Abstract
Effects of endocrine disruptors on reproductive variables of top predators, such as alligators and crocodiles, have long been cited. Due to their long life span, these predators provide us with historic contaminant annals. In this study we tried to test whether lifestyle (free-ranging vs. farm animals) and reproductive age of Morelet’s crocodiles in Campeche, Mexico, affect the bioaccumulation of organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs). Subsequently, we tested to see whether their concentration was related to steroid hormones (testosterone and estradiol-17β) levels once normal cyclic hormone variation and reproductive age had been taken into account. From the group of contaminants considered (analyzed as families), only frequency of hexachlorocyclohexanes (∑HCH) and ∑PCB permitted analyses. Whereas there was a greater concentration of ∑HCH bioaccumulated by free-ranging crocodiles, ∑PCB was found in equal quantities in free-ranging and farm animals. No difference was observed in relation to reproductive age for any of the contaminants. However, ∑PCB concentrations were related to testosterone levels among female crocodiles. This androgenic effect of ∑PCB has not been reported previously. Because testosterone promotes aggressive behavior in vertebrates, excessive aggression during the estrous season, or when female crocodiles should be caring for their young, could result in reproductive failure in Morelet’s crocodiles and potential long-term decline of the population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Endocrine disruption caused by organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) has been witnessed since the 1960s (Colborn et al. 1993). Wildlife studies indicate that even though the body burdens of some persistent chemicals (PCs) have decreased as a result of either a ban or severe limitation of their use (e.g., DDT and its metabolites; dieldrin), the decrease among certain populations has been slow, and biologically significant levels are still observed in many populations (Schmitt et al. 1990; Miller et al. 1992; Tillitt et al. 1992). This is particularly evident in long-lived animals that have experienced the different historic stages of these chemicals (Rowe 2008).
Because of their lipid-soluble character, PCs tend to increase at higher trophic levels (Lintelmann et al. 2003), and it is precisely because of this and owing to the major accidental pesticide spill that took place in Lake Apopka, FL, in 1980 (Woodward et al. 1993), that alligators and crocodiles have frequently been employed as subjects to study their effect on reproduction (Guillette et al. 2000). These studies have shown that among the effects that these contaminants have on reptiles, crocodiles from contaminated lakes manifest developmental defects that are detectable at the time of hatching and persist throughout juvenile life stages. They show decreased clutch viability and increased juvenile crocodile mortality (Woodward et al. 1993); hatchling and juvenile crocodiles demonstrate alterations in plasma estradiol-17 β, testosterone, dihydrotestosterone and thyroxine concentrations, as well as morphological abnormalities of the testis and ovary (Guillette et al. 1994); and juvenile male crocodiles have decreased phallus size, coincident with lower plasma testosterone levels (Guillette et al. 1996; Gunderson et al. 2004). It has also been reported that no clear relationship exists between estradiol and total length in Apopka female crocodiles or between testosterone and total length in Apopka male crocodiles (Crain et al. 1998). Reports also testify that serum contaminant concentrations are not correlated with sex steroid (estradiol-17 β and testosterone) concentrations (Guillette et al. 1999).
Sex hormones are the most potent regulators of reproductive cycles among vertebrates (McLachlan 1993). Testosterone and estradiol-17 β are particularly important for regulating the development and function of reproductive activity and behavior in both sexes. Hormone levels in vertebrates are not constant throughout the life span and/or throughout the year as individuals experience different reproductive states. The reproductive cycle for the American alligator (Alligator mississippiensis) was described by Lance (1989), who cited maximal reproductive activity during late spring, whereas Guillette et al. (1997) demonstrated that female crocodiles exhibit increased ovarian steroidogenesis in September and October. Ovarian and hepatic activity is suppressed during the winter months but resumes rapidly with significant increases in plasma estradiol-17 β during the spring before ovulation in May or June. Likewise, juvenile animals display seasonal variation in terms of plasma sex steroids (Rooney et al. 2004), with the degree of response depending on body size. Alligators exceeding a size threshold of approximately 38 cm from snout to vent begin to show pronounced seasonal variation in estradiol-17ß and testosterone, suggesting a peripubescent period. In the case of Morelet’s crocodiles, it has been indicated that male and female crocodiles reach sexual maturity an age of approximately 7 years and a length of 1.5 m (Platt 1996; Platt and Thorbjarnarson 2000). A recent work that also acknowledges this physiological cyclicity states that future studies of endocrine disruption in ectotherms should consider size-specific responses to endocrine-disrupting chemicals (Crain et al. 1998). It also has previously been reported that alligators and crocodiles of varying sizes may manifest differences in terms of their steroid concentrations (Milnes et al. 2002; Rainwater 2003).
Because differences in lifestyle may result in differences in exposure risk in these long-lived animals, in this study we investigated concentrations of OCPs and PCBs bioaccumulated by wild and farm female and male Morelet’s crocodiles. We also evaluated their steroid hormone levels at two times during the year. Specifically we (1) compared OCP and PCBs bioaccumulated by wild and farmed female and male crocodiles; (2) analyzed whether larger animals showed greater concentrations of contaminants and steroid hormones; and (3) to some extent explored whether concentrations of bioaccumulated contaminants related to hormone levels.
Materials and Methods
Study Areas and Sample Collection
Crocodiles (>75 cm) were sampled during four successive nights at two different sites in Campeche, Mexico (Fig. 1): the Champotón River (19º16″ to 19º22″N and 90º43″ to 90º27″W; 47 km and 650 km2) and the Biosphere Reserve of Los Petenes (20º32″ to 20º42″N and 90º20″ to 90º30″ W). Measurements of OCPs and PCBs found in sediments from these two sites were reported in Gonzalez-Jauregui (2008). Crocodiles were also sampled at the Wildlife Management Unit from the Centro de Estudios Tecnológicos del Mar (CETMar) 02, Campeche.
Samples were collected between October 2005 and May 2006, comprising the posthatch season (November) until the mating season (February) and during the nesting period (May to July) of the reproductive cycle of Morelet’s crocodile (Alvarez del Toro and Sigler 2001). Animals were hand or noose-captured at night from a boat on the Champotón River and by traveling in a truck throughout the Los etenes (collector permit no. SGPA/DGVS/00671). Immediately after capture, a blood sample (approximately 5 mL) was taken from the postcranial sinus and transferred to a lithium heparin-treated Vacutainer. Each crocodile was permanently marked for future identification by removing a unique series of caudal scutes using a sterile scalpel (Rainwater et al. 2007). Subsequently the extracted scutes were covered with aluminum foil and retained for contaminant analysis.
The sex of each animal was determined by cloacal examination of the genitalia (Allsteadt and Lang 1995) and measurements of total body length (TL; measured ventrally), snout–vent length (SVL; measured ventrally from tip of snout to anterior margin of the cloaca) and weight were obtained. Once measurements and sample collections had been completed, each crocodile was released at the site of capture.
Vacutainers and covered scutes were kept in a polyethylene, portable, low-temperature chest until delivery to the Laboratorio de Contaminación e Impacto Ambiental of Centro EPOMEX-UAC. Once in the laboratory, blood samples were centrifuged at 3000 rpm for 5 min, and the plasma supernatant was transferred to a cryotube. Plasma and scutes were kept frozen at −20°C until steroid hormone and PCs analysis.
OC Pesticide and PCB Analyses
We employed the method proposed by Bargar et al. (1999) with certain modifications. All visible fat was removed from each scute, weighed, minced, and allowed to dry for 24 h. Each fat sample was then mixed with anhydrous sodium sulfate and added to 5.0 mL hexane and acetone (1:1) in a P-Selecta ultrasound bath (Barcelona, Spain) for 1 h. Samples were evaporated down to 1 mL before cleanup. The concentrates were loaded onto a chromatographic column (45 cm × 20 mm) containing a glass wool plug, 5 cm activated Florisil, and 2 cm Na2SO4. The column had previously been washed with 50 mL n-hexane. The column was sequentially eluted with 20 mL n-hexane, 20 mL n-hexane and dichloromethane (DCM; 1:1) and 20 mL DCM at a flow rate of 2 mL/min.
The solution of standards Organochlorine Pesticide Mixture from Ultra Scientific (North Kingstown, RI) consisting of α, β, γ, and δ hexachlorocyclohexane (HCH), heptachlor, aldrin, heptachlor epoxide, endosulfan I, dieldrin, p,p DDE, endrin, endosulfan II, endrin aldehido, p,p′ DDD, endosulfan sulphate y p,p′ DDT, and CEN PCB Congener Mix-1 of SUPELCO (Bellefonte, PA) solution along with 2,4′,5-trichlorinated biphenyl, 2,4,4′-trichlorinated biphenyl, 2,2′,5,5′-tetrachlorinated biphenyl, 2,2′,3,5′-tetrachlorinated biphenyl, 2,2′,4,5,5′-pentachlorinated biphenyl, 2,3′,4,4′,5-pentachlorinated biphenyl, 2,2′,4,4′,5,5′-hexachlorinated biphenyl, 2,2′,3,4,4′,5′-hexachlorinated biphenyl, 2,2′,3,3′,4,4′,5,5′-octachlorinated biphenyl, 2,2′,3,4,4′,5,5′-heptachlorinated biphenyl and 2,2′,3,3′,4,4′,5,5′-octachlorinated biphenyl standards was used. Two quality-control samples and one reagent-blank sample were analyzed for every batch of 10 samples. The quality-control samples were spiked with each one of the standards to monitor the efficiency of extraction and analysis. Organic solvents were pesticide or gas chromatography/mass spectrometry grade. A Varian 3800 gas chromatograph from Agilent Technologies, Inc (Wilmington, DE) equipped with a 63Ni electron capture detector and a 30 m × 0.32 mm DB-5 column was used to separate and quantify the PCs. Inlet and detector temperatures were 250°C and 315°C, respectively. The temperature program was as follows: initial temperature 100°C; increased from 100°C to 180°C at 25°C/min; increased from 180°C to 220°C at 3°C/min with a 3-min hold; and increased from 220°C to a final temperature of 300°C at 11°C/min with an 8-minute hold. PCs were identified using congruence of standard, and unknown retention times and were quantified using integration of peak areas. Average recovery percentage equaled 77%; however, sample concentrations were not adjusted for extraction efficiency. The limit of detection for PCs (based on detector response for p,p′-DDE) in scute fat was 3.0 ng/g. All pesticide concentrations were recorded according to dry weight (ng/g).
Steroid Hormone Analysis
Plasma samples were shipped to the Reproductive Endocrine Laboratory of the Department of Reproductive Biology, Universidad Autónoma Metropolitana-Iztapala, for analysis.
Steroid hormones were measured, without further extraction procedures, in duplicate from each sample employing enzyme immunoassay. Testosterone (17β-hydroxy-4-androsten-3-one) and estradiol (1,3,5(10)-estratriene-3,17β-diol) kits from Diagnostic Systems Laboratories (Webster, TX) were used according to the manufacturer instructions. Both procedures use 50 μl plasma (either female or male samples) added to wells in a Microplate Reader MR 600 color spectrophotometer from Dynatech Products (Alexandria, VA). Linearity and antibody specificity were tested with dilutions of known concentration samples and purified testosterone and estradiol-17β standards (Sigma Chemical, St. Louis, MO). Recovery was 94.5% ± 0.88% for testosterone and 89.4% ± 2.4% for estradiol-17β. Cross-reactivity of the testosterone kit was 100% with testosterone and 0.64% ± 2% for estradiol. The estradiol kit had 100% cross-reactivity with estradiol but went undetected with testosterone. Sensitivity was 40 pg/μL for testosterone and 7 pg/μL for estradiol.
Data Analysis
Contaminant concentrations were analyzed as families (∑HCH; ∑heptachlor; ∑drines; ∑endosulphanes; ∑DDT and ∑PCB), but individual values are reported in Table 1. Results did not conform to normality; therefore, nonparametric tests or transformed data in parametric tests were used. For statistical purposes, nondetected values are considered (and included) as zeros.
Because wild animals were captured in two different sites, we ensured they could be treated as a single free-ranging category by performing a Mann–Whitney U-test. Then differences between free-ranging and farmed animals, as well as sex-related differences, were analyzed with independent Mann–Whitney U-tests.
To analyze whether larger animals showed greater concentrations of contaminants and steroid hormones, animals were classified as juvenile (<1.5 m) and adult (>1.5 m). Mann–Whitney U-tests were used in the case of contaminants, and log-transformed hormone concentrations were compared with a two-way analysis of variance. In this case, two periods were included: reproduction (March to April) and posthatch (October to December).
A simple regression analysis was run between rank-transformed (Conover & Iman 1981) contaminant concentrations and log-transformed steroid hormone levels. Analyses were performed independently for female and male crocodiles. All statistics were processed using the computer program Statistica version 7.1 (StatSoft 1984–2006; Tulsa, OK).
Results
A total of 37 animals were captured, and approximately 50% of them (n = 16) showed detectable concentrations of bioaccumulated contaminants: 6 (16.2%) exhibited OCPs, whereas 18 (48.65%) showed PCBs (Table 2).
In terms of the pesticides measured, only ∑HCH were detected in sufficient samples to permit analyses. The other pesticides (∑drines, ∑endosulphanes, ∑heptachlor, and ∑DDT) were found in only one or two individual animals. Consequently, these data were not analyzed.
Wild Versus Farmed Crocodiles PCs
Neither concentrations of ∑HCH or ∑PCB measured in animals from the Champotón River, nor those from Petenes, differed (HCHs: U = 45, n 1 = 6, n 2 = 17, Z = 0.544, p = 0.587 and PCBs: U = 47, n 1 = 6, n 2 = 17, Z = 0.309, p = 0.757); thus, all of the samples were included in a single free-ranging category in all of the analyses.
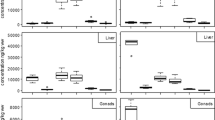
Concentrations of ∑HCH were significantly greater in free-ranging animals (U = 119, n 1 = 23, n 2 = 14, Z = 2.049, p = 0.040; Fig. 2a), but no differences between free-ranging and farmed animals were found in the case of ∑PCB (U = 149.5, n 1 = 23, n 2 = 14, Z = 0.392, p = 0.695; Fig. 2b).
Age–PC and Age–Hormone Concentrations
Because HCH concentrations differed between wild and farm animals that precluded the comparison using other variables due to small sample size. However, PCBs did not differ between the two lifestyles, and all available data were used to compare concentrations between juvenile and adult animals. No differences between juvenile and adult female crocodiles (U = 30, n 1 = 8, n 2 = 9, Z = 0.577, p = 0.518; Fig. 3a), nor between juvenile and adult male crocodiles (U = 46.5, n 1 = 9, n 2 = 11, Z = 0.244, p = 0.807; Fig. 3b), were found.
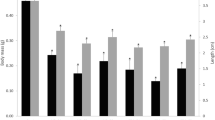
Sex hormones did not present variation between juvenile and adult female crocodiles (E2: F (1,14) = 0.451, P = 0.513; T: F (1,14) = 0.199, p = 0.662) nor for the periods considered (E2: F (1,14) = 0.326, P = 0.577; T: F (1,14) = 0.025, p = 0.877). In the case of the male crocodiles, even though neither estradiol nor testosterone differed between the periods considered (E2: F (1,17) = 2.430, P = 0.137; T: F (1,17) = 0.227, P = 0.640) and estradiol did not differ between juvenile and adult animals (E2: F (1,17) = 1.059, P = 0.318), testosterone did differ between juvenile and adult animals (T: F (1,17) = 9.685, P = 0.006).
PCs and Steroid Hormone Concentrations
Because testosterone levels in male crocodiles varied according to the age of the animals, no further analyses were possible with our data because of lack of statistical power. However, lineal regression analyses indicated that neither estradiol in male (R 2 = 0.000628, F (1,18) = 0.01131, p = 0.916; Fig. 4a) nor in female crocodiles (R 2 = 0.0886, F (1,15) = 1.4577, p = 0.2460; Fig. 4b) covaried with PCB concentrations. However, female testosterone levels significantly varied with PCB levels (R 2 = 0.2637, F (1,15) = 5.3716, p = 0.0350; Fig. 4c).
Discussion
Results from this study indicate that all 15 of the persistent contaminants considered are found in at least one sampled animal. However, only ∑HCH and ∑PCB were found in enough samples to allow comparisons according to the factors considered. Whereas HCHs are found in greater concentrations in free-ranging animals, PCB contaminants are found in wild and farm animals in similar concentrations, and they seem to have an androgenic effect on the female crocodiles.
PC Contaminants: Lifestyle Determinants
Of the contaminants considered in this study, ∑HCH and ∑PCB were most frequently found, but their concentration pattern associated with lifestyle differed. ∑HCH were present only among free-ranging animals, whereas they were not found in the animals from the Wildlife Management Unit at CETMar (02). This finding is particularly interesting because it suggests that wild animals are generally at greater risk of being intoxicated with these pesticides than animals kept in captivity. Thus, it is prey obtained from the wild (insects, aquatic invertebrates, amphibians, fish, reptiles, and mammals [Platt et al. 2006]) that pass the contaminants to the crocodiles. During their early life, crocodiles consume invertebrates, detritus-feeder crustaceans, or prey that feed directly on these organisms; thus, at this stage they are likely to absorb ∑HCH. Although sugar cane plantations located along the riverbanks are probably the source of ∑HCH in the Champotón River, it is not clear where they come from in the case of animals of the Reserve of Los Petenes.
Contrastingly, ∑PCB were found in equal quantities in animals from all of the sites. The source of PCBs in the general study area is not clear; however, although the production of PCBs has ceased, these compounds continue to be detected in environmental samples from all around the world (Breivik et al. 2002). PCB residues have been reported in sediments along the Gulf Coast of Mexico. In the Términos Lagoon, located 150 km north of Champotón river, PCB concentrations ranged from 15.6 to 355.8 pg g−1 (Carvalho et al. 2009). In sediments from the Champotón River, the concentration of these compounds fluctuated from 36 to 7722 pg g−1, and in the Petenes area they ranged from undetectable to ≤515.7 pg g−1 (Gonzalez-Jauregui 2008).
Whereas ∑PCB are employed in the riverine zone of the Champotón River and subsequently deposited in the river with run off, they are probably brought to the Petenes area by demersal fish. This type of fish spends part of its life in the estuarine region and probably acquires ∑PCB on the continental slope of the Gulf of Mexico, where many activities associated with the oil industry takes place. Crocodiles in the wild feed on these fish and they are also used for human consumption. Crocodiles at the wildlife-management unit are fed with fish remains, and this may be the way in which they absorb PCBs. Crocodiles may acquire ∑PCB in the Champotón River when detritus feeders and soil invertebrates bring them into the food web.
PC Contaminants: Ecological Determinants
Crocodile, juvenile and adult, both female and male, did not show significant differences in bioaccumulated PCBs. However, juvenile animals show a greater variation in PCBs bioaccumulated compared with adult animals both in the female and male crocodiles. Our results indicate that although most male crocodiles registered only one contaminant, certain individuals carry a large number of toxic compounds (i.e., 9 or 13 chemicals bioacummulated), whereas female crocodiles present only a small number (i.e., 0 to 3 chemicals bioacummulated). This difference may be due to sex-related (Crain 1998) ecological behaviors that result in differential exposure to contaminants. It is possible that crocodiles and alligators exhibit a similar dispersal pattern to that of other polygynic vertebrates, where male crocodiles migrate far from their natal area, compared with female crocodiles who show fidelity to their nesting sites (Johnson & Gaines 1990; Davies 1991). C. johnstoni male crocodiles in Australia disperse two to three times the distance of female crocodiles (Tucker et al. 1998), whereas C. acutus and C. intermedius female crocodiles are phylopatric (Thorbjarnarson and Hernández 1993; Casas-Andreu 2003). This contrasting behavior may result in varying exposure to particular contaminants.
Our findings showed that approximately 50% of samples were free of PC contaminants and that there was usually only one contaminant present compared with other quantifications of contaminants in the scutes of Morelet’s crocodile (Rainwater et al. 2007), where multiple contaminants were recorded for all of the samples collected. In these studies (developed in Costa Rica), endrin, methoxychlor, p,p′-DDE, and p,p′-DDT occurred in 100% of the scutes analyzed, whereas in Mexico these contaminants do not appear to be as frequent. Of the frequent ∑HCH quantified in our samples, only lindane was tested for and not found in Costa Rica. Concentrations of contaminants found in these two sites cannot be compared because our results are recorded according to fat content (∑drines 0.99 μg/g; ∑DDT 2.01 μg/g), whereas in these other reports whole scute weights are used (∑drines 0.37 μg/g; ∑DDT 0.60 μg/g).
Hormones: Reproductive State and Age
Compared with previous findings in American alligators where seasonal fluctuations are clear (Lance 1989; McLachlan 1993; Guillette et al. 1997; Rooney et al. 2004), neither estradiol nor testosterone (in female or male crocodiles) showed significant variation according to the two periods considered: reproduction and posthatch. This is most probably a result of the distribution of sampled individuals along the months, but it may also be the result of the periods chosen. That is, spring and fall (October) are reported as times of increased steroids (Lance 1989) and we may have just missed times when these levels decreased. Because testosterone is a precursor of estradiol (Norman and Litwack 1997; Norris 1997), this lack of difference in the two hormones may be associated with the steroidogenesis pathways of estrogens.
For male crocodiles, we found an increase in testosterone concentrations related to reaching reproductive maturity. Increased steroid hormones concentrations have been previously reported in adult (>180 cm) versus juvenile (56 to 172 cm) alligators (Rooney et al. 2004).
Endocrine Disruption in Morelet’s Crocodiles?
Estrogen mimicry is the most frequently reported endocrine action resulting from environmental contaminants (McLachlan and Arnold 1996; Crain et al. 1997; Guillette et al. 1994, 2000), and it is probable that often an estrogenic effect has been emphasized because of Bitman et al.’s discovery (1968) that the pesticide DDT is estrogenic.
In our case, compared with previous reports (Guillette et al. 1999), which found no relationship between serum contaminant concentrations and sex steroid levels in alligators, we found that ∑PCB were related to testosterone levels among female crocodiles. This apparent androgenic effect of PCBs may be caused by an increase in gonadal testosterone production (Guillette et al. 2000). This effect has not been reported previously for PCBs, but an estrogenic effect was found in the case of the red eared turtle (Bergeron et al. 1994).
The effect of endocrine disruptors on testosterone levels has previously been reported among male alligators (Guillette et al. 1994, 2000), including an effect on anatomical structures dependant on testosterone for growth and differentiation (Crain 1998). Other species showing similar relations include rats (Guillette et al. 1996); Dall’s porpoises (Phocoenoides dalli [Gross et al. 1995]) and goldfish (Carassius auratus [Connor et al. 1996]). In particular, sexual differentiation in reptiles, which is temperature dependant, may be biased due to hormonal disruptors (Salame-Méndez et al. [2008] for a review).
Conclusion
This study indicates that ∑HCH and ∑PCB are the prevalent contaminants bioaccumulated by Morelet’s crocodiles from the Southern Gulf of Mexico. Concentration of ∑HCH is greater in free-ranging animals, but ∑PCB is found in equal quantities in wild and captive animals. Because ∑PCB have an androgenic effect on female crocodiles, there should be concern about its long-term influence on crocodile populations. Because testosterone promotes aggressive behavior in vertebrates (Bouissou 1983; Nelson 2005), excessive aggression during the estrous season, or when female crocodiles should be caring for their young, could result in reproductive failure.
References
Allsteadt J, Lang JW (1995) Sexual dimorphism in the genital morphology of young American alligators, Alligator mississippiensis. Herpetology 51:314–325
Alvarez del Toro M, Sigler L (2001) Los Crocodylia de Mexico. IMENAR, PROFEPA, Mexico
Bargar TA, Sills-McMurry C, Dickerson RL, Rhodes W, Cobb GP (1999) Relative distribution of polychlorinated biphenyls among tissues of neonatal American alligators (Alligator mississippiensis). Arch Environ Contam Toxicol 37:364–368
Bergeron JM, Crews D, McLachlan JA (1994) PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect 102:780–781
Bitman J, Cecil HC, Harris SJ, Fries GF (1968) Estrogenic activity of o,p′-DDT in the mammalian uterus and avian oviduct. Science 162:371–372
Bouissou ME (1983) Androgens, aggressive behavior and social relationships in greater mammals. Horm Res 18:43–61
Breivik K, Sweetman A, Pacyna JM, Jones KC (2002) Towards a global historical emission inventory for selected PCB congeners—a mass balance approach 1. Global production and consumption. Sci Total Environ 290:181–198
Carvalho FP, Villeneuve JP, Cattini C, Rendón J, Mota de Oliveira J (2009) Pesticide and PCB residues in the aquatic ecosystems of Laguna de Terminos, a protected area of the coast of Campeche, Mexico. Chemosphere 74:988–995
Casas-Andreu G (2003) Ecología de la anidación de Crocodylus acutus (Reptilia: Crocodylidae) en la desembocadura del Río Cuitzmala, Jalisco, México. Acta Zool Mex 89:111–128
Colborn T, vom Saal FS, Soto AM (1993) Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101:378–384
Connor K, Howell J, Chen I, Liu H, Berhane K, Sciaretta C et al (1996) Failure of chloro-s-triazine-derived compounds to induce estrogen receptor-mediated responses in vivo and in vitro. Fundam Appl Toxicol 30:93–101
Conover WJ, Iman RL (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 35:124–129
Crain DA, Guillette LJ Jr, Rooney AA, Pickford DB (1997) Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect 105:1030–1032
Crain DA, Guillette LJ Jr, Pickford DB (1998) Sex-steroid and thyroid hormone concentrations in juvenile alligators (Alligator mississippiensis) from contaminated and reference lakes in Florida, USA. Environ Toxicol Chem 17:446–452
Davies NB (1991) Mating systems. In: Krebs JR, Davies NB (eds) Behavioral ecology: an evolutionary approach. Blackwell, Oxford, pp 263–294
Gonzalez-Jauregui (2008) Relación de concentraciones residuales de una mezcla de plaguicidas organoclorados y policlorobifenilos con la concentración de hormonas sexuales de dos poblaciones de Crocodylus moreletii. Master’s thesis, Institut de Ecología, A.C. Xalapa, Mexico
Gross TS, Crain DA, Bjorndal KA, Bolten AB, Carthy RR (1995) Identification of sex in hatchling loggerhead turtles (Caretta caretta) by analysis of steroid concentrations in chorioallantoic/amniotic fluid. Gen Comp Endocrinol 99:204–210
Guillette LJ Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR (1994) Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect 102:680–688
Guillette LJ Jr, Pickford DB, Crain DA, Rooney AA, Percival HF (1996) Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol 101:32–42
Guillette LJ Jr, Woodward AR, Crain DA, Masson GR, Palmer BD, Cox MC et al (1997) The reproductive cycle of the female American alligator (Alligator mississippiensis). Gen Comp Endocrinol 108:87–101
Guillette LJ Jr, Brock JW, Rooney AA, Woodward AR (1999) Serum concentrations of various environmetal contaminants and their relationship to sex steroid concentrations and phallus size in juvenile American alligators. Arch Environ Contam Toxicol 36:447–455
Guillette LJ, Crain DA, Gunderson MP, Kools SAE, Milnes MR, Orlandod EF et al (2000) Alligators and endocrine disrupting contaminants: a current perspective. Am Zool 40:438–452
Gunderson MP, Bermudez DS, Bryan TA, Degala S, Edwards TM, Kools SAE et al (2004) Variation in sex steroid and phallus size in juvenile American alligator (Alligator mississippiensis) collected from 3 sites witting the Kissmmee-Everglades drainage in Florida (USA). Chemosphere 56:335–345
Johnson ML, Gaines MS (1990) Evolution of dispersal: theoretical models and empirical tests using birds and mammals. Annu Rev Ecol Syst 21:449–480
Lance VA (1989) Reproductive cycle of the American alligator. Am Zool 29:999–1018
Lintelmann J, Katayama A, Kurihara N, Shore L, Wenzel A (2003) Endocrine disruptors in the environment. Pure Appl Chem 75:631–681
McLachlan JA (1993) Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect 101:386–387
McLachlan JA, Arnold SF (1996) Environmental estrogens. Am Sci 84:452–461
Miller MA, Madenjian CP, Masnado RG (1992) Patterns of organochlorine contamination in lake trout from Wisconsin waters of the Great Lakes. J Great Lakes Res 18:742–754
Milnes MR, Woodward AR, Rooney AA, Guillette LJ (2002) Plasma steroid concentrations in relation to size and age in juvenile alligators from two Florida lakes. Comp Biochem Physiol A 131:923–930
Nelson RJ (2005) An introduction to behavioral endocrinology. Sinauer Associates, Sunderland
Norman AW, Litwack G (1997) Hormones. Academic Press, California
Norris DO (1997) Vertebrate endocrinology. California Academic Press, California
Platt SG (1996) Ecology and status of Morelet’s crocodile in Belize. Clemson University, Clemson
Platt SG, Thorbjarnarson (2000) Population status and conservation of Morelet’s crocodile, Crocodylus moreletii, in northern Belize. Biol Conserv 96:21–29
Platt SG, Rainwater TR, Finger AG, Thorbjarnarson JB, Anderson TA, McMurry ST (2006) Food habits, ontogenetic dietary partitioning and observations of foraging behavior of Morelet’s crocodile (Crocodylus moreletii) in Northern Belize. Herpetol J 16:281–290
Rainwater TR (2003) Ecotoxicoloy of Morelet’s crocodile in Belize. Doctoral dissertation, Texas Tech University, TX
Rainwater TR, Wu TH, Finger AG, Cañas JE, Yu L, Reynolds KD et al (2007) Metals and organochlorine pesticides in caudal scutes of crocodiles from Belize and Costa Rica. Sci Total Environ 373:146–156
Rooney AA, Crain DA, Woodward AR, Guillette LJ Jr (2004) Seasonal variation in plasma sex steroid concentrations in juvenile American alligators. Gen Comp Endocrinol 135:25–34
Rowe CL (2008) The calamity of so long life: life histories, contaminants, and potential emerging threats to long-lived vertebrates. Bioscience 58:623–631
Salame-Méndez A, Méndez-de la Cruz F, Aguirre-León G, Serrano H (2008) Disrupción endocrina de la diferenciación sexual. ContactoS 70:43–49
Schmitt CJ, Zajicek JL, Peterman PH (1990) National contaminant biomonitoring program: residues of organochlorine chemicals in U.S. freshwater fish, 1976–1984. Arch Environ Contam Toxicol 19:748–781
Thorbjarnarson JB, Hernández G (1993) Reproductive ecology of the Orinoco crocodile (Crocodylus intermedius) in Venezuela. II. Reproduction and social behavior. J Herpetol 27:371–379
Tillitt DE, Ankley GT, Giesy JP, Ludwig JP, Kurita-Matsuba H, Weseloh DV et al (1992) Polychlorinated biphenyl residues and egg mortality in double-crested cormorants from the Great Lakes. Environ Toxicol Chem 11:1281–1288
Tucker AD, McCallum HI, Limpus CJ, McDonald KR (1998) Sex-biased dispersal in a long-lived polygynous reptile (Crocodylus johnstoni). Behav Ecol Sociobiol 44:85–90
Woodward AR, Jennings ML, Percival HE, Moore CT (1993) Low clutch viability of American alligators on Lake Apopka. Florida Sci 56:52–63
Acknowledgments
M. G.-J. held a CONACyT scholarship for master’s studies (no. 190352) during the period of this investigation. We are extremely thankful to Javier Omar Gomez, Sergio Padilla, and Ernesto Perera for their invaluable help with manipulating the animals. We also thank the personnel from the Centro de Estudios Tecnológicos del Mar 02 (CETMar 02), at Campeche, Mexico, for permission to use their animals in this study. Caroline Karlaske improved the language.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gonzalez-Jauregui, M., Valdespino, C., Salame-Méndez, A. et al. Persistent Organic Contaminants and Steroid Hormones Levels in Morelet’s Crocodiles From the Southern Gulf of Mexico. Arch Environ Contam Toxicol 62, 445–454 (2012). https://doi.org/10.1007/s00244-011-9716-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-011-9716-5