chemistry. Environ Pollut 51:241–258
Hair JF, Anderson RE, Tatham RL, Black WC (1998) Multivariate data analysis, 5th edn. Prentice Hall, Upper Saddle River, pp 87–138
Hashmi MZ, Malik R, Shahbaz M (2013) Heavy metals in eggshells of cattle egret (Bubulcus ibis) and little egret (Egretta garzetta) from the Punjab province, Pakistan. Ecotoxicol Environ Saf 89:158–165
Jonchere V, Brionne A, Gautron J, Nys Y (2012) Identification of uterine ion transporters for mineralisation precursors of the avian eggshell. BMC Physiol 12:10
Kamiński P (1998) The impact of calcium and heavy metals upon the nest development of the tree sparrow (Passer montanus) [in Polish] Wyd. UMK, Toruń
Kamiński P, Warot L (2005) Chemical element interactions in Jackdaw Corvus monedula nestlings. In: Jerzak L, Kavanagh B, Tryjanowski P (eds) Corvids of Poland [in Polish with English abstract]. Bogucki Wyd Nauk, Poznań, pp 173–184
Kasprzykowski Z (2003) Habitat preferences of foraging Rooks Corvus frugilegus during the breeding period in the agricultural landscape of eastern Poland. Acta Ornithol 38:27–31
Kasprzykowski Z (2007) Reproduction of the rook Corvus frugilegus in relation to the colony size and foraging habitats. Folia Zool 56:186–193
Mahaffey KR, Capar SG, Gladen BC, Fowler BA (1981) Concurrent exposure to lead, cadmium, and arsenic. Effects on toxicity and tissue metal concentrations in the rat. J Lab Clin Med 98:463–481
Malmberg T (1973) Pesticides and the rook Corvus frugilegus in Scania, Sweden between 1955 and 1970. Oikos 24:377–387
Miljeteig C, Gabrielsen GW, Strom H, Gavrilo MW, Lie E, Jenssen B (2012) Eggshell thinning and decreased concentrations of vitamin E are associated with contaminants in eggs of ivory gulls. Sci Total Environ 431:92–99
Möller G (1996) Biogeochemical interactions affecting hepatic trace element levels in aquatic birds. Environ Toxicol Chem 15:1025–1033
Morera M, Sanpera C, Crespo S, Jover L, Ruiz X (1997) Inter and intraclutch variability in heavy metals and selenium levels in Audouin’s Gull eggs from Ebro Delta, Spain. Arch Environ Contam Toxicol 33:71–75
Nys Y, Gautron J, Garcia-Ruiz JM, Hincke MT (2004) Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. Gen Palaeontol (Palaeobiochemistry) C. R. Palevol 3:549–562
Orłowski G, Czapulak A (2007) Different extinction risks of the breeding colonies of Rook Corvus frugilegus in rural and urban areas of SW Poland. Acta Ornithol 42:145–155
Orłowski G, Kamiński P, Kasprzykowski Z, Zawada Z (2013) Relationships between stomach content and concentrations of essential and nonessential elements in tissues of omnivorous nestling Rooks Corvus frugilegus: is the size and composition of stomach content relevant? Folia Zool 62:281–288
Orłowski G, Kamiński P, Kasprzykowski Z, Zawada Z, Koim-Puchowska B, Szady-Grad M et al (2012a) Essential and nonessential elements in nestling Rooks Corvus frugilegus from eastern Poland with a special emphasis on their high cadmium contamination. Arch Environ Contam Toxicol 63:601–611
Orłowski G, Kamiński P, Kasprzykowski Z, Zawada Z (2012b) Metal interactions within and between tissues of nestling rooks Corvus frugilegus. Biologia 67:1211–1219
Orłowski G, Kasprzykowski Z, Dobicki W, Pokorny P, Polechonski R (2010) Geographical and habitat differences in concentrations of copper, zinc and arsenic in eggshells of the Rook Corvus frugilegus in Poland. J Ornithol 151:279–286
Orłowski G, Kasprzykowski Z, Dobicki W, Pokorny P, Wuczyński A, Polechoński Z, Mazgajski TD. Residues of chromium, nickel, cadmium and lead in Rook Corvus frugilegus from urban and rural areas of Poland. (submitted)
Orłowski G, Kasprzykowski Z, Zawada Z, Kopij G (2009) Stomach content and grit ingestion by Rook Corvus frugilegus nestlings. Ornis Fenn 86:117–122
Parsiegla KL, Katz JL (1999) Calcite growth inhibition by copper(II) II. Effect of solution composition. J Cryst Growth 213:368–380
Pendleton GW, Whitworth MR, Olsen GH (1995) Accumulation and loss of arsenic and boron, alone and in combination, in mallard ducks. Environ Toxicol Chem 14:1357–1364
Pinowska B, Kraśnicki K, Pinowski J (1981) Estimation of the degree contamination of granivorous birds with heavy metals in agricultural and industrial landscape. Ekol Pol A7:137–149
Pinowski J, Pinowska B, Kraśnicki K, Tomek T (1983) Chemical composition of growth in nestling Rooks Corvus frugilegus. Ornis Scand 14:289–298
Pinowski J, Barkowska M, Kruszewicz AH, Kruszewicz AG (1994) The causes of the mortality of eggs and nestlings of Passer spp. J Biosci 19:441–451
Ratcliffe DA (1970) Changes attributable to pesticides in egg breakage frequency and eggshell thickness in some British birds. J Appl Ecol 7:67–115
Rodriguez-Navarro AB, Gaines K, Romanek C, Masson G (2002) Mineralization of Clapper Rail eggshells from a contaminated salt marsh system. Arch Environ Contam Toxicol 43:449–460
Scheuhammer AM (1996) Influence of reduced dietary calcium on the accumulation and effects of lead, cadmium, and aluminum in birds. Environ Pollut 94:337–343
Skalická M, Korenekova B, Nad P, Saly J (2008) Influence of chromium and cadmium addition on quality of Japanese quail eggs. Acta Vet Brno 77:503–508
Svendsgaard DJ, Hertzberg RC (1994) Statistical methods for the toxicological evaluation of the additivity assumption as used in the Environmental Protection Agency Chemical Mixture Risk Assessment Guidelines. In: Yang RSH (ed) Toxicology of chemical mixtures. Academic, San Diego, pp 599–642
Wada N, Yamashita K, Umegaki T (1995) Effect of divalent cationsupon nucleation, growth and transformation of calcium carbonatepolymorphs under conditions of double diffusion. J Cryst Growth 148:297–304
Zhou QX, Zhang QR, Liang JD (2006) Toxic effects of acetochlor and methamidops on earthworm Eisenia fetida in Phaeozem, Northeast China. J Environ Sci 18:741–745
Acknowledgments
We are grateful to W. Bena, P. T. Dolata, S. Jędrzejewski, K. Kujawa, R. Kruszyk, S. Kuźniak, K. and U. Martini, R. Piekarski, J. Pietrowiak, M. Rachel, J. Ratajczak, M. Słupek, A. Sokołowska, T. Stawarczyk, P. Tryjanowski, T. Wilżak, T. Zarzycki, and the management of the landscape parks in Sieradz for participating in the ecotoxicological study of the Rook in Poland. Thanks are also due to the anonymous reviewers for giving valuable comments. We also appreciate the improvements in English use made by Peter Senn and Bruce Peterson through the Association of Field Ornithologists’ programme of editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orłowski, G., Kasprzykowski, Z., Dobicki, W. et al. Trace-Element Interactions in Rook Corvus frugilegus Eggshells Along an Urbanisation Gradient. Arch Environ Contam Toxicol 67, 519–528 (2014). https://doi.org/10.1007/s00244-014-0030-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0030-x