Abstract
Pesticide residues in breeding ponds can cause avoidance by at least some amphibian species. So far, outdoor experiments have been performed only with artificial pools in areas where the focus species usually occur and new colonization has been observed. Results of this kind of study are potentially influenced by natural disturbances and therefore are of limited comparability. We used an easily manufactured and standardizable arena approach, in which animals in reproductive condition for some hours had a choice among pools with different concentrations of a contaminant. Because there has been much debate on the potential environmental impacts of glyphosate-based herbicides, we investigated the impact of glyphosate isopropylamine salt (GLY-IS), Roundup LB PLUS (RU-LB-PLUS), and glyphosate’s main metabolite aminomethylphosphonic acid (AMPA) on individual residence time in water. The following European amphibian species were tested: Common frog (Rana temporaria), Palmate newt (Lissotriton helveticus), and Alpine newt (Ichthyosaura alpestris). The residence time in water was not significantly affected by concentrations below or slightly above the European Environmental Quality Standards for AMPA or the German “worst-case” expected environmental concentrations for GLY-IS and RU-LB-PLUS. Occasionally, microclimatic cofactors (nightly minimum ground temperature, water temperature) apparently influenced the residence time. The major drawback of such quick behavior studies is that results can only be transferred to perception and avoidance of contaminated water but not easily to site selection by amphibians. For example, testing oviposition site selection requires more natural water bodies and more time. Hence, to develop a standard procedure in risk assessment, an intermediate design between an arena approach, as presented here, and previously performed field studies should be tested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Amphibian populations are decreasing at alarming rates worldwide (Houlahan et al. 2000; Stuart et al. 2008). Environmental contamination is one of the supposed causes for the observed decreases (Boone et al. 2007; Stuart et al. 2008). Pesticides are especially suspected to affect freshwater systems, which are exploited by most amphibian species for reproduction and/or part of their annual activity phase (Relyea and Hoverman 2006). Contamination of aquatic but also terrestrial amphibian habitats with pesticides can occur by way of direct over-spraying (Relyea 2005; Dinehart et al. 2009), drift (Davidson et al. 2002, 2004), or runoff (Browner 1994). Acute (Relyea 2005; Relyea and Jones 2009) and chronic effects (Cauble and Wagner 2005; Howe et al. 2004) on amphibian health have been reported. However, we currently know little about indirect effects, e.g., how pesticides can alter aquatic site selection (Vonesh and Buck 2007). Chemically contaminated ponds were avoided by some anuran (i.e., frogs and toads) species (Takahashi 2007; Vonesh and Buck 2007). Thereby, one of the supposed causes for amphibian decline, e.g., environmental contamination (Boone et al. 2007), can act simultaneously with others, especially the main problem of habitat loss (Stuart et al. 2008). Conversely, adults of the species, which apparently are able to perceive pesticides, could protect themselves as well as their offspring from the effects of contaminants (Vonesh and Kraus 2009).
Up until now, data on amphibian responses to contaminated water bodies have resulted from field experiments. Artificial ponds were created within the area where the focus species occurs and reproduces, and subsequent colonization (measured by number of eggs per water body) was investigated (Takahashi 2007; Vonesh and Buck 2007; Vonesh and Kraus 2009). Such field studies are effective and useful, but they are of limited comparability because their design must fit the given landscape architecture. Furthermore, they are potentially influenced by nonassessable and unpredictable factors. Apart from this potential uncontrolled disturbance, some aspects that might affect animals’ responses remain unknown or are difficult to assess, e.g., population size, sex ratio, etc. Therefore, we tested the effects of contamination on aquatic site selection by amphibians under standardized conditions. Arena approaches can be used to standardize animal behavior experiments. For example, these are widely used in orientation studies in which a defined number of animals can choose among different sources (Landler and Gollmann 2011). We transferred this design to test residence time of amphibians in water in response to different grades of contamination with a substance, i.e., potential avoidance of contaminated water. We chose three widespread European species as test organisms. Apart from one anuran species, the Common frog (Rana temporaria), we included for the first time two newt species: the Palmate newt (Lissotriton helveticus) and the Alpine newt (Ichthyosaura alpestris). All tested animals were adults in reproductive condition so they were attracted by water. Hence, experiments took place during the particular breeding time of the test species. The two newts have a prolonged aquatic phase during several months that exceeds their breeding activity. In contrast, the Common frog is an “explosive breeder,” i.e., most adults of a population reproduce within a short time period (see Wells 1977).
We chose to relate our study to glyphosate-based herbicides (GBHs). These have been suggested to dominate the worldwide herbicide market, and their use is increasing (Duke and Powles 2008). Several studies are available on the effects of glyphosate (GLY) and GBHs on anuran embryos (Perkins et al. 2000), larvae (Relyea and Jones 2009; Fuentes et al. 2011), and juvenile or adult animals (Mann and Bidwell 1999; Relyea 2005; Dinehart et al. 2009), and they include chronic effects (Howe et al. 2004) and indirect impacts (Jones et al. 2010). Interestingly, no studies are available on the effects of GLY’s main metabolite, aminomethylphosphonic acid (AMPA; Rueppel et al. 1977).
In our arena approach with newts, we chose glyphosate isopropylamine salt (GLY-IS), which is the active ingredient of most GBHs (Chemical Abstracts Service [CAS] no. 38641-94-0), and a GBH formulation named Roundup LB PLUS (RU-LB-PLUS), which includes 16 % of an unknown surfactant. Common-frog experiments were performed using AMPA, which is not only the main metabolite of GLY but also of other phosphonate compounds (CAS no. 1066-51-9) (Skark et al. 1998).
Material and Methods
Common Frog Experiments
Effects of contamination with AMPA on residence time in water were tested for male frogs. Fifty male Common frogs were sampled on March 10 and 11, 2012, when they migrating to their breeding site, a small pond near Trier, Germany. Their reproductive condition was recognized by the presence of black nuptial pads (Schlüpmann and Günther 1996).
Frogs were immediately taken to an experimental site within the area of Trier University. Each 10 male animals were kept in buckets (approximately 1 m in diameter) outdoors until the start of the experiment (the latest one started on March 28). The bottom of the buckets contained humid soil, and pieces of turf served as hiding places. Plenty of fresh water was sprayed daily to prevent dehydration of animals.
AMPA (99.5 % purity) was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Stock solutions were prepared daily with distillated water, and four concentrations were tested: 0, 5, 50, and 500 μg/L. The highest concentration corresponded to AMPA’s European Environmental Quality Standard (EQS) for the protection of aquatic biota and against which monitoring data should be assessed. The Water Framework Directive of the European Union (Directive 2000/60/EC) provides a procedure to set EQS. The annual average EQS for AMPA is 450 μg/L. In line with the rounded guide value for surface water proposed by the Swedish authorities, we chose the highest test concentration of 500 μg/L (http://www.egeis.org). To the best of our knowledge, 400 μg/L is the highest environmental concentration that has been reported in water (Coupe et al. 2012). Based on the high concentration, we tested two lower concentrations (50 and 5 μg/L). These concentrations are more frequently found in surface waters, including amphibian ponds (Struger et al. 2008; Battaglin et al. 2009). Hence, the tested concentrations are not only legally but also environmentally relevant.
Experiments were performed in arenas with 10 replicates/night from March 12 to 28. An arena (1.5 × 1.5 m) was defined by an amphibian drift fence and consisted of a hiding place (leaves and planks) and four artificial pools [plastic pans of 34 × 34 cm2, each with a 10-L capacity and a 13-cm maximum depth (Fig. 1)]. Arenas were located in a sunny location. This design was chosen per Common frog reproduction behavior, i.e., the size of the breeding water is less important, and shallow sunny water is preferred (e.g., Schlüpmann and Günther 1996). During heavy rain, a roof protected against decrease of the tested concentrations. The artificial pools contained well water (average pH 7.7, 341 μs/cm, 10.4 mg O2/L, 8.7°dH). The four concentrations were applied randomly, one to each of the four pools in an arena. Concentrations were renewed every 24 h because AMPA has a half-life of 7–14 days in water depending on local conditions (Giesy et al. 2000). In addition, we are aware that AMPA can adsorb to plastic and that plastic can release substances over time.
To avoid the influence of conspecifics, site selection was investigated by setting just one male animal into an arena. Because the species is primarily nocturnal, animals were set into the arenas at 19:00. After an adaption period of 1 h, the position of each male animal was recorded every 30 min to 01:00 the following day (i.e., residence time of each individual within 5 h was recorded). After 01:00, the activity in these poikilothermic animals was remarkably decreased or had even stopped. After the experiments, all animals were released to their natural habitat.
Newt Experiments
Effects of water contamination with GLY-IS and RU-LB-PLUS on residence time were tested with both newt species. A total of each 120 Palmate and Alpine newts were sampled in two ponds near Trier, Germany, using water traps. Sampling was performed between March 29 and April 13, 2012, and always when animals were “required.” Because these sexually dimorphic species are common, sexes could easily be balanced. Study design and methods were the same as described for Common frogs because both newt species are known to accept small water bodies, e.g., cartwheel traces (Winkler and Heunisch 1997). However, newts were set pairwise in each arena (male, female) because it is widely unknown which sex chooses suitable water bodies first or if these animals can perceive the presence of an animal of the other sex in water. Depending on the species, half of the animals (n = 60) were tested with one of the two substances. GLY-IS was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany), and RU-LB-PLUS was purchased from a local hardware store. In Germany, the estimated worst-case concentration of GLY in surface water after application of the highest approved rate and without buffer strip is approximately 0.9 mg a.e./L (BVL 2010). Based on this worst-case expected environmental concentration, we used 1 mg a.e./L as a high concentration (again with two lower and more environmentally relevant concentrations of 0.1 and 0.01 mg a.e./L) for both the formulation and the active ingredient. The annual average EQS for GLY is approximately 100 μg/L (http://www.egeis.org), i.e., our second highest concentration.
Statistical Analyses
We used Kolmogorov–Smirnov tests to test for normal distribution of the data. To compare the residence time (min) of individuals in the four groups of contamination, we used Kruskal–Wallis tests followed by Wilcoxon rank-sum tests (because these data were not normally distributed). Furthermore, we tested with generalized linear models (GLM) if the different concentrations or considered environmental cofactors influenced the percentage residence time of animals of each trial (i.e., percentage residence time of all individuals tested in 1 night) as proposed for such behavioral studies where uninteresting behavior (here time spent on land) is excluded from analysis (Everitt and Howell 2005). Considered environmental cofactors were average water temperature (as measured with a laboratory thermometer; TFA, Wertheim, Germany), nightly minimum ground temperature, precipitation, relative air humidity (all data were obtained from the weather station Petrisberg, which is situated approximately 1 km airline to the experimental site). Animals that did not enter the water (n = four frogs) were excluded from analysis. The software R was used for statistical analyses (R Development Core Team 2009).
Results
All frogs and newts survived the experiments, except for one frog, an individual of such poor body condition that we did not link its death to our experiment.
Experiments With Common Frogs
On average, male Common frogs stayed on land 54 % of the total observed time, 14.6 % in the control, 10.2 % in the low concentration, 11.8 % in the medium concentration, and 9.4 % in the highest AMPA concentration (Fig. 2). The frogs usually started calling after staying in a pool for more than half an hour, but they also changed pools during observation. On land, they were sitting or migrating most of the time. Some individuals occasionally tried to climb out of the arena, but they usually did not hide.
Kruskal–Wallis and the Wilcoxon rank-sum tests on the residence time in the four contamination groups did not show significant differences; likewise, the GLM for percentage residence time did not show a significant influence of any predictor.
Newt Experiments
Palmate Newt
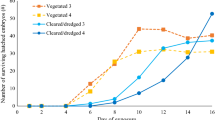
In the experiments with GLY-IS, animals stayed on land 65.2 % of the total observed time, 7 % in the control, 11.2 % in the low concentration, 7.8 % in the medium concentration, and 8.8 % time in the highest GLY-IS concentration (Fig. 3a). On land, animals were hiding most of the time; the rest of the time they were walking on the grass. No animal tried to climb out of the arena. Male animals usually followed female animals into the same pool, but occasionally they were the first to enter the water. After staying several minutes in a pool together, male animals often started courtship behavior.
In the experiments with RU-LB-PLUS, Palmate newts stayed on land for 62.1 % of the total observed time, 7.7 % in the control, 12.2 % in the low concentration, 7.3 % in the medium concentration, and 10.7 % in the highest RU-LB-PLUS concentration (Fig. 3b). Animals showed the same behavior on land and in the water as in the previous experiment. For the results of both experiments, Kruskal–Wallis and the Wilcoxon rank-sum tests on the residence time did not show significant differences, and GLM on percentage of residence time did not show significant impacts of any predictor.
Alpine Newt
Behavior of Alpine newts was similar to that of Palmate newts. During the experiments with GLY-IS, animals stayed on land for 63.9 % of the total observed time, 11.2 % in the control, 8.7 % in the low concentration, 10.2 % in the medium concentration, and 6 % in the highest GLY-IS concentration (Fig. 3c). No significant differences between residence time in the four contamination groups were found, but the GLM showed a significantly positive impact of air temperature (p < 0.001, z = 4.53) and a significantly negative impact of water temperature (p < 0.05, z = −3.11) on percentage of residence time in the pools.
In the experiments with RU-LB-PLUS, newts stayed on land 76 % of the total observed time, 8.5 % in the control, 3 % in the low concentration, 6 % in the medium concentration, and 6.5 % in the highest RU-LB-PLUS concentration (Fig. 3d). Here again, neither Kruskal–Wallis and the Wilcoxon rank-sum tests on residence time nor GLM for percentage of residence time showed significant impacts of any predictor.
Discussion
Species Responses to Contaminants
High survival rates were expected because AMPA is considered to be no more toxic than its parent GLY to fish and other standard test organisms (Carey et al. 2008), and LC50 values of diluted GLY on adult anurans are approximately 100 mg a.e./L (Mann and Bidwell 1999). Toxicity data of diluted Roundup formulations on metamorphs and adult amphibians range between 49.4 and 88.7 mg a.e./L (Mann and Bidwell 1999).
Frogs and newts entered artificial pools but remained on land for more than half of the total observed time. In all cases, water contamination with the tested environmentally relevant concentrations did not seem to lead to avoidance of contaminated pools. Conversely, all three species tested in these experiments entered water contaminated at concentrations that slightly exceeded the annual average European EQS (AMPA) or EEC (GLY [Roundup]). Whether this may cause chronic effects remains to be studied. It is also notable that the surfactant in the Roundup formulation was either not perceived by the animals or the animals were not bothered by it. In one case, microclimatic cofactors were found to have significant effects on percentage of residence time in newts. This is not surprising because the activity of most amphibians is weather-dependent (Duellman and Trueb 1986). For example, positive and negative effects of greater air and water temperature could be related to greater activity rates in the newts such that they did not hide on land but entered or changed pools more often.
It must be first said that comparability between our study and other amphibian site-selection studies is hampered because in all previous studies artificial ponds were created in known breeding areas. Furthermore, explicitly female responses (i.e., oviposition site selection) were not studied, and male site selection (as studied by us for frogs) and female oviposition site selection are not mandatorily correlated in anurans (Resetarits and Wilbur 1991).
In other anuran site-selection studies, species responded differently to contamination with GBH. North American Gray tree frogs (Hyla versicolor–chrysoscelis complex) strictly avoided ponds contaminated with Roundup (Takahashi 2007). However, only one relatively high concentration (2.4 mg a.e./L) was tested; only five female animals oviposited; and it was not observed if animals entered the contaminated ponds by night time. Both the active ingredient and the formulation of an insecticide (Sevin and its active ingredient carbaryl, also tested at worst-case scenario concentrations) decreased oviposition site selection by Gray treefrogs compared with water and acetone (solvent) controls (Vonesh and Buck 2007; Vonesh and Kraus 2009). Here, the sample size was large, i.e., >100 female animals accepted the newly created ponds, but again a relatively high concentration was used (7 mg/L). Conversely, no effects of the active ingredient and the formulation on site selection by Northern cricket frogs (Acris crepitans) were found by Vonesh and Kraus (2009).
The European newt species tested in our study apparently did not perceive the tested concentrations or did not mind them, but results from Gertzog et al. (2010) suggest that terrestrial Eastern red-backed salamanders (Plethodon cinereus) were able to detect a Roundup formulation as well as two other herbicides sprayed on soil at approved application rates.
It is possible that some amphibians can perceive contamination due to the olfactory sense, which plays a main role in the orientation and communication behavior of most amphibians (Duellman and Trueb 1986). There is also (indirect) evidence that Wood frogs (Lithobates sylvaticus) use chemical cues from predators (fish) for site selection (Hopey and Petranka 1994), which partly support the above-mentioned explanation. Another explanation could be that some species perceive contamination through their permeable skin, so they must enter the water to assess its quality. For example, it has been suggested that amphibians can “taste” and examine water with their skin before absorbing it (Smith et al. 2007).
Suitability of the Arena Approach
It may be taken into account that the studies by Takahashi (2007), by Vonesh and Buck (2007), and by Vonesh and Kraus (2009) not only used different contaminants but also used relatively high concentrations (e.g., estimated after direct applications on the water’s surface), whereas our experimental design was based on relatively low but environmentally and legally relevant concentrations. We did not have a “clear” positive control—e.g., Roundup concentrations ≥2.4 mg a.e./L, which were avoided by treefrogs in Takahashi’s (2007) study—to demonstrate. Such a positive control was not possible in our case due to wild animal welfare rights in Germany; furthermore, they may be far from realistic concentrations in most cases.
Furthermore, the absence of statistically supported effects could simply be an artifact of the experimental design. For instance, the animals could have been stressed (artifact no. 1); the arenas could have been too small such that animals did not perceive the four pools as distinct water bodies (artifact no. 2); the sample size or scoring procedures could have been inadequate (artifact no. 3); Common frogs have relatively strong home site fidelity, which could have influenced their selection (artifact no. 4); and the used pools were too unnatural (artifact no. 5). Calling activity in frogs and courtship behavior of newts argue for lack of stress, so artifact no. 1 can be disregarded. In addition, artifact no. 5 can disregarded because of our arena approach; however, in a parallel experiment, frog pairs were tested and only 2 of 50 females spawned (data not shown). This could have several reasons, among others (e.g., artifacts no. 4 and 5). Further arena approaches should address artifacts no. 2 and 3, although it may be noted that in terrestrial site–selection studies with different vertebrates, animals were usually smaller or similar arenas were used (e.g., Keen 1982; Smith et al. 2003). A larger sample size (arenas, nights, and animals) may change the results. This may be appreciated with the help of a power analysis. In our study design (four different concentrations in one replicate), a power analysis was impossible.
Because animals stayed longer on land than in the water, a longer observation time during the night would be desirable: Before 20:00 and after 1:00, no or nearly no movement could be observed because all three species are only active at night and are poikilothermic. In summary, it seems necessary to validate the arena approach with further experiments.
Perception and avoidance (shown by residence time) of contaminated water bodies should be investigated by arena approaches; however, to study other (relevant) behavior, such as oviposition site selection, more sophisticated designs are needed (at least for some species). The alternative to arena approaches would be mesocosm approaches, field tests (as have already been performed with North American species), or contamination of natal ponds. Contamination of natal ponds would be unethical. Regarding field tests, one could not rely on a sufficiently high sample size for comparability, they are relatively work- and cost intensive, and the performance is difficult in many cases. Hence, mesocosm experiments with (semi)natural ponds, more space, longer time, and more animals should be preferred. In conclusion, assessing the risk of environmental contaminants on habitat selection of amphibians under standardized conditions remains a difficult task.
References
Battaglin WA, Rice KC, Focazio MJ, Salmons S, Barry RX (2009) The occurrence of GLY, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa, and Wyoming, 2005–2006. Environ Monitor Assess 155:281–307
Boone MD, Cowman D, Davidson C, Hayes T, Hopkins W, Relyea R, Schiesari L, Semlitsch R (2007) Evaluating the role of environmental contaminants in amphibian population declines. In: Gascon C, Collins JP, Moore RD, Church DR, McKay JE, Mendelson JR III (eds) Amphibian conservation action plan. IUCN/SSC Amphibian Specialist Group, Gland and Cambridge, pp 32–35
Browner CM (1994) The administration’s proposals. EPA J 20:6–9
Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL) (2010) Glyphosate—Comments from Germany on the paper by Paganelli, A. et al. (2010) Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling. BVL, Braunschweig, Germany, pp 1–8
Carey S, Crk T, Flaherty C, Hurley P, Hetrick J, Moore K, et al. (2008) Risk of glyphosate use to federally threatened California red-legged frog (Rana aurora draytonii). United States Environmental Protection Agency (USEPA) Environmental Fate and Effects Division, Washington, DC
Cauble K, Wagner RS (2005) Sublethal effects of the herbicide glyphosate on amphibian metamorphosis and development. Bull Environ Contam Toxicol 75:429–435
Coupe RH, Kalkhoff SJ, Capel PD, Gregoire C (2012) Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag Sci 68:16–30
Davidson C (2004) Declining downwind: Amphibian population declines in California and historical pesticide use. Ecol Appl 14:1892–1902
Davidson C, Shaffer HB, Jennings MR (2002) Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conserv Biol 16:1588–1601
Dinehart SK, Smith LM, McMurry ST, Anderson TA, Smith PN, Haukos DA (2009) Toxicity of a glufosinate- and several glyphosate-based herbicides to juvenile amphibians from the Southern High Plains, USA. Sci Total Environ 407:1065–1071
Duellman WE, Trueb L (1986) Biology of amphibians. John Hopkins University Press, Baltimore, MD
Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64:319–325
Everitt B, Howell D (2005) Encyclopedia of statistics in behavioral science. Wiley, New York, NY
Fuentes L, Moore LJ, Rodgers JH Jr, Bowerman WW, Yarrow GK, Chao WY (2011) Comparative toxicity of two glyphosate formulations (original formulation of Roundup® and Roundup WeatherMAX®) to six North American larval anurans. Environ Toxicol Chem 30:2756–2761
Gertzog BJ, Kaplan LJ, Nichols D, Smith GR, Rettig JE (2010) Avoidance of three herbicide formulations by Eastern red-backed salamanders (Plethodon cinereus). Herp Conserv Biol 6:237–241
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundup® herbicide. Rev Environ Contam Toxicol 167:35–120
Hopey ME, Petranka JW (1994) Restriction of wood frogs to fishfree habitats: how important is adult choice? Copeia 1994:1023–1025
Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL (2000) Quantitative evidence for global amphibian population declines. Nature 404:752–755
Howe CM, Berrill M, Pauli BD, Helbing CC, Werry K, Veldhoen N (2004) Toxicity of glyphosate-based pesticides to four North American frog species. Environ Toxicol Chem 23:1928–1938
Jones DK, Hammond JI, Relyea RA (2010) Roundup® and amphibians: the importance of concentration, application time, and stratification. Environ Toxicol Chem 29:2016–2025
Keen WH (1982) Habitat selection and interspecific competition in two species of plethodontid salamanders. Ecology 63:94–102
Landler L, Gollmann G (2011) Magnetic orientation of the common toad: establishing an arena approach for adult anurans. Front Zool 8:6
Mann RM, Bidwell JR (1999) The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch Environ Contam Toxicol 36:193–199
Perkins PJ, Boermans HJ, Stephenson GR (2000) Toxicity of glyphosate and triclopyr using the frog embryo teratogenesis assay-Xenopus. Environ Toxicol Chem 19:940–945
R Development Core Team (2009) R: a language and environment for statistical computing. R foundation for statistical computing. R Development Core Team, Vienna
Relyea RA (2005) The lethal impact of Roundup® on aquatic and terrestrial amphibians. Ecol Appl 15:1118–1124
Relyea R, Hoverman JT (2006) Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecol Lett 9:1157–1171
Relyea RA, Jones DK (2009) The toxicity of Roundup OriginalMAX® to 13 species of larval amphibians. Environ Toxicol Chem 28:2004–2008
Resetarits WJ Jr, Wilbur HM (1991) Calling site choice by Hyla chrysoscelis: effect of predators, competitors, and oviposition sites. Ecology 72:778–786
Rueppel ML, Brightwell BB, Schaefer J, Marvel JT (1977) Metabolism and degradation of glyphosate in soil and water. J Agric Food Chem 25:517–528
Schlüpmann M, Günther R (1996) Grasfrosch—Rana temporaria LINNAEUS, 1758. In: Günther R (ed) Die Amphibien und Reptilien Deutschlands. Gustav Fischer, Jena, pp 412–454
Skark C, Zullei-Seibert N, Schottler U, Schlett C (1998) The occurrence of glyphosate in surface water. Int J Environ Anal Chem 70:93–104
Smith GR, Todd A, Rettig JE, Nelson F (2003) Microhabitat selection by Northern cricket frogs (Acris crepitans) along a west-central Missouri creek: field and experimental observations. J Herpetol 37:383–385
Smith PN, Cobb GP, Godard-Codding C, Hoff D, McMurry ST, Rainwater TR, Reynolds KD (2007) Contaminant exposure in terrestrial vertebrates. Environ Pollut 150:41–64
Struger J, Thompson D, Staznik B, Martin P, McDaniel T, Marvin C (2008) Occurrence of glyphosate in surface waters of Southern Ontario. Bull Environ Contam Toxicol 80:378–384
Stuart SN, Hoffmann M, Chanson JS, Cox NA, Berridge RJ, Ramani P et al (2008) Threatened amphibians of the world. Lynx Editions, Barcelona
Takahashi M (2007) Oviposition site selection: pesticide avoidance by gray treefrogs. Environ Toxicol Chem 26:1476–1480
Vonesh RJ, Buck JC (2007) Pesticide alters oviposition site selection by bray treefrogs. Oecologia 154:219–226
Vonesh RJ, Kraus JM (2009) Pesticide alters habitat selection and aquatic community composition. Oecologia 160:379–385
Wells KD (1977) The social behaviour of anuran amphibians. Anim Behav 25:666–693
Winkler C, Heunisch G (1997) Fotografische Methoden der Individualerkennung bei Bergmolch (Triturus alpestris) und Fadenmolch (Triturus helveticus) (Urodela, Salamandridae). In: Henle K, Veith M (eds) Naturschutzrelevante Methoden der Feldherpetologie. Deutsche Gesellschaft für Herpetologie und Terrarienkunde, Rheinbach, pp 71–77
Acknowledgments
Permissions to conduct the experiments were obtained by the Landesuntersuchungsamt (Koblenz, Germany) and the Struktur und Genehmigungsdirektion Nord (Koblenz, Germany). N. W. is grateful for financial support from the Graduiertenkolleg at Trier University, which was funded by the German Research Foundation. We are grateful to Willy Werner and Frank Thomas for creating the testing area at the Department of Geobotany, Trier University, available to us. Rainer Ruff and Theresa Scheuren helped with observations and construction of the arenas. Ulrich Schulte helped with frog and newt collecting. Furthermore, we are grateful to Bernd Fontaine for technical support and to Kate Pond for improving the language of the manuscript. Axel Hochkirch, Katharina Wollenberg Vallero, and Michael Veith provided important discussion on the study design and analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagner, N., Lötters, S. Effects of Water Contamination on Site Selection by Amphibians: Experiences from an Arena Approach With European Frogs and Newts. Arch Environ Contam Toxicol 65, 98–104 (2013). https://doi.org/10.1007/s00244-013-9873-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-013-9873-9