Abstract
The aim of the study was to evaluate the effect of heavy-metal contamination on two fish species (Channa striatus and Heteropneustes fossilis) inhabiting a small freshwater body of northern India. After being captured, each specimen was weighed, measured, and analyzed for heavy metals (chromium [Cr], nickel [Ni], and lead [Pb]). Accumulation of heavy metals was found to be significantly greater (p < 0.05) in different tissues (gill, liver, kidney, and muscle) of fishes captured from the reservoir than from the reference site. Levels of heavy-metal contamination in Shah jamal water was Cr (1.51 mg/l) > Ni (1.22 mg/l) > Pb (0.38 mg/l), which is significantly greater than World Health Organization standards. Bioaccumulation factor was calculated, and it was observed that Pb was most detrimental heavy metal. Condition factor was also influenced. Micronucleus test of fish erythrocytes and comet assay of liver cells confirmed genotoxicity induced by heavy-metal contamination in fishes. Heavy metals (Cr, Ni, and Pb) were increased in both fish species as determined using recommended values of Federal Environmental Protection Agency for edible fishes. This raises a serious concern because these fishes are consumed by the local populations and hence would ultimately affect human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Aquatic fauna contributes to the major part of our dietary needs. In recent years, increasing amounts of aquatic contamination resulting from discharges of industrial, agricultural, and urban waste into aquatic habitats poses a serious concern for public health due to consumption of fish from these water bodies (Bickham et al. 2000; Mayon et al. 2006). Heavy metals—such as cadmium (Cd), arsenic (As), mercury (Hg), lead (Pb), copper (Cu), nickel (Ni), chromium (Cr), and zinc (Zn), etc. as well as other persistent toxic substances—are the leading cause of aquatic contamination (Gurcu et al. 2010). Small lakes and reservoirs are deteriorated more frequently by heavy-metal contamination due to human interference compared with rivers (Sabhapandit et al. 2010; Bhat et al. 2012). Some metals in minimal concentrations are essential for living organisms. But persistent heavy-metal tissue bioaccumulation, due to their redox cycling and their ability to deteriorate tissue sulfhydryl groups, leads to oxidative stress, which can be seen through genotoxic parameters such as micronucleus text (MNT) and comet assay (Ali et al. 2009). It is evident from the earlier studies that Cr, Ni, and Pb present in these local water bodies accumulates and biomagnifies in fish tissues due to chronic exposure in their living habitat (Sen et al. 2011; Javed and Usmani 2012). Similar heavy metals were studied by Fatima and Usmani (2013) in Yamuna river for Channa striatus and Heteropneustes fossilis showed marked hepatotoxicity and nephrotoxicity. These metals have been grouped as hazardous metals and have Hazardous Substance Databank (HSDB) numbers, viz., Cr (910), Ni (1096), and Pb (231), due to their widespread toxicities (Hazardous Substances Data Bank 2006).
Because there is growing concern about the presence of genotoxins in local water bodies, the development of sensitive biomarkers has gained importance (Hayashi et al. 1998). MNT, which is a suitable parameter, is used in bioindication for the generation of genetic material fragments known as micronuclei (MN). These fragments appear in the cytoplasm when the parts of the chromosomes or entire chromosome are not rapidly incorporated in the nuclei of the daughter cells in mitosis because these fragments do not have centromeres; these fragments left behind are incorporated in the secondary nuclei called “micronuclei” (Schmid 1975; Heddle et al. 1983). Thus, this test helps to examine the genotoxic effects of contaminants that are present in the aquatic environment (Tucker and Preston 1996). The count of MN has served as an index of chromosome breaks and mitotic spindle apparatus dysfunction (Hooftman and De Raat 1982).
Many types of damage can occur in DNA molecules as a result of the endogenous production of reactive oxygen species (ROS), interactions with xenobiotics, irradiation, etc. The most prevalent causative agent of oxidative DNA damage is the •OH ion, which can be produced as a result of a reaction between O2·− and iron (Pb; Beckman and Ames 1997). Damage products include double-strand breaks, single-strand breaks, interstrand and intrastrand cross-links, and adduct and DNA protein cross-links to name a few (Wood et al. 2001). DNA damage by xenobiotics occurs in three basic steps wherein the first is the formation of adducts with toxic molecules. The next stage, secondary modifications of DNA, includes single- and double-strand breakage, changes in DNA repair, base oxidation, and cross-links. Xenobiotics may induce these secondary modifications by way of ROS production. In the third stage, cells show altered function, which can lead to cell proliferation and, consequently, cancer (Monserrat et al. 2007). Those DNA modifications most commonly used as biomarkers in studies addressing effects of xenobiotic exposure in fish include oxidative base damage and the formation of DNA adducts. Thus, the development of another genotoxic hallmark of DNA strand breaks is the comet assay (single-cell gel electrophoresis), which has sensitivity for detecting low levels of DNA damage (0.1 DNA break/109 Da) (Gedic et al. 1992). This sensitive assay gives an idea of the impact of the toll of heavy metal-induced free radicals on DNA molecules because there are breakages of phosphodiester linkages within DNA molecules (Shugart 2000). DNA repair in aquatic organisms is slower than that in mammalian cells, which confers an advantage to measure induced DNA breakages in toxicant exposed fishes (Walton et al. 1984; Maccubbin 1994).
Aligarh is an urban area and is best known for its lock industry. The objective of the present study was to evaluate the effects of effluents from nearby lock factories into the Shahjamal reservoir. Concentrations of Cr, Ni, and Pb were estimated in water samples and two important fish species namely, C. striatus and H. fossilis.
Earlier, C. punctatus was used as a genotoxic model by Ali et al. (2009) to evaluate the mutagenic effect of chlorpyrifos in freshwater. Although H. fossilis was used by Ahmed et al. (2013) to study the effects of hexavalent chromium on genotoxicity. C. striatus (a demersal fish) and H. fossilis (a catfish) are usually found in reservoirs and ditches in northern India. They are comestible fish species and are a good source of protein. H. fossilis also have medicinal value. These fishes were captured to assess the bioaccumulation of heavy metals in their tissues and their genotoxic potential as data regarding the genotoxic and mutagenic nature of composite heavy metals on fish, which is lacking, from natural aquatic ecosystem. Furthermore, the experiment aimed to evaluate the sensitivity and suitability of blood cells through MNT and of liver cells through comet assay of two selected fish species indwelling a heavy metal-contaminated aquatic ecosystem.
Materials and Methods
Site of Collection
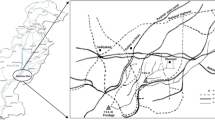
Shahjamal, Aligarh, Northern India (27°52’51”N longitude and 78°3’14”E latitude) is dominated by many small-scale lock factories, which discharge their waste into nearby water bodies. We have selected one such effluent-dominated water body for the current study, i.e., Shahjamal reservoir. The water quality of the water body used for the study was found to be loaded with discharge from a small-scale lock-manufacturing industry. Jawan canal lies at coordinates 28°02′26.1″N longitude and 78°06′49″E latitude. It is low in pollution and was used to compare the impact of an effluent-dominated water body on fish genotoxicity. A map showing the reference and experimental sites is shown in Fig. 1.
Sampling of Water
Water was collected from the reference and experimental sites. Systematic water sampling was performed to obtain unbiased profiling of the reservoir. Zonal sampling from four sides of the reservoir was performed for heavy-metal analysis. Water was stored at 4 °C in clean sampling glass bottles.
Fish Procurement
Fish species C. striatus and H. fossilis were collected from reference and reservoir water with the help of local fisherman and brought to the laboratory.
Sample Processing for Heavy-Metal Estimation
Digestion of Water Samples
Heavy-metal estimation in water was performed using the acidic digestion method as described by Cabrera et al. (1992) with slide modifications. Water sample (100 ml) was taken in a 250-ml volumetric flask and acidified with 5 ml of HNO3 (55 %). Then the acidified water was evaporated on a hot plate to approximately 20 ml. Then 5 ml of HNO3 (55 %) and 10 ml of perchloric acid (70 %) were added along with a few glass beads (the sample was evaporated until brown fumes changes to dense white fumes of perchloric acid). It was cooled to room temperature and diluted with distilled water (DW) in a 100 ml volumetric flask.
Digestion of Tissue Sample
Fishes were dissected and organs (liver, kidney, gill, muscle) isolated and processed as described by Du Preez and Steyn (1992). Thawed frozen tissue sample was rinsed in DW and blotted with blotting paper. The known weight of tissue was shifted to a 250 ml volumetric flask. Nitric acid (5 ml [55 %]) and perchloric acid (1 ml [70 %]) were also added and kept overnight. Then HNO3 (5 ml [55 %]) and perchloric acid (4 ml [70 %]) was added further in a volumetric flask and kept on a hot plate at 200–250 °C. Transparent clear solution was subsequently obtained as brown fumes converted to dense white fumes, which indicated complete digestion. The sample was cooled and diluted to 10 ml with DW by proper rinsing of the digested flask. The sample was stored until heavy-metal detention in washed glass bottles. Heavy metals were analyzed using a atomic absorption spectrophotometer (AAS; 2280, Perkin-Elmer, USA) according to the American Public Health Association (1989). All chemicals (sulphuric, nitric, and perchloric acid) used were of analytical grade.
Quality Control
A certified reference material (CRM) (Cr [02733], Ni [42242], and Pb [16595]; TraceCERT CRMs for AAS; Sigma Aldrich, USA) and blanks were used to check the accuracy of the instrument. Analysis of blanks and reference material replicates showed good accuracy, and reference material concentrations found were within 98–102 % of the certified values for all measured heavy metals.
Bioaccumulation Factor
The bioaccumulation factor (BAF) is described as the ratio of concentration of pollutant (Heavy metal) in an organism to its concentration in the water body (Authman and Abbas 2007).
The BAF was calculated using the formula: BAF = concentration of heavy metal in fish tissue (μg/g)/concentration of heavy metal in water body (mg/L).
Condition Factor
Condition factor (CF) was calculated using the length and weight of fish and expressed as mean ± SD. The formula used was CF = weight (g)/length3 (cm)3 × 100 as described by Fulton (1904).
MNT
MN frequency in erythrocytes was evaluated according to the method described by Fenech (1993). Blood was collected from fish by way of cardiac puncture and smeared on clean slides, dried at room temperature for 24 h, and subsequently fixed with 100 % methanol. Samples were stained with 10 % Giemsa solution for 10 min, air dried, and fixed permanently.
A total 2,000 erythrocytes were examined for each specimen under a light microscope (Nikon eclipse E200) with oil immersion at 1,000× magnification. Blind scoring of MN was performed on randomized and coded slides. Criteria described by Fenech et al. (2003) were taken into consideration.
Other nuclear and cellular anomalies—such as nuclear buds, erythrocytes bearing more than a single micronucleus, blebbed nuclei, lobed nuclei, notched nuclei, vacuolated cytoplasm, and microcytes—were recorded separately on the basis of the criteria described by Da Silva and Fontanetti (2006). MN frequency = (no. of cells containing MN) (1,000)/total number of cells counted.
Preparation of Single-Cell Suspension and Comet Assay
DNA damage was assessed using alkaline comet assay as described by Singh et al. (1988) with slight modifications. All of the comet assay experiment was performed under dim-light conditions to avoid additional DNA damage. Freshly collected liver samples were washed with chilled phosphate buffered saline-calcium magnesium free (PBS-CMF). Liver tissues were minced with PBS-CMF 20 mM ethylene diamine tetraacetic acid (EDTA) and 10 % dimethyl sulphoxide (DMSO) at pH 7.4 and filtered through a 100 µm mesh strainer. Tissues were collected in a tube, and 2 ml of PBS-CMF was added followed by centrifugation at 2,000 rpm at 4 °C for 10 min. Cell pellet was collected and resuspended in PBS-CMF. Cell-viability test was performed using the Trypan blue exclusion method (Anderson and Wild 1994), and samples showing viability >80 % were considered for comet assay. Cell suspensions were suspended in 0.5 % low melting point-agarose (LMPA) overlaid on slides precoated with a fine layer of 1.25 % normal-melting agarose. A third layer of 0.75 % LMPA was poured, and slides were immersed in lysing solution (2.5 M NaCl, 100 mM EDTA, 10 mM Trizma base, 0.2 mM NaOH, 1 % Triton X-100, and DMSO [pH 10]) for 1 h at 4 °C to lyse cells and facilitate DNA unfolding. This was followed by electrophoresis buffer (300 mM NaOH, 1 mM EDTA [pH > 13]) for 20 min to allow DNA unwinding, and electrophoresis was performed at 25 V and 300 mA current in the same buffer for 30 min. After electrophoresis, slides were neutralized with neutralizing buffer (0.4 M Tris buffer [pH 7.5]).
Slides were dried and stained with ammoniacal silver nitrate solution. Photographs were obtained at 400×. Ten fishes per species were analyzed, and 50 cells were scored (500 cells/species per sites) were scored randomly and analyzed using Cometscore software (version 1.5; TriTek, Sumerduck). Degree of DNA damage was represented as percent DNA in tail.
Statistical Analysis and Calculation of Induction Factor
Parameters (mean and SD) were calculated for data obtained for the physiochemical properties of water and the presence of heavy metals in water and fish samples. One-way analysis of variance was applied to compare the difference amongst the means; p values <0.05 were considered significant. For heavy-metal bioaccumulation, Duncan’s new multiple range test was applied (Duncan 1955) as post hoc test for multiple comparisons.
For genotoxic parameters, the statistical significance of the differences in mean values between experimental and reference fishes was determined using Student t test. Induction factor for MN frequency and comet assay parameters (percent cells with tail, tail length, and percent DNA in tail) was calculated as ratio of MN frequency or comet assay parameter between experimental and reference samples (Polard et al. 2011). Assumptions of normality (Shapiro–Wilk test) and homogeneity (Levene’s test) of data were verified (Zar 1984).
Results
Physicochemical Properties of Reference and Reservoir Water
Physiochemical properties of reference and experimental sites are listed in Table 1. For reference site, temperature (22.64 °C), pH (7.4), and dissolved oxygen (DO) (5.80) were found to be in standard level. However, for the experimental site water temperature and pH ranged between 23.8 and 24.9 °C and 6.98–7.78, respectively. The DO concentration (2.5–3.80 mg/l) was considerably low. The concentrations of heavy metal was in the order Cr (1.51 ± 0.14) > Ni (1.22 ± 0.09) > Pb (0.38 ± 0.07) in water samples collected from the Shahjamal reservoir. All heavy metals were present in levels beyond recommended values set by World Health Organization (1985) (Table 1). Heavy metals (Cr, Ni, and Pb) were below detection limit (BDL), i.e., lower than in experimental sites. Therefore, Jawan canal was taken as the reference site for the study.
Heavy-Metal Bioaccumulation in Fish
The two fish species, C. striatus and H. fossilis, collected were dissected to remove tissues (liver, kidney, gill, muscle) for the determination of bioaccumulation (µg/g wet weight) of heavy metals. The bioaccumulation of heavy metals (Cr, Ni, and Pb) in (µg/g) wet weight in different tissues of C. striatus collected from the experimental site is listed in Table 2. There was heavy bioaccumulation of all of the heavy metals in fish tissues (Cr accumulated the most). The pattern of bioaccumulation was found to be Cr (gills [94.82 µg/g] > kidney [38.51 µg/g] > liver [12.33 µg/g] > muscle [3.04 µg/g]) followed by lead (kidney [38.45 µg/g] > gills [32.23 µg/g] > liver [8.76 µg/g] > muscle [2.7 µg/g]), and Ni, which accumulated the least (gills [11.99 µg/g] > kidney [9.67 µg/g] > liver [9.08 µg/g] > muscle [0.65 µg/g]). The bioaccumulation of heavy metal (Cr, Ni, and Pb) in (µg/g) wet weight in different tissues of H. fossilis collected from experimental site is listed in Table 3.
In H. fossilis, the pattern of accumulation was found comparable with that in C. striatus. Cr accumulated most (gills [97.75 µg/g] > liver [33.34 µg/g] > kidney [27.57 µg/g] > muscle [6.85 µg/g]) followed by Pb [gills [41.67 µg/g] > kidney [32.57 µg/g] > liver [16.75 µg/g] > muscle [1.67 µg/g]), whereas the pattern for bioaccumulation of Ni was gills (54.19 µg/g) > kidney (8.89 µg/g) > liver (7.54 µg/g) > muscle (2.9 µg/g)].
In both fish species, gills were the most highly influenced organ, and amongst the heavy metals, Cr bioaccumulated the most in all tissues. In addition, the values for all of the heavy metals in muscle (edible part of fish) were much greater than the values recommended by Federal Environmental Protection Agency (1999) (footnotes in Tables 2 and 3).
BAF
Heavy metals present in the water and values accumulated in various tissues were used to calculate BAFs. The BAF values for C. striatus are listed in Table 4. In C. striatus, the highest BAF was observed in kidney (101.18) for Pb. The BAF in H. fossilis tissue samples is listed in Table 5. The highest BAF was observed in liver (109.65) of H. fossilis for Pb.
CF
Length, weight (wet weight), and CF of C. striatus and H. fossilis are listed in Table 6. Results show that CF for both fish species was significantly different in the site under study (experimental) compared with its corresponding reference site.
MNT
Frequency of MN and other nuclear abnormalities in reference and fish collected from the experimental site are listed in Table 7 (Fig. 2). There was a significant increase in MN frequency (1.88×) and nuclear abnormalities (1.29×) in C. striatus. For H. fossilis, MN frequency (1.65×) and other abnormalities (1.47×) were increased in the Shahjamal site compared with the reference site.
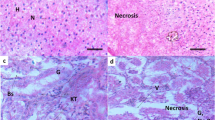
Representative of MN and other nuclear abnormalities in erythrocytes of a C. striatus reference fish, b C. striatus collected from the experimental site, c H. fossilis reference fish (d), and H. fossilis collected from the experimental site. NE normal erythrocytes, VC vacuolated cytoplasm, NB nuclear bud, MC microcyte, MMN multiple MN, LN lobed nucleus
Comet Assay
DNA damage in reference fish and fish collected from the experimental site is listed in Table 8 as percent cells with tail, tail length (pixels), and percent DNA in tail. Tail length and percent DNA in tail showed a significant increase in DNA damage (Fig. 3a, c). Induction factor (the toll of heavy metal-dominated water bodies on DNA damage is visualized by percent tail DNA) of 1.66 for C. striatus and 1.31 for H. fossilis. Thus, C. striatus showed a greater level of genotoxic damage (Fig. 3b) with greater mean tail length (8.25 pixels) than did H. fossilis (6.33 pixels) (Fig. 3d). However, a greater number of cells with tail was induced in H. fossilis (1.5×) than in C. striatus (2.07×) compared with their corresponding reference fish.
Discussion
In this study, we found high levels of contamination in Shahjamal reservoir, which can be attributed to the fact that lock factories are discharging their waste directly into this local water body with no proper water treatment. Effluents from industries discharge mainly heavy metals (Cr, Ni, and Pb). Heavy metals are the major cause of pollution in water bodies due to their ubiquitous distribution and tremendous potential for bioaccumulation in aquatic biota (Yohannes et al. 2013; Szefer et al. 1990). Cr is an important component of the lock industry. Chromium-plating liquid contains mostly hexavalent chromium, which causes most of the toxic effects. Hexavalent chromium is a more potent skin penetrant than the trivalent form (Wahlberg and Skog 1965). Perhaps due to this, Cr was found high amounts in the reservoir water and in tissues of both fish species. Cr accumulated the most in the tissues, and this corroborates with the findings of other investigators where Cr accumulated in greater concentrations (Fatima and Usmani 2013; Eneji et al. 2011; Taweel et al. 2011).
Ni is the second most-used heavy metal in lock polishing by this industry. It was found in moderate concentrations in water but accumulated least in tissues as has also been noted by other investigators (Canli et al. 1998; Vinodhini and Narayan 2008; Eneji et al. 2011; Javed 2005). Ni toxicity is responsible for allergic skin reactions and has been reported to be one of the most common causes of allergic toxicities as reflected by positive dermal patch tests (Clarkson 1988; Kitaura et al. 2003; Cavani 2005).
Pb concentration was the lowest in the reservoir water but accumulated in moderate amounts as has also been reported by other investigators (Canli et al. 1998; Vinodhini and Narayan 2008; Abdel-Baki et al. 2011; Javed 2005). It is toxic even at low concentrations because it mimics essential elements (e.g., calcium, magnesium, Pb, and Zn) with consequent effects on enzymes containing SH groups (Jennette 1981), increased incorporation of erroneous nucleotides (Johnson 1998), and accumulation of free radicals due to alteration of the oxidative processes of cells; it also effects repair mechanisms in which Pb has been implicated as a co-carcinogen (Fracasso et al. 2002).
Most of the studies performed on the induction of heavy metals in fish have been performed in a laboratory (Ferraro et al. 2004; Liney et al. 2006; Ahmed et al. 2013). However, there are a few studies where fish were collected from a natural ecosystem (Canli et al. 1998; Javed 2005; Javed and Usmani 2012).
Many investigators have also reported concentrations of heavy metals in a water body and its concentration in fish tissues, but they did not estimate the toxicity of these heavy metals by their presence in the tissue-to-water ratio. Fishes dwelling in heavy metal-loaded urban reservoir water accumulated these toxic metals in various tissues with biomagnifications of greater factors. Usually other investigators have focused on wet-weight heavy-metal concentrations in various fish tissues, but BAF is a more reliable means to estimate toxicity caused by heavy metals. C. striatus and H. fossilis are relished by the local population. Thus, when BAF was performed, it was observed that in both fish species, Pb, followed by Cr and Ni, accumulated the most in gills and kidney. Gills were affected the most; this could be due to subcellular partitioning of toxicants and other factors that influence the uptake, retention, and bioaccumulation of heavy metals in fish tissues (Nesto et al. 2007; Zhao et al. 2012). This fact corroborates with the finding by Wang and Rainbow (2006) that metal bioaccumulation in fish is controlled by a balance between uptake and elimination and differential accumulation of these metals in various tissues.
Accumulation of these heavy metals lead to altered physiology and can be visualized through the use of a growth biomarker, i.e., CF. CF signifies the general health and growth of the fish and forms the gross health index. CF is a low-cost and simple tool to obtain information on the ability to tolerate by aquatic animals. Both fish species in the present study showed a marked decrease in CF with their respective references due to the heavy-metal toll. Similar observations, i.e., a decrease in CF due to metal pollution in highly degraded natural ecosystems, have been made by Hodson et al. (1992) in white sucker fish, Betancur et al. (2009) in eight fish species, and Omar et al. (2012) in Oreochromis niloticus and Mugil cephalus, This parameter may serve as a gross screening biomarker to indicate exposure and effects or to provide information on energy reserves (Mayer et al. 1992).
Channa striatus and H. fossilis are relished by the local population. Hence, these two fish species were chosen for the study for genotoxic studies from effluent-dominated water bodies of Shahjamal. The accumulation of heavy metals leads to the deterioration of genetic material and can be visualized through biomarkers, i.e., MNT and comet Assay. Here, chronic exposure to heavy metals in water bodies lead to nuclear damage; thus, for confirmation, MNT was performed. MNT in erythrocytes is a widely used tool for chronically metal-exposed fish with different types of pollutants having clastogenic and aneugenic properties (Udroiu 2006). In the present study, MN induction was significantly greater (p < 0.05) at 1.88× and 1.65× in C. striatus and H. fossilis, respectively, compared with reference fish. MN frequency was greater in C. striatus (0.47/1000 erythrocytes) than in H. fossilis (0.33/1000erthrocytes). This indicates that C. striatus is more sensitive to polluted water, and this can also be attributed to the factors such as interspecies sensitivity, metabolic capacity, DNA repair, and defence mechanisms (Rodriguez-Cea et al. 2003). Similarly, high MN values have been reported for different fishes exposed to polluted waters (Sanchez-Galan et al. 2001; Bagdonas et al. 2003; Betancur et al. 2009; Omar et al. 2012). Such marked MN inductions have also been reported by several investigators (Minissi et al. 1996; De Lemos et al. 2001; Betancur et al. 2009; Dillon et al. 1998) when fish were exposed to heavy metals.
Nucleus disturbances that we found through MNT, such as chronic genotoxicity, was further confirmed by comet assay, which is the most popular and sensitive biomarker for DNA strand breaks in aquatic fauna (Frenzilli et al. 2009). Comet assay showed significantly (p < 0.05) greater damage in terms of percent cells with tail, tail length, and percent DNA in tail in both fish species compared with reference fish. Of both species, C. striatus was found to be more sensitive to polluted reservoir water with greater mean percent DNA (11.49 %) than seen in H. fossilis (7.48 %). Similarly, other investigators have also reported high DNA fragmentation in liver cells from natural water studies (Rajaguru et al. 2003; Liney et al. 2006) and from induced studies (Ahmed et al. 2013; Akter et al. 2009). Comet assay is a sensitive biomarker of oxidative damage to cells because it induces changes in tissue structure and function earlier than those induced by MNT (Deventer 1996). This test is also an indirect tool to detect oxidative stress in tissues because reducible heavy metals lead to the formation of hydroxyl radicals, which in turn leads to base alteration and deoxyribosephosphate backbone breakage. In turn, GSH activity is increased, thus leading to increased tail DNA because GSH decreases more and more reducible heavy metals to form free radicals (Li et al. 2010). Therefore, DNA adduct formation is visible as comet formation in cells.
Conclusion
The effluent from the lock factory in the Shahjamal reservoir has the potential to adversely affect human health through the consumption of contaminated fish. The results of the present study show that heavy metals are affecting fish health and that metal tissue residue levels exceed the recommended values of Federal Environmental Protection Agency (1999) for edible fish. The heavy metal-laden waste material first pollutes the aquatic water body. This disturbs the physicochemical parameters of the water as well as the fauna. Thus, the current study was focused on different parameters that systematically correlate the impact of heavy metals on fish health because fish respond to toxic agents present in water bodies similar to greater vertebrates and can allow the assessment of substances that are potentially hazardous to humans.
CF gives idea of gross health condition of dweller fishes, whereas more reliable genotoxic damage was studied through the use of MNT, which detects cell injuries that have undergone at least one mitotic cycle and measures chromosome loss, whereas comet assay detects reparable injuries (alkali-labile sites) by direct measurement of DNA strand breakage. Therefore, both tests together are recommended for genotoxic studies to understand underlying mechanisms of genetical disruptions. The effluent from lock factories should be monitored regularly, and mitigation measures should be put in place to protect the ecosystem and human health.
References
Abdel-Baki AS, Dkhil MA, Al-Quraishy S (2011) Bioaccumulation of some heavy metals in tilapia fish relevant to their concentration in water and sediment of Wadi Hanifah, Saudi Arabia. Afr J Biotechnol 10:2541–2547
Ahmed MK, Kundu GK, Al-Mamun MH, Sarkar SK, Akter MS, Khan MS (2013) Chromium (VI) induced acute toxicity and genotoxicity in freshwater stinging catfish, Heteropneustes fossilis. Ecotoxicol Environ Saf 92:64–70
Akter MS, Ahmed MK, Akhand AA, Ahsan N, Islam MM, Khan MS (2009) Arsenic and mercury induce death of Anabas testudineus (Bloch) involving fragmentation of chromosomal DNA. Terr Aquat Environ Toxicol 3:42–47
Ali D, Nagpure NS, Kumar S, Kumar R, Kushwaha B, Lakra WS (2009) Assessment of genotoxic and mutagenic effects of chlorpyrifos in freshwater fish Channa punctatus (Bloch) using micronucleus assay and alkaline single-cell gel electrophoresis. Food Chem Toxicol 47(3):650–656
American Public Health Association (1989) Standard methods for the examination of water and wastewater, 17th edn. American Water Works Association and Water Pollution Control Federation, New York
Anderson SL, Wild GC (1994) Linking genotoxic responses and reproductive success in ecotoxicology. Environ Health Perspect 102:9–12
Authman MM, Abbas HH (2007) Accumulation and distribution of copper and zinc in both water and some vital tissues of two fish species (Tilapia zillii and Mugil cephalus) of Lake Qarun, Fayoum Province. Egypt Pak J Zool 10:2106–2122
Bagdonas E, Bukleskis E, Lazutka JR (2003) Frequency of micronucleated erythrocytes in wild fish from natural freshwater bodies. Ekologija 1:67–71
Beckman KB, Ames BN (1997) Oxidative decay of DNA. J Biol Chem 272(32):19633–19636
Betancur IP, Palacio Baena JA, Guerrero MC (2009) Micronuclei test application to wild tropical ichthyic species common in two lentic environments of the low zones in Colombia. Actual Biol 31:67–77
Bhat MM, Narain K, Andrabi SZA, Shukla RN, Yunus M (2012) Assessment of heavy metal pollution in urban pond ecosystems. UJERT 2:225–232
Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R (2000) Effects of chemical contaminants on genetic diversity in natural populations: implications for biomonitoring and ecotoxicology. Mutat Res 463:33–51
Cabrera F, Conde B, Flores V (1992) Heavy metals in the surface sediments of the tidal river Tinto (SW Spain), Fresenius. Environ Bull 1:400–405
Canli M, Ay O, Kalay M (1998) Levels of heavy metals (Cd, Pb, Cu, Cr and Ni) in Tissue of Cyprinus carpio, Barbus capito, and Chondrostoma regium from the Seyhan River, Turkey. Turk J Zool 22:149–157
Cavani A (2005) Breaking tolerance to nickel. Toxicology 209:119–121
Clarkson TW (1988) Biological monitoring of toxic metals. Plenum Press, New York, pp 265–282
Da Silva Souza T, Fontanetti CS (2006) Micronucleus test and observation of nuclear alterations in erythrocytes of Nile tilapia exposed to waters affected by refinery effluent. Mutat Res 605:87–93
De Lemos CT, Rodel PM, Terra NR, Erdtmann B (2001) Evaluation of basal micronucleus frequency and hexavalent chromium effects in fish erythrocytes. Environ Toxicol Chem 20:1320–1324
Deventer K (1996) Detection of genotoxic effects on cells of liver and gills of B. rerio by means of single cell gel electrophoresis. Bull Environ Contam Toxicol 56:911–918
Dillon CT, Lay PA, Bonin AM, Cholewa M, Legge GJ, Collins TJ et al (1998) Permeability, cytotoxicity, and genotoxicity of chromium (V) and chromium (VI) complexes in V79 Chinese hamster lung cells. Chem Res Toxicol 11:119–129
Du Preez HH, Steyn GJ (1992) A preliminary investigation of the concentration selected metals in the tissues and organs of the tiger fish (Hydrocynus vittatus) from the Oilfants River, Kruger National Park, South Africa. Water SA 18:131–136
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
Eneji IS, Rufus SA, Annune PA (2011) Bioaccumulation of heavy metals in fish (Tilapia Zilli and Clarias Gariepinus) organs from River Benue, North-Central Nigeria. Pak J Anal Environ Chem 12:25–31
Fatima M, Usmani N (2013) Histopathology and bioaccumulation of heavy metals (Cr, Ni and Pb) in fish (Channa striatus and Heteropneustes fossilis) tissue: a study for toxicity and ecological impacts. Pak J Biol Sci 16:412–420
Federal Environmental Protection Agency (1999) Guidelines to standard for Environment Pollution Control in Nigeria. Lagos, Nigeria
Fenech M (1993) The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res 285:35–44
Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E (2003) HUMN project: detailed description of the scoring criteria for the cytokinesis block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534:65–75
Ferraro MV, Fenocchio A, Mantovani M, Ribeiro C, Cestari MM (2004) Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests. Genet Mol Biol 27:103–107
Fracasso ME, Perbellini L, Solda S, Talamini G, Franceschetti P (2002) Lead induced DNA strand breaks in lymphocytes of exposed workers: role of reactive oxygen species and protein kinase C. Mutat Res 515:159–169
Frenzilli G, Nigro M, Lyons BP (2009) The Comet assay for the evaluation of genotoxic impact in aquatic environments. Mutat Res 681:80–92
Fulton TW (1904) The rate of growth of fishes. Fish Board Scotl Ann Rep 22:141–241
Gedic CM, Ewen SWB, Collins AR (1992) Single cell gel electrophoresis applied to analysis of UV-C damage and its repair in human cells. Int J Radiat Biol 62:313–320
Gurcu B, Yildiz S, Koca YBG, Koca S (2010) Investigation of histopathological and cytogenetic effects of heavy metals pollution on Cyprinus carpio (Linneaus, 1758) in the Gölmarmara Lake, Turkey. J Anim Vet Adv 9:798–808
Hayashi M, Ueda T, Uyeno K et al (1998) Development of genotoxicity assay systems that use aquatic organisms. Mutat Res 399:125–133
Hazardous Substances Data Bank (2006) Broad scope in human and animal toxicity, safety and handling, environmental fate, and more. http://toxnet.nlm.nih.gov/cgibin/sis/htmlgen?HSDB
Heddle JA, Hite M, Kirkhart B (1983) The induction of micronuclei as a measure of genotoxicity. A report of the US Environmental Protection Agency Gene-Tox Program. Mutat Res 123:61–118
Hodson PV, Thivierge D, Levesque MC, McWhirter M, Ralph K, Gray B et al (1992) Effects of bleached kraft mill effluent on fish in the St. Maurice River, Quebec. Environ Toxicol Chem 11(11):1635–1651
Hooftman RN, De Raat WK (1982) Induction of nuclear anomalies micronuclei in the peripheral blood erythrocytes of the eastern mud minnow Umbra pygmea by ethyl methane-sulphonate. Mutat Res 104:147–152
Javed M (2005) Heavy metal contamination of freshwater fish and bed sediments in the river Ravi stretch and related tributaries. Pak J Biol Sci 8:1337–1341
Javed M, Usmani N (2012) Uptake of heavy metals by Channa Punctatus from sewage-fed aquaculture pond of Panethi, Aligarh. Global J Res Eng Chem Eng 12:27–34
Jennette KW (1981) The role of metals in carcinogenesis: biochemistry and metabolism. Environ Health Perspect 40:233–252
Johnson FM (1998) The genetic effects of environmental lead. Mutat Res 410:123–140
Kitaura H, Nakao N, Yoshida N, Yamada T (2003) Induced sensitization to nickel in guinea pigs immunized with mycobacteria by injection of purified protein derivative with nickel. New Microbiol 26:101–108
Li J, Wang Z, Ma M, Peng X (2010) Analysis of environmental endocrine disrupting activities using recombinant yeast assay in wastewater treatment plant effluents. Bull Environ Contam Toxicol 85:529–535
Liney KE, Hagger JA, Tyler CR, Depledge MH, Galloway TS, Jobling S (2006) Health effects in fish of long-term exposure to effluents from wastewater treatment works. Environ Health Perspect 114:81
Maccubbin AE (1994) DNA adducts analysis in fish: laboratory and field studies. In: Malins DC, Ostrander GK (eds) Aquatic toxicological, molecular, biochemical and cellular perspectives. Lewis, Boca Raton, pp 267–294
Mayer FL, Versteeg DJ, Mckee MJ, Folmar LC, Graney RL, Mccume DC et al (1992) Physiological and nonspecific biomarkers. In: Hugget RJ, Kimerle RA, Mehrle PM Jr, Bergman HL (eds) Biomarker, biochemical, physiological and histological markers of anthropogenic stress. Lewis, Ann Arbor, pp 5–85
Mayon N, Bertrand A, Leroy D, Malbrouck C, Mandiki SNM, Silvestre F et al (2006) Multiscale approach of fish responses to different types of environmental contaminations: a case study. Sci Total Environ 367:715–773
Minissi S, Ciccotti E, Rizzoni M (1996) Micronucleus test in erythrocytes of Barbus plebejus (Teleostei, Pisces) from two natural environments: a bioassay for the in situ detection of mutagens in freshwater. Mutat Res 367:245–251
Monserrat JM, Martinez PE, Geracitano LA, Lund Amado L, Martinez Gaspar Martins C, Lopes Leaes Pinho G et al (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol C 146:221–234
Nesto N, Romano S, Moschino V, Mauri M, Da Ros L (2007) Bioaccumulation and biomarker responses of trace metals and micro-organic pollutants in mussels and fish from the Lagoon of Venice, Italy. Mar Pollut Bull 55:469–484
Omar WA, Zaghloul KH, Abdel-Khalek AA, Abo-Hegab S (2012) Genotoxic effects of metal pollution in two fish species, Oreochromis niloticus and Mugil cephalus, from highly degraded aquatic habitats. Mutat Res 746:7–14
Polard T, Jean S, Gauthier L, Laplanche C, Merlina G, Sanchez-Perez JM et al (2011) Mutagenic impact on fish of runoff events in agricultural areas in south-west France. Aquat Toxicol 101:126–134
Rajaguru P, Suba S, Palanivel M, Kalaiselvi K (2003) Genotoxicity of a polluted river system measured using the alkaline comet assay on fish and earthworm tissues. Environ Mol Mutagen 41:85–91
Rodriguez-Cea A, Ayllon F, Garcia-Vazquez E (2003) Micronucleus test in freshwater fish species: an evaluation of its sensitivity for application in field surveys. Ecotoxicol Environ Saf 56:442–448
Sabhapandit P, Saikia P, Mishra AK (2010) Statistical analysis of heavy metals from water samples of Tezpur sub-division in Sonitpur District, Assam, India. Appl Biol Pharmacol Technol 3:946–951
Sanchez-Galan S, Linde AR, Ayllon F, Garcia-Vazquez E (2001) Induction of micronuclei in eel (Anguilla anguilla L.) by heavy metals. Ecotoxicol Environ Saf 49:139–143
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Sen I, Shandi A, Shrivastava V (2011) Study for determination of heavy metals in fish species of the river Yamuna (Delhi) by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Adv Appl Sci Res 2:161–166
Shugart LR (2000) DNA damage as a biomarker of exposure. Ecotoxicology 9:329–340
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Szefer P, Szefer K, Skwarzec B (1990) Distribution of trace metals in some representative fauna of the Southern Baltic. Mar Pollut Bull 21:60–62
Taweel A, Shuhaimi-Othman M, Ahmad AK (2011) Heavy metals concentration in different organs of tilapia fish (oreochromis niloticus) from selected areas of Bangi, Selangor, Malaysia. Afr J Biotechnol 10:11562–11566
Tucker JD, Preston RJ (1996) Chromosome aberrations, micronuclei, aneuploidy, sister chromatid exchanges and cancer risk assessment. Mutat Res 365:147–159
Udroiu I (2006) The micronucleus test in piscine erythrocytes. Aquat Toxicol 79:201–204
Vinodhini R, Narayan M (2008) Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). J Environ Sci Technol 5:179–182
Wahlberg JE, Skog E (1965) Percutaneous absorption of trivalent and hexavalent chromium: a comparative investigation. Arch Dermatol 92:315–318
Walton DG, Acton AB, Stich HF (1984) DNA repair synthesis following exposure to chemical mutagens in primary liver, stomach, and intestinal cells isolated from rainbow trout. Cancer Res 44:1120–1121
Wang WX, Rainbow PS (2006) Subcellular partitioning and the prediction of cadmium toxicity to aquatic organisms. Environ Chem 3:395–399
Wood RD, Mitchell M, Sgouros J, Lindahl T (2001) Human DNA repair genes. Science 291(5507):1284–1289
World Health Organization (1985) Guidelines for drinking water quality. WSO, Geneva
Yohannes Y, Ikenaka Y, Saengtienchai A, Watanabe K, Nakayama SM, Ishizuka M (2013) Occurrence, distribution, and ecological risk assessment of DDTs and heavy metals in surface sediments from Lake Awassa-Ethiopian Rift Valley Lake. Environ Sci Pollut Res 20:1–9
Zar JH (1984) Biostatistical analysis. Prentice-Hall, Englewood Cliffs, pp 253–260
Zhao S, Feng C, Quan W, Chen X, Niu J, Shen Z (2012) Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Mar Pollut Bull 64:1163–1171
Acknowledgments
The authors gratefully acknowledges the chairman, Department of Zoology, AMU, Aligarh, for providing facilities for undertaking the studies. The authors are also grateful to the Maulana Azad National Fellowship (GRANT No. F.40-3(M/S)/2009(SA-III/MANF), University Grant Commission, New Delhi, for financial assistance (awarded Senior Research Fellowship).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatima, M., Usmani, N., Mobarak Hossain, M. et al. Assessment of Genotoxic Induction and Deterioration of Fish Quality in Commercial Species Due to Heavy-Metal Exposure in an Urban Reservoir. Arch Environ Contam Toxicol 67, 203–213 (2014). https://doi.org/10.1007/s00244-014-0024-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0024-8