Abstract
Copper is an essential metal, but its toxic effects are pronounced when organisms are exposed to it in excessive amounts. However, information about the effects of chronic copper exposure on the cuticle ultrastructure of organisms is insufficient. Studies of the model organism, Caenorhabditis elegans, could further our understanding of the effect of chronic excessive copper exposure on human health. In this study, the cuticle surface ultrastructure of C. elegans was observed using scanning electron microscopy after excessive copper exposure. In addition to this, some biological functions, such as chemotaxis, reproduction, and development, were also analyzed. After chronic excessive copper exposure, the worms’ body surface from vulva to tail was extensively wrinkled and folded along with the annulus. The worm’s vulva size was significantly decreased, and the middle ridge of the alae was disrupted. Furthermore, some of the biological functions of nematodes were also affected: the chemotaxis index was partially changed, bags-of-worms were induced, development was delayed, and egg-laying number was decreased by copper treatment. The results of the present study shed new light on the effects of copper on C. elegans cuticle as well as some biological functions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Copper occurs naturally in the environment; however, its contamination rates are accelerating with various anthropogenic activities. Copper was widely used in coinage and weapons in the Chinese feudal era, and approximately 0.65 million tons of copper were released into the atmosphere (Yan et al. 2010). Recently, a number of copper-containing compounds in China, including copper sulfate, copper sulfate plus citric acid, and chelated copper compounds (e.g., Cutrine-Plus Algaecide/Herbicide; Guangzhou yiooo Biological Technology Co., Ltd., China), have become oversupplied as additives in fertilizers, pesticides, and manure with the rapid development of agriculture; thus, the risk of soil copper pollution has become more serious (Xiong et al. 2010). As a result, copper can accumulate in biological tissues and increase human exposure through bioaccumulation in food chains (Subathra and Karuppasamy 2008). Information regarding the negative impacts of excessive copper to organisms, e.g., lipid metabolism, decreased growth, decreased reproduction, and acute and chronic toxicity, is currently being addressed by ongoing research (Chen et al. 2013; Rogevich et al. 2009; Hashemi et al. 2008). Even low levels of chronic copper intake can produce oxidative harmful effects and DNA damage in humans (Jomova and Valko 2011; De Olivera et al. 2012). Information regarding the impact of excessive copper sulfate exposure on the cuticle surface ultrastructure is still insufficient.
Caenorhabditis elegans, one of the important model organisms, has a number of features (free-living, soil-dwelling, etc.) that make it quite powerful as a model for toxicological research. It has also been used as an indicator of diverse pollutants (Donkin and Dusenbery 1993; Peredney and Williams 2000; Graves et al. 2005; Chu et al. 2005; Ma et al. 2009). The indicator end points used generally were lethality, development, intestinal development, egg-laying, reproduction, behavior, and fluorescence levels. Some toxicological biology processes have also been documented (Anderson et al. 2001; Leung et al. 2008; Zhao et al. 2013). For almost all multicellular organisms, a surface barrier is essential for maintaining the organism’s internal environment. It generally has been considered to be impervious to most substances. For C. elegans, the annulus is indented in the cuticle surface. The alae are composed of three closely spaced tread-like projections and run along the lateral sides of the worm (Cox et al. 1981). The cuticle becomes wrinkled with age, and antioxidant enzymes play important roles in that process (Masaki 2010). In addition, it has been noted that many metals affect behaviors of C. elegans, such as feeding, movement, learning, chemotaxis, and frequency of swing (Anderson et al. 2001; Boyd and Willams 2003; Boyd et al. 2003; Wang and Wang 2008; Wang et al. 2009; Zhang et al. 2010). Excessive amounts of copper are known to cause nerve damage in C. elegans, which slows the speed of the organism (Williams and Dusenbery 1990b; Graves et al. 2005; Gaggelli et al. 2006). Cerium oxide nanoparticles can cause significant growth inhibition (Arnold et al. 2013). In response to harsh environmental conditions, including high population density, limited food supply, and increased temperature, C. elegans larvae undergo dauer development to increase their resistance to multiple stress factors, and the eggs are retained and hatched inside the body of the parent (Golden and Riddle 1984; Trent et al. 1983).
In C. elegans, it has been reported that copper could not only cause lethality, decreased brood size, decreased life span, decreased feeding, and abnormal behavior as with other metals (Anderson et al. 2004; Harada et al. 2007; Song et al. 2008; Yang et al. 2012), it can also induce germline apoptosis; all of these defect are transferable between generations (Wang and Wang 2007; Wang et al. 2009). Currently, there are few studies about the ultrastructure of the cuticle surface and chemotaxis defects after copper exposure. This study investigated the following after copper exposure: (1) ultrastructure of the cuticle, (2) relationship between bags-of-worms (BW) and F1 larvae survival, (3) chemotaxis behavior, (4) internal physiological development, and (5) reproduction. The results of this study may shed light on the effects of excessive copper exposure on human health and aid in the development of a copper-pollution monitor.
Materials and Methods
Preparation of Nematode Cultures
Experiments were performed with worms originally obtained from the Caenorhabditis Genetics Center. They were maintained on agar nematode growth medium (NGM 1.7 % agar, 2.5 g/L peptone, 25 mmol/L NaCl, 25 mmol/L KPO4 buffer [pH 6.0], 5 mg/L cholesterol, 1 mmol/L CaCl2, and 1 mmol/L MgSO4) plates seeded with Escherichia coli OP50 at 20 °C (Brenner 1974). Age-synchronous populations of worms (stage L4 larvae) were collected as previously described (Donkin and Williams 1995). Only wild-type N2 and unc-2(ra612) were used in this study.
Copper Exposure
Copper sulfate salt and an analytical reagent (Sigma-Aldrich [St. Louis, MO]; Shanghai Zhizhen Chemical Co., Ltd.) with ≥99 % purity was used to make the 1 mol/L stock solutions in sterilized distilled water. Cuticle surface, BW photographs and chemotaxis behavior were observed with chronic copper exposure (Khanna et al. 1996), which was performed in NGM with 2.5 mmol/L copper sulfate for 20 generations to test transferable bioaccumulation effect. The BW frequencies, large vacuole, shriveled germlines, and spermatheca were observed with acute copper exposures, which were performed in K-medium (50 mmol/L NaCl and 30 mmol/L KCl) with live OP50 (Williams and Dusenbery 1990a) and 800 μmol/L CuSO4 for 24 h. Body length and development experiments were performed in K-medium with 0, 100, 200, 250, 300, and 350 μmol/L CuSO4 for 4 days. Egg-laying experiment was performed with 0, 100, and 200 μmol/L CuSO4 for 6 days. The worms were observed and photographed using an Olympus Bx51 microscope.
Examined Cuticle Surface Ultrastructures Using Scanning Electron Microscopy
Epidermal ultrastructures were mainly observed with a scanning electron microscope (SEM) as previously described (Cox et al. 1981). Twelve to 16 h after stage L4 hermaphrodites developed, they were transferred to unseeded plates and washed with M9 buffer 5–6 times (2,000 r/m, 1 min) to remove bacteria. The worms were fixed overnight with 3 % glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) and stained in 1 % osmium tetroxide in 0.1 M sodium phosphate buffer (pH 7.2). Then the worms were dehydrated in a series of ethanol solutions (35, 55, 75, 85, 95, and 100 %) and tert butyl alcohol. The worms were critical point-dried with CO2 and coated with gold using a Sputter JEC-1100. The worms were observed and photographed on a JSM-35CF SEM. Vulva size was measured by Image J software.
Chemotaxis Assay
Chemotaxis assay was performed as previously described (Emily et al. 1997). The tested media were 10 mmol/L CuSO4, 0.05 mmol/L methomyl, 10 mmol/L quinine, 100 mmol/L NH4Cl, and 10 mmol/L histidine. The worms were collected with K-medium and then placed into chemotaxis medium composed of control medium and test medium. The worms were numbered separately on control medium and test medium after 2 h. The worms between the 1 cm middle lines were not counted. The chemotaxis index was Iche = (Nt − Nc)/(Nt + Nc).
Development and Egg-Laying Assay
Eggs were put on K-medium with different copper concentrations (0, 100, 200, 250, 300, 350, and 400 μmol/L). Body length was measured every day for a total of 4 days. The worms were simply placed on the pad and photographed using the microscope. The length of the worms was measured using Image J software. Eggs were numbered after being transferred from the parent to fresh agar plates (0, 100, 200 μmol/L, respectively) every 12 h for a total of 96 h after reaching the L4 stage.
Statistical Analysis
All data were expressed as mean ± SE. Graphs were generated using Sigmaplot 10.0 software (Systat Software, Inc., USA). Analysis of variance (ANOVA [one-way]) followed by Dennett’s t test that was used to determine the significance of the differences between the groups in microsoft excel (Excel version in Microsoft Office 2010 for Windows). A probability level of 0.05 was considered statistically significant. Three to four replicates for each treatment were performed for all experiments.
Results
Cuticle Ultrastructure After Chronic Copper Exposure
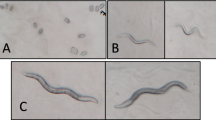
In the chronic copper treatment group, the front of the body was smooth, but the rest of the body was severely wrinkled from vulva to tail (Fig. 1e) compared with the control group (Fig. 1a). In addition, the skin was wrinkled and folded along with the annulus, which was more extensively indented (Fig. 1f) than that in the control group (Fig. 1b). The middle ridge of the alae was disrupted (Fig. 1g) although it was continuous in the control group (Fig. 1c). The vulva was dried and wrinkled to the extent that eggs could not be laid normally, and the overall size of the vulva was smaller (Fig. 1h) than that in the control group (Fig. 1d). The vulva size was decreased by approximately 47 % (Fig. 2b) compared with that in the control group.
Surface of worms exposed to excessive copper as determined by SEM. a–d Wild-type N2 worms. e–h Copper-treated worms. a The body is smooth and glossy. e The body is wrinkled from vulvae to tail. b The cuticle is flat and sleek. f The cuticle is wrinkled and folded along with the annulus. c The alae is flat, sleek, and continuous. g The alae has a projecting ridge, and the middle ridge is disrupted. d The vulva is smooth and glossy. h The vulva is small, dried, and folded. White bar = 250 μm; black bar = 6 μm
Internal egg-hatching was induced by copper exposure. a Eggs retained and hatched inside the parent’s body, a phenomenon termed “bags-of-worms,” in the chronic copper treatment group is marked by black arrowheads (bottom panel). Embryos inside a parent body in the control group (marked by white arrowheads) are normal. b Vulva size is decreased significantly as measured using Image J software (n = 12 for the control group, and n = 15 for the treatment group). c BW frequencies greatly were increased dependent on copper concentration in unc-2 (ra612) compared with N2, and the survival of larval F1 was relatively increased in unc-2 (ra612) compared with N2. BW frequencies were tested after CuSO4 exposure for 24 h. The survival of F1 larvae were tested after CuSO4 exposure for 48 h. The bar represents 20 μm. *P <0.05, **P <0.01, Dunnett’s t test. Error bars represent SE
BW Were Induced by Chronic and Acute Copper Treatment
BW were also observed during the 4-day reproductive period after chronic copper exposure for 20 generations. Internal egg-hatching in worms (i.e., BW) was induced by chronic exposure to copper, whereas the control eggs hatched outside of the parent’s body (Fig. 2a). BW were also induced by acute copper exposure (Fig. 3h), whereas this phenomenon was absent in the control group (Fig. 3d). To understand the relationship between BW and the survival ratio of F1 larvae, we tested differences in BW frequency and survival ratio of F1 larvae under exposure to copper between N2 and unc-2(ra612) treatments. BW frequencies were significantly increased dependent on copper concentration in both N2 and unc-2(ra612) treatments (Fig. 2c). However, BW frequencies were greater in unc-2(ra612) compared with N2 at copper concentrations of 50, 100, 200, and 400 μmol/L, respectively. At the same time, the umber of surviving F1 larvae per parent worm were also greater in unc-2(ra612) compared with N2 at copper concentrations of 100, 200, and 400 μmol/L, respectively (Fig. 2c). At the 800 μmol/L copper treatment, there was greater BW frequency and decreased numbers of surviving F1 larvae in unc-2(ra612) compared with N2.
Severe osmoregulation disruption and shriveled germlines were induced by acute excessive copper exposure. a–d Control worms. e–h Treated worms (800 μmol/L CuSO4 in K-medium [24 h]). a Pinocytotic vacuoles in the head (white arrowheads). e Large vacuoles (black arrowheads). b Spermatheca filled with densely packed round spermatids. f Shriveled spermatheca. c Control germlines were normal. g Treated germlines were shriveled. d Normal egg stage in controls. h BW. Bar = 10 μm
Chemotaxis Behavior Was Partially Changed by Chronic Copper Treatment
The avoidance index of the chronic copper treatment group was the same as that of the control group for the quinine, methomyl, and copper sulfate treatments. The attraction to histidine and ammonium chloride of the chronic copper treatment group was opposite that of the control group (Table 1).
Other Phenotypes After Excessive Copper Exposure
In addition, after acute copper exposure (800 μmol/L) for 24 h, a fraction of the worms displayed osmoregulatory alterations, such as large vacuoles in the cavity (Fig. 3e), which could be related to kidney and bladder defects observed in humans (Huang and Lin 2004), whereas in control group there were some pinocytotic vacuoles (Fig. 3a). The morphology of the spermatheca and germlines was shriveled more extensively (Fig. 3f, g) compared with those in the control group.
Development Was Delayed and Egg-Laying Was Decreased
The results showed that the development of C. elegans was delayed depending on copper exposure concentration (Fig. 4a, b). The percentage of worms that developed into adults also decreased (Fig. 4c). When the CuSO4 concentration was increased to 300 μmol/L, the worms’ growth was stopped, and almost all worms were arrested at the L1 stage (Fig. 4a). Growth recovery occurred after the worms had been transferred to NGM from the medium with copper in 4 days. All of the worms were dead when the CuSO4 concentration was increased to 400 μmol/L.
Dose and time-dependent development delays and lethality were caused by supplemental CuSO4. a Developmental stages of worms on different at different CuSO4 concentrations. b Worm body length was measured at different CuSO4 concentrations. All worms were dead at 400 μmol/L CuSO4. c Most animals that did not survive to adulthood died at stage L1 accompanying the time and dose treatment. Worms that progressed to the adult stage within 4 days were defined as surviving to adulthood. Bar = 50 μm
Total egg number was significantly lower for the worms exposed to 100 or 200 μmol/L CuSO4 than for the control worms in K-medium (Table 2). The spawning stage was delayed from 48 to 96 h after the L4 stage in the 100 μmol/L CuSO4 treatment group. Although the 200 μmol/L CuSO4 treatment did not delay spawning, it was significantly lower than that in the 100 μmol/L treatment group and the control group.
Discussion
The C. elegans cuticle as a model skin (Chisholm and Xu 2012), its wrinkles in the worms exposed to copper are similar to those seen as a result of ageing (Masaki 2010). It can also be supported that inflammatory cytokine secretion and necrosis were induced by CuO nanoparticles in human skin organ cultures (Cohen et al. 2013). It is possible that this effect on cuticle might be solely due to direct interaction with copper (or some combination of direct interaction with absorption). Furthermore, studies using synchrotron X-ray fluorescences reported that copper enters C. elegans mainly through the gut and not the cuticle (Jackson et al. 2005). Thus, we wanted to know the internal effect that occurred after copper exposure. The following phenotypes were also observed in the following studies: BW, chemotaxis behavior, development, egg-laying, and osmoregulation.
The determination of BW (Fig. 2) allowed for advanced insight in the degree of wrinkled cuticle (Fig. 1) and decreased vulva (Fig. 2b) size caused by copper exposure. To determine that the development of BW was just a defect in egg-laying or may have been a protective mechanism of the parent’s body for larvae against a harsh environment with excessive copper, unc-2 was used for the test. unc-2(ra612), which indicates a failure to adapt either to paralysis by dopamine or to stimulation of egg-laying by serotonin, causes hyperactive egg-laying (Schafer and Kenyon 1995). The results showed that copper exposure could cause BW dependent on copper concentrations in both N2 and unc-2(ra612) treatments. Although progeny hatching inside of the mother might be a defect in egg-laying and not a parental protective mechanism, the greater occurrence of BW still showed greater survival in F1 larvae in unc-2(ra612) compared with N2. It could also indicate that the parent’s body might protect the larvae against a harsh environment with excessive copper. This supported the hypothesis that BW an adaptive response of C. elegans to excessive copper in the environment because the parent’s body can provide physical protection and enough food in a toxic environment for the larvae to reach the resistant dauer stage (Mosser et al. 2011). Internal hatching also decreased the reproductive output of the worms.
The worms adaptively responded to a variety of chemical stimuli in the environment by exhibiting different behaviors (i.e., avoidance or attraction). Changes in the behavior of C. elegans is believed to be a more effective indicator of neurotoxicity than morphology at low concentrations of toxins (Williams and Dusenbery 1990b). Recently, copper has also been shown to be a neurotoxin (Hedges 2010), and it was observed to cause DNA damage in copper-melting workers (De Olivera et al. 2012). The results showed damage in the attraction to histidine and ammonium chloride, which is consistent with that fact there are severe defects of chemotaxis to water-soluble attractants (NaCl, cAMP, and biotin) after copper exposure (Xing et al. 2009). The reversed attraction seen in worms exposed to copper might have been caused by neuronal damage. Wang and Wang reported that copper, zinc, lead, and barium, etc., can affect C. elegans body bending or learning behavior or cause severe deficits in neuronal survival and synaptic functions (Zhang et al. 2010; Graves et al. 2005; Wang and Wang 2008). However, the avoidance index to copper was not changed by chronic copper exposure. Thus, there might be stabilization synaptic differentiation of avoidance to copper that perhaps existed in C. elegans because half of the nematodes were not able to cross the copper barrier to move to diacetyl (Li et al. 2011). We have also identified a gene that is involved in copper sensing (data not shown).
The decreased egg-laying number was concordant with that in our previous study (Song et al. 2008). Many data exist regarding the ability of copper to decrease the reproduction of C. elegans (Anderson et al. 2001; Boyd and Willams 2003; Harada et al. 2007). Anderson et al. (2001) reported that the EC50 value for reproduction defect caused by copper was 39 μmol/L. The values not being identical may be due to the difference caused by the fact that we used CuSO4 and they used CuCL2. After exposure to 100 μmol/L CuSO4, the number of eggs was similar to that reported by Harada et al. (2007). These results were also supported by a study reporting that copper exposure induces germline apoptosis (Wang et al. 2009), egg-laying defects as a result of vulva protrusion (Fig. 3h), and atrophy of germlines and spermatheca (Fig. 3f, g) in C. elegans. Because retained larvae could damage the parent’s gonads, offspring production was significantly decreased; however, the survival rate of the internally hatched larvae was as high or even greater than that of externally hatched larvae (Fig. 2c). Thus, the eggs hatched in the parent for the long-term survival of the dauer larvae.
The development delay and recovery were the same as those presented in a previous report (Harada et al. 2007; Yu et al. 2012). At the same time, it was observed that C. elegans growth is inhibited by cerium oxide nanoparticle (Arnold et al. 2013). Thus, dauer formation might also be related to copper sulfate exposure. Copper can also inhibit the snail’s growth and reproduction (Rogevich et al. 2009).
In general, wrinkles are associated with ageing, and the ageing process is believed to be influenced by the formation of reactive oxygen species (ROS) (Baumann 2007). The influence of copper on extrinsic cuticle ageing might also be partially caused by its role in potentially altering the normal course of intrinsic (also known as “natural” or “cellular”) ageing through gut uptake. Our previous studies tested this hypothesis. In C. elegans, we previously showed that after chronic copper exposure, malondialdehyde content is 2.2 times greater (Song et al. 2008). Thus, oxidative damage was caused by copper treatment and might be relevant in copper-induced cuticle ageing, including the formation of wrinkled cuticle, abnormal alae, and smaller vulva. Wrinkles were also pronounced in human skin cells after ROS stress induced by ultraviolet radiation (Masaki 2010). The results of this research suggests that wrinkles in the cuticle of C. elegans might be associated with copper-induced ageing. In another study, the double mutant daf-16; unc-75 C. elegans strain, which is defective in genes controlling dauer formation, longevity, and response to ROS, was identified to have a superior sensitivity to heavy metals (Chu et al. 2005). The high oxidative damage caused by chronic copper exposure might induce defects in cuticle wrinkling, chemotaxis, BW development, dauer arrest, development delay, and egg-laying.
Conclusion
This research offers the first detailed description of the surface ultrastructure of C. elegans exposed to excessive copper. Copper exposure can cause multiple biological deficits. It could be concluded that the effect of copper on cuticle might be due to a combination of direct interaction with the copper and the internal effect of copper absorption. The cuticle wrinkling, BW occurence, dauer arrest, and chemotaxis behavior could be regarded as reliable individual population-level reporters of copper toxicity. Because there was no direct evidence correlating the defects caused by copper exposure with ROS stress, it cannot definitively be concluded that the structural defects of the cuticle, vulva, and other parts of the worm were caused by cell damage and ROS when the worms were exposed to copper. Future works will be performed with C. elegans antioxidant enzyme deletion mutants.
References
Anderson GL, Boyd WA, Williams PL (2001) Assessment of sublethal endpoints for toxicity testing with the nematode Caenorhabditis elegans. Environ Toxicol Chem 20(4):833–838
Anderson GL, Cole RD, Williams PL (2004) Assessing behavioral toxicity with Caenorhabditis elegans. Environ Toxicol Chem 23:1235–1240
Arnold MC, Badireddy AR, Wiesner MR, Di Giulio RT, Meyer JN (2013) Cerium oxide nanoparticles are more toxic than equimolar bulk cerium oxide in Caenorhabditis elegans. Arch Environ Contam Toxicol 65(2):224–233
Baumann L (2007) Skin ageing and its treatment. J Pathol 211(2):241–251
Boyd WA, Willams PL (2003) Comparison of the sensitivity of three nematode species to copper and their utility in aquatic and soil toxicity tests. Environ Toxicol Chem 22(11):2768–2774
Boyd WA, Cole RD, Anderson GL, Williams PL (2003) The effects of metals and food availability on the behavior of Caenorhabditis elegans. Environ Toxicol Chem 22(12):3049–3055
Brenner S (1974) The genetics of Caenorhabidits elegans. Genetics 77:71–94
Chen QL, Luo Z, Liu X, Song YF, Liu CX, Zheng JL et al (2013) Effects of waterborne chronic copper exposure on hepatic lipid metabolism and metal-element composition in Synechogobius hasta. Arch Environ Contam Toxicol 64(2):301–315
Chisholm AD, Xu S (2012) The Caenorhabditis elegans epidermis as a model skin. II: differentiation and physiological roles. Wiley interdisciplinary reviews. Dev Biol 1(6):879–902
Chu KW, Chan SK, Chow KL (2005) Improvement of heavy metal stress and toxicity assays by coupling a transgenic reporter in a mutant nematode strain. Aquat Toxicol 74(4):320–332
Cox GN, Kusch M, Edgar RS (1981) Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J Cell Biol 90(1):7–17
De Olivera JVD, Boufleur LA, Santos CEID, Dias JF, Squeff CH, Silva GRG et al (2012) Occupational genotoxicity among copper smelters. Toxicol Ind Health 28(9):789–795
Donkin SG, Dusenbery DB (1993) A soil toxicity test using the nematode Caenorhabditis elegans and an effective method of recovery. Arch Environ Contam Toxicol 25(2):145–151
Donkin SG, Williams PL (1995) Influence of developmental stage, salts and food presence on various end points using Caenorhabditis elegans for aquatic toxicity testing. Environ Toxicol Chem 14(12):2139–2147
Emily RT, Bruce EK, Bargmann CI (1997) Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91:161–169
Gaggelli E, Kozlowski H, Valensin D, Valensin G (2006) Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev 106(6):1995–2044
Golden JW, Riddle DL (1984) The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol 102(2):368–378
Graves A, Boyd W, Williams P (2005) Using transgenic Caenorhabditis elegans in soil toxicity testing. Arch Environ Contam Toxicol 48(4):490–494
Harada H, Kurauchi M, Hayashi R, Eki T (2007) Shortened lifespan of nematode Caenorhabditis elegans after prolonged exposure to heavy metals and detergents. Ecotoxicol Environ Safe 66:378–383
Hashemi S, Blust R, De Boeck G (2008) Combined effects of different food rations and sublethal copper exposure on growth and energy metabolism in common carp. Arch Environ Contam Toxicol 54(2):318–324
Hedges KA (2010) Copper exposure damages neurons and induces paralysis in the nematode, Caenorhabditis elegans, far below lethal concentrations. Master’s thesis, Humboldt State University, Arcata, CA
Huang WH, Lin JL (2004) Acute renal failure following ingestion of manganses containing fertilizer. J Toxicol Clin Toxicol 42:305–307
Jackson BP, Williams PL, Lanzirotti A, Bertsch PM (2005) Evidence for biogenic pyromorphite formation by the nematode Caenorhabditis elegans. Environ Sci Technol 39(15):5620–5625
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87
Khanna N, Cressman CP III, Tatara CP, Williams PL (1996) Tolerance of the nematode Caenorhabditis elegans to pH, salinity, and hardness in aquatic media. Arch Environ Contam Toxicol 32(1):110–114
Leung MCK, Williams PL, Benedetto A, Au C, Helmke KJ, Aschner M et al (2008) Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci 106:5–28
Li YX, Wang Y, Hu YO, Zhong JX, Wang DY (2011) Modulation of the assay system for the sensory integration of 2 sensory stimuli that inhibit each other in nematode Caenorhabditis elegans. Neurosci Bull 27(2):69–82
Ma H, Glenn TC, Jagoe CH, Jones KL, Williams PL (2009) A transgenic strain of the nematode Caenorhabditis elegans as a biomonitor for heavy metal contamination. Environ Toxicol Chem 28(6):1311–1318
Masaki H (2010) Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci 58(2):85–90
Mosser T, Matic I, Leroy M (2011) Bacterium-induced internal egg hatching frequency is predictive of life span in Caenorhabditis elegans populations. Appl Environ Microbiol 77(22):8189–8192
Peredney CL, Williams PL (2000) Utility of Caenorhabditis elegans for assessing heavy metal contamination in artificial soil. Arch Environ Contam Toxicol 39(1):113–118
Rogevich E, Hoang T, Rand G (2009) Effects of sublethal chronic copper exposure on the growth and reproductive success of the Florida apple snail (Pomacea paludosa). Arch Environ Contam Toxicol 56(3):450–458
Schafer WR, Kenyon CJ (1995) A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature 375:73–78
Song S, Guo Y, Yin K, Wu H, Zhang X, Yang M et al (2008) Copper on long-term role of nematode physiological characteristics. Sichuan J Zool 27(5):832–834
Subathra S, Karuppasamy R (2008) Bioaccumulation and depuration pattern of copper in different tissues of mystus vittatus related to various size groups. Arch Environ Contam Toxicol 54(2):236–244
Trent C, Tsung N, Horvitz HR (1983) Egg-laying defective mutant of the nematode Caenorhabditis elegans. Genetics 104(4):619–647
Wang Y, Wang DY (2007) Copper exposure causes transferable defects of phenotypes and behaviors in C. elegans. J Saf Environ 7(2):10–14
Wang DY, Wang Y (2008) Phenotypic and behavioral defects caused by barium exposure in nematode Caenorhabditis elegans. Arch Environ Contam Toxicol 54(3):447–453
Wang S, Wu L, Wang Y, Luo X, Lu Y (2009) Copper-induced germline apoptosis in Caenorhabditis elegans: the independent roles of DNA damage response signaling and the dependent roles of MAPK cascades. Chem Biol Interact 180(2):151–157
Williams PL, Dusenbery DB (1990a) Aquatic toxicity testing using the nematode, Caenorhabditis elegans. Environ Toxicol Chem 9(10):129–1285
Williams PL, Dusenbery DB (1990b) A promising indicator of neurobehavioural toxicology using the nematode Caenorhabditis elegans and computer tracking. Toxicol Ind Health 6:425–440
Xing XJ, Du M, Zhang YF, Wang DY (2009) Adverse effects of metal exposure on chemotaxis towards water-soluble attractants regulated mainly by ASE sensory neuron in nematode Caenorhabditis elegans. J Environ Sci 21:1684–1694
Xiong X, Yanxia L, Wei L, Chunye L, Wei H, Ming Y (2010) Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Resour Conserv Recycl 54(11):985–990
Yan H, Sun L, Wang Y, Liu X, Qiu S, Cheng W (2010) A 2000-year record of copper pollution in South China Sea derived from seabird excrements: a potential indicator for copper production and civilization of China. J Paleolimnol 44(2):431–442
Yu ZY, Zhang J, Yin DQ (2012) Toxic and recovery effects of copper on Caenorhabditis elegans by various food-borne and water-borne pathways. Chemosphere 87(11):1361–1367
Zhang Y, Ye B, Wang D (2010) Effects of metal exposure on associative learning behavior in nematode Caenorhabditis elegans. Arch Environ Contam Toxicol 59(1):129–136
Zhao YL, Wu QL, Li YP, Wang DY (2013) Translocation, transfer, and in vivo safety evaluation of engineered nanomaterials in the non-mammalian alternative toxicity assay model of nematode Caenorhabditis elegans. RSC Adv 3:5741–5757
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31071980) and the Nature Science Foundation of Shanxi Province (Grant No. 2011011033-1). The authors give special thanks to Chonglin Yang for help with this study. The nematode strain used in this work was provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, S., Guo, Y., Zhang, X. et al. Changes to Cuticle Surface Ultrastructure and Some Biological Functions in the Nematode Caenorhabditis Elegans Exposed to Excessive Copper. Arch Environ Contam Toxicol 66, 390–399 (2014). https://doi.org/10.1007/s00244-013-9991-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-013-9991-4